Key Points
Platelets and PAR-4 contribute to the progression of APAP-induced liver injury in mice through independent pathways.
Abstract
Acetaminophen (APAP)-induced liver injury in humans is associated with robust coagulation cascade activation and thrombocytopenia. However, it is not known whether coagulation-driven platelet activation participates in APAP hepatotoxicity. Here, we found that APAP overdose in mice caused liver damage accompanied by significant thrombocytopenia and accumulation of platelets in the liver. These changes were attenuated by administration of the direct thrombin inhibitor lepirudin. Platelet depletion with an anti-CD41 antibody also significantly reduced APAP-mediated liver injury and thrombin generation, indicated by the concentration of thrombin-antithrombin (TAT) complexes in plasma. Compared with APAP-treated wild-type mice, biomarkers of hepatocellular and endothelial damage, plasma TAT concentration, and hepatic platelet accumulation were reduced in mice lacking protease-activated receptor (PAR)-4, which mediates thrombin signaling in mouse platelets. However, selective hematopoietic cell PAR-4 deficiency did not affect APAP-induced liver injury or plasma TAT levels. These results suggest that interconnections between coagulation and hepatic platelet accumulation promote APAP-induced liver injury, independent of platelet PAR-4 signaling. Moreover, the results highlight a potential contribution of nonhematopoietic cell PAR-4 signaling to APAP hepatotoxicity.
Introduction
Acetaminophen (APAP) hepatotoxicity is the leading cause of drug-induced liver injury and acute liver failure in the United States and other developed countries.1-4 Even though APAP hepatotoxicity has been recognized for more than 50 years, the main treatment options for overdose have been limited to early treatment with N-acetylcysteine and liver transplantation in the most severe cases. Many studies have been conducted to understand the mechanisms of APAP hepatotoxicity and to seek alternative therapies. It is commonly understood from early studies in murine models that APAP overdose leads to excessive production of the reactive metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), and consequent depletion of hepatic glutathione (GSH), which is responsible for detoxifying NAPQI. This depletion of GSH permits NAPQI binding to cellular proteins, and this initiating event is followed by a myriad of cellular and molecular events involving parenchymal and nonparenchymal cells that ultimately determine the severity of liver damage.5-10 Evidence supports a role for numerous mediators in the pathogenesis of APAP-induced liver injury, including nitric oxide (NO) and peroxynitrite formation, oxidative stress, mitochondrial injury, alteration in hepatic blood flow, and the innate immune response.8-11
APAP overdose is associated with activation of the coagulation cascade in mice and in humans.12-14 Indeed, consumptive coagulopathy is one of the major clinical signs in acute liver failure from APAP poisoning.12,13 Generation of the coagulation protease thrombin occurs rapidly after administration of a hepatotoxic dose of APAP to mice, and this procoagulant response is tissue factor-dependent.14,15 Coinciding with coagulation cascade activation in clinical APAP-induced liver damage is a decrease in blood platelet concentration, and it has been suggested that platelets contribute to acute liver failure.16 Although some molecules capable of affecting platelet function have been investigated in experimental APAP hepatotoxicity, the role of platelets in APAP-induced liver damage, particularly the link to coagulation, has not been directly investigated.
Thrombin is a direct and potent activator of platelets through the protease-activated receptors (PARs).17-19 Although PAR-1 is the primary thrombin receptor mediating platelet activation in humans,17 mouse platelets respond to thrombin primarily through a complex of PAR-3/PAR-4.19 Thus, our previous observation that PAR-1 deficiency reduced APAP-induced liver injury14 cannot be explained by altered thrombin-mediated platelet activation. Indeed, to date, the role of platelets and thrombin-mediated platelet activation (ie, via PAR-4) in APAP hepatotoxicity has not been explored. In this study, we used a combination of pharmacologic, genetic, and cell-specific depletion strategies to test the hypothesis that platelets and PAR-4 contribute to the procoagulant and hepatotoxic responses accompanying APAP overdose in mice.
Materials and methods
Animals
Male mice between 8 and 16 weeks of age were used for experiments. Wild-type (WT) C57Bl/6J mice (9 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) and were used for lepirudin and for platelet depletion studies. They were acclimated for at least 1 week at approximately 22°C with alternating 12-hour light/dark cycles and access to standard irradiated chow diet (Teklad 22/5 Rodent Diet 8940: Harlan Laboratories) and spring water ad libitum. All procedures were carried out with the approval of the Michigan State University Institutional Animal Care and Use Committee. PAR-4−/− and WT mice of an identical genetic background (N8 C57Bl/6J) were maintained by homozygous breeding, and aged-matched males were used for experiments.20 These mice were maintained in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International at Michigan State University.
Experimental protocols
Mice were fasted for approximately 15 hours before being treated with APAP (300 mg/kg) or saline by intraperitoneal (IP) injection. Two distinct thrombin inhibition studies were performed. For the first, mice were given lepirudin (1 mg/kg, dissolved in saline, IP) or its saline vehicle at −0.5 hour and +0.5 hour, +2.5 hours, and +4.5 hours relative to administration of APAP. For the second study, to avoid any confounding effects of lepirudin on APAP metabolism, lepirudin or saline was administered at +1.5, +2.5, and +4.5 hours after APAP injection. Platelet depletion was accomplished according to the method of Luyendyk et al21 with some modification. Briefly, low-endotoxin and azide-free anti-CD41 antibody (2 mg/kg, mixed in phosphate-buffered saline [PBS]), isotype control rat IgG1κ (2 mg/kg, mixed with PBS), or PBS was injected IP at 12 hours and 2 hours before administration of APAP.
At the termination of the experiments, mice were anesthetized with sodium pentobarbital (50 mg/kg IP) or isoflurane, and blood and liver tissues were collected immediately. Blood was drawn from the caudal vena cava into a syringe containing 3.2% sodium citrate (final concentration, 0.32%) for blood platelet concentration determination and plasma preparation. The left lateral liver lobe was collected and fixed with 10% neutral-buffered formalin for 24 hours and transferred to 70% ethanol prior to embedding for histopathology. The median lobe was collected and flash-frozen in liquid nitrogen for GSH measurement and RNA analysis. The right lobe was collected and embedded in Tissue-Tek O.C.T. (or optimal cutting temperature) medium and frozen in liquid nitrogen-chilled isopentane for immunofluorescence studies.
Bone marrow transplantation
To ablate endogenous bone marrow cells, 8-week-old WT C57Bl/6J mice were exposed to 5.5 Gy, twice, separated by 2 hours, using an X-RAD320 small animal irradiator (Precision X-ray, North Branford, CT). Bone marrow cells (2 × 106) were isolated from the femur and tibia of male mice, WT or PAR-4−/− , as described previously,22 and were injected into the irradiated mice via the retro-orbital sinus. Mice were housed in specific pathogen-free conditions and allowed ad libitum access to sterile rodent chow and acidified water containing sulfatrim (1 mg/mL) for 6 weeks. Then the mice were allowed antibiotic-free water for the next 2 weeks. In preliminary dose-response studies, we found that bone-marrow transplantation increased sensitivity of fasted (15 hours) WT mice to APAP-induced liver injury. Thus, for these studies we used a slightly lower dose of APAP (250 mg/kg), which produced liver injury similar to previous studies. Plasma and liver samples were collected 3 and 24 hours after APAP administration, as described above. Effective reconstitution of donor bone marrow was confirmed by PAR-4 genotyping of anticoagulated whole blood collected from the caudal vena cava. Primers for genotyping were 5′CAGATGTTTCCTGGGCTGGGTG3′; 5′ATTGTGGGTGCCTCAGTGTCCC3′; 5′CAGGGTTTTCCCAGTCACGACG3′.
Liver injury
Liver injury was assessed by plasma alanine aminotransferase (ALT) activity, determined spectrophotometrically, and by morphometric evaluation of left lateral liver lobes. Plasma hyaluronic acid (HA) concentration was determined using a commercial ELISA (Corgenix Inc., Broomfield, CO). Formalin-fixed liver samples were embedded in paraffin, sectioned 5 μm thick and stained with hematoxylin-eosin. Slides were scanned and digitized using the Virtual Slide System VS110 (Olympus, Hicksville, NY) with a ×20 objective. An automated, random subsampling of 60 images was performed on the digitized slides with NewCast software (Visiopharm, Hoersholm, Denmark) at ×200 magnification. Calculation of the percentage of area with hepatocellular necrosis/degeneration (ie, moderate-to-severe irregular vacuolar degeneration adjacent to necrosis) was carried out using a morphometric approach in which areas of necrosis were measured in at least 10 ×40 images in a masked fashion using ImageJ or with the STEPanizer software (http://www.stepanizer.com).23 For the latter analysis, a point grid was superimposed over the images, and points intersecting normal liver parenchyma and hepatocellular necrosis/degeneration were counted. The percentage of liver tissue with hepatocellular necrosis/degeneration was estimated by dividing the number of points intersecting hepatocellular necrosis/degeneration by the number of total points on liver tissue (ie, the sum of the points of normal liver parenchyma and hepatocellular necrosis/degeneration). The accumulation of red blood cells (RBCs) in liver tissue was evaluated similarly.
Platelet accumulation in the liver
Platelet accumulation was performed as described previously24 using an anti-CD41 antibody and fluorescence detection on frozen liver sections. Slides were photographed using an Olympus IX71 inverted fluorescent microscope, with the following fluorescence filter sets: CD41-antibody, excitation 535 nm, emission 645 nm; 4′,6 diamidino-2-phenylindole: excitation 350 nm, emission 460 nm. Quantification of fluorescence was performed using Image J software (imagej.nih.gov/ij/). The integrated intensity of CD41 fluorescence from at least 3 representative microscopic fields per liver section was measured using a ×10 objective, and the average was calculated as one replicate.
Immunohistochemical detection of neutrophils and nitrotyrosine-protein adducts in liver
Tissue fixed in formalin for 24 hours and then immersed in 70% ethanol was embedded in paraffin. Each section was cut 5 μm thick. Sections were rehydrated by placing slides in a container in a water bath at 60°C for 30 minutes, and were then incubated successively with 100% xylazine, 95% ethanol, 80% ethanol, H2O, and PBS for 3 minutes each. Immunohistochemical labeling for neutrophils was performed by the Michigan State University Investigative Histopathology Laboratory, as described previously.25 The sections were labeled with antinitrotyrosine antibody (1:400 diluted with antibody diluent), using universal LSAB+ kit, peroxidase, and protein blocking solution. First, the control slides (no APAP) were evaluated for the background labeling. Afterward, the slides were evaluated without knowledge of treatment using a grading system based on the area of labeling and to a lesser degree, on the labeling intensity relative to controls. Scoring of the area of labeling was as follows: 0 = no to rare hepatocyte labeling; 1 = labeling of 1 to 2 layers of hepatocytes around few to many central venules; 2 = labeling of 2 to 4 layers of hepatocytes around most central venules with no bridging of labeled areas; 3 = labeling of 2 to 4 layers of hepatocytes around most central venules, with infrequent and narrow bridging of labeled areas; 4 = labeling of ≥5 layers of hepatocytes around most central venules, with moderat-to-extensive bridging. Intensity of labeling was graded as follows: 0 = no labeling; 1.2 = mild; 1.5 = moderate; 1.8 = marked. Scores from the area and intensity of the labeling were multiplied together to obtain a summary score; intensity-weighting factors were chosen to be evenly distributed between 1 and 2 such that intensity would augment but not dominate area scores at each grade (supplemental Figure 1, available on the Blood Web site).
Statistical analysis
Data are expressed as mean ± standard error of the mean. Data that were not normally distributed were subjected to appropriate transformation (eg, logarithmic, square root, arcsin squared). Student t test was used to compare two means, and analysis of variance was used when more than 2 means were compared. If a difference was detected with analysis of variance, appropriate post hoc testing (Holm-Sidak) was performed. If data transformation failed to produce a normal distribution, nonparametric tests were performed (Mann-Whitney U test for comparison of 2 means, and Kruskal-Wallis 1-way analysis of variance for multiple comparisons). P < .05 was set as the criterion for statistical significance.
Details of experimental methods, including materials, measurement of blood platelet concentration, liver GSH and APAP-Cys adduct measurement, and real-time polymerase chain reaction are described in the supplemental Data.
Results
APAP overdose in mice causes depletion of blood platelets and accumulation of platelets in liver
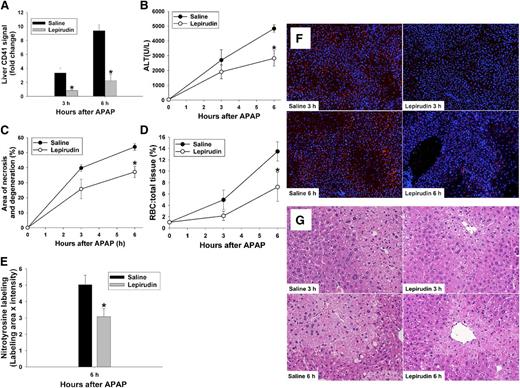
In agreement with previous studies,14 plasma ALT activity increased within 3 hours of APAP administration and continued to increase thereafter (Figure 1A). APAP administration caused a decrease in blood platelet concentration within 6 hours (Figure 1B). Hepatic accumulation of platelets (ie, CD41 detected in liver by immunofluorescence) in APAP-treated mice began between 0.5 and 2 hours and continued through 6 hours (Figure 1C). The accumulation began as a diffuse increase in signal (Figure 1D) at 2 hours and progressed to more concentrated signals that appeared to radiate from the central venules (Figure 1D) at 3 and 6 hours.
Platelet accumulation during development of APAP-induced liver injury in mice. C57BL/6J mice were treated with 300 mg/kg APAP or with saline vehicle (0 hr) IP. (A) ALT activity in plasma. (B) Concentration of platelets in blood. (C) Quantification of CD41 signal in the liver at 0.5, 2, 3, and 6 hours. Signal intensity of CD41 was normalized to the CD41 signal at time 0. (D) Representative photomicrographs of frozen liver sections labeled with anti-CD41 antibody to identify platelets; 4′,6 diamidino-2-phenylindole stain was used to identify nuclei. Platelets are pseudocolored red; nuclei are pseudocolored blue. (Original magnification ×100) #Significantly different from the saline-treated group (n = 5 mice [0 hour], 5 mice [0.5 hours], 3 mice [2 hours], 7 mice [3 hours], and 5 mice [6 hours]).
Platelet accumulation during development of APAP-induced liver injury in mice. C57BL/6J mice were treated with 300 mg/kg APAP or with saline vehicle (0 hr) IP. (A) ALT activity in plasma. (B) Concentration of platelets in blood. (C) Quantification of CD41 signal in the liver at 0.5, 2, 3, and 6 hours. Signal intensity of CD41 was normalized to the CD41 signal at time 0. (D) Representative photomicrographs of frozen liver sections labeled with anti-CD41 antibody to identify platelets; 4′,6 diamidino-2-phenylindole stain was used to identify nuclei. Platelets are pseudocolored red; nuclei are pseudocolored blue. (Original magnification ×100) #Significantly different from the saline-treated group (n = 5 mice [0 hour], 5 mice [0.5 hours], 3 mice [2 hours], 7 mice [3 hours], and 5 mice [6 hours]).
APAP-induced hepatic platelet accumulation and liver injury are thrombin-dependent
We demonstrated previously that inhibition of thrombin with heparin reduces APAP-mediated hepatic damage,14 but heparin could impact liver injury through its nonanticoagulant effects.26 Thus, to assess better whether thrombin activity contributes to platelet accumulation in APAP-treated mice, we used the direct and specific thrombin inhibitor, lepirudin.27 Lepirudin pretreatment significantly reduced hepatic platelet accumulation 3 and 6 hours after APAP administration (Figure 2A,F). To evaluate liver injury, plasma ALT activity and area of necrosis/degeneration were determined (Figure 2B-C,G). Hepatic necrosis was characterized as centrilobular coagulative necrosis, and degeneration of hepatocytes was occasionally observed bordering the necrotic area. Lepirudin pretreatment significantly reduced the increase in plasma ALT activity and area of necrosis/degeneration that occurred 6 hours after APAP treatment. There was a significant decrease in accumulation of RBCs, mostly adjacent to necrotic areas, in the livers of lepirudin-pretreated mice (Figure 2D,G). Qualitative histologic evaluation suggested that the increase in RBCs was due to congestion within the sinusoids at 3 and 6 hours.
Effect of lepirudin on APAP hepatotoxicity. Mice were treated with 300 mg/kg APAP or vehicle IP at time 0 hour. Lepirudin (1 mg/kg) or saline was given IP at −0.5 hour, and at +0.5, +2.5, and +4.5 hours relative to administration of APAP. (A) Hepatic CD41 signal at 3 and 6 hours, intensity normalized to the CD41 signal of WT mice that were not treated with either APAP or lepirudin. (B) ALT activity in plasma. (C) Area of necrosis and degeneration as a percentage of total liver area. (D) RBC to total cell (%) in sections of liver tissue. (E) Nitrotyrosine label at 6 hours quantified as described in “Materials and methods.” (F) Representative photomicrographs of frozen liver sections labeled with anti-CD41 antibody to identify platelets, using 4,6 diamidino-2-phenylindole stain to identify nuclei. Platelets are pseudocolored red; nuclei are pseudocolored blue. (Original magnification ×100) (G) Representative liver sections from each group at 3 and 6 hours after APAP injection. (Original magnification ×200). *Significantly different from the saline-pretreated group at same time (N = 4 mice per group for each treatment/time).
Effect of lepirudin on APAP hepatotoxicity. Mice were treated with 300 mg/kg APAP or vehicle IP at time 0 hour. Lepirudin (1 mg/kg) or saline was given IP at −0.5 hour, and at +0.5, +2.5, and +4.5 hours relative to administration of APAP. (A) Hepatic CD41 signal at 3 and 6 hours, intensity normalized to the CD41 signal of WT mice that were not treated with either APAP or lepirudin. (B) ALT activity in plasma. (C) Area of necrosis and degeneration as a percentage of total liver area. (D) RBC to total cell (%) in sections of liver tissue. (E) Nitrotyrosine label at 6 hours quantified as described in “Materials and methods.” (F) Representative photomicrographs of frozen liver sections labeled with anti-CD41 antibody to identify platelets, using 4,6 diamidino-2-phenylindole stain to identify nuclei. Platelets are pseudocolored red; nuclei are pseudocolored blue. (Original magnification ×100) (G) Representative liver sections from each group at 3 and 6 hours after APAP injection. (Original magnification ×200). *Significantly different from the saline-pretreated group at same time (N = 4 mice per group for each treatment/time).
Nitrotyrosine-protein adducts arising from NO generation appear early in livers of mice overdosed with APAP.28 Thrombin increases expression of inducible NO synthase in glioma cells,29 and it activates endothelial NO synthase and causes NO release from endothelial cells through activation of PARs.30,31 Thus, the enhancement of NO production through thrombin may contribute to peroxynitrite formation and consequent nitrotyrosine-protein adducts in liver tissue. Accordingly, immunohistochemistry labeling for nitrotyrosine-protein adducts was evaluated as a biomarker of peroxynitrite formation in liver tissue. Treatment with lepirudin significantly reduced the nitrotyrosine signal in the livers of APAP-treated mice (Figure 2E).
Depletion of GSH has been used extensively as a biomarker for the formation of the reactive metabolite, NAPQI.5,9,32,33 APAP administration caused more than 80% reduction in liver GSH, and this was unaffected by lepirudin pretreatment (supplemental Figure 2A), suggesting that inhibition of APAP-induced liver injury by lepirudin was not a function of altered APAP metabolism.
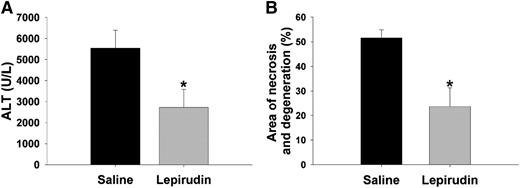
APAP bioactivation and covalent binding of NAPQI to liver proteins occurs in mice within 1.5 hours after a toxic dose of APAP.34 To test the effect of a thrombin inhibitor given after APAP bioactivation had occurred, lepirudin treatment was started 1.5 hours after APAP administration. The increase in plasma ALT activity and histopathologic changes 6 hours after APAP were less severe in mice given lepirudin compared with mice given saline vehicle (Figure 3A-B).
Effect of lepirudin given after APAP on hepatotoxicity. Mice were treated with 300 mg/kg APAP IP at time 0 hour. Lepirudin (1.5 mg/kg) or saline was given at 1.5, 2.5, and 4.5 hours after APAP or saline. Liver injury was determined 6 hours after APAP administration. (A) Plasma ALT activity. (B) Area of necrosis and degeneration. *Significantly different from the saline-pretreated group (N = 7 mice per group for each treatment/time).
Effect of lepirudin given after APAP on hepatotoxicity. Mice were treated with 300 mg/kg APAP IP at time 0 hour. Lepirudin (1.5 mg/kg) or saline was given at 1.5, 2.5, and 4.5 hours after APAP or saline. Liver injury was determined 6 hours after APAP administration. (A) Plasma ALT activity. (B) Area of necrosis and degeneration. *Significantly different from the saline-pretreated group (N = 7 mice per group for each treatment/time).
Platelets contribute to APAP-induced liver injury and coagulation
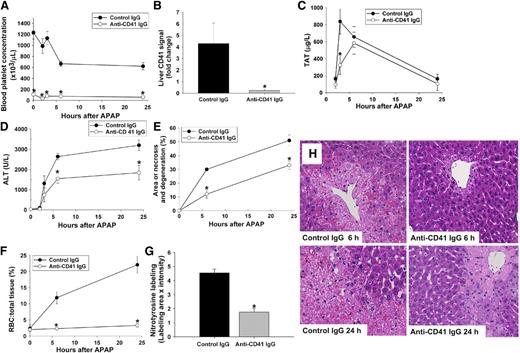
To evaluate whether platelets are required for APAP-induced liver damage, platelets were depleted in mice using an antibody to the platelet-specific integrin, CD41. Pretreatment with anti-CD41 antibody decreased the platelet concentration in blood at the time of APAP administration (0 hours) by >90% compared with control IgG-pretreated mice (Figure 4A). This difference was apparent before and up to 24 hours after APAP administration and was accompanied by attenuation of hepatic platelet accumulation and thrombin generation (estimated from plasma thrombin-antithrombin [TAT] concentration) in APAP-treated mice (Figure 4B-C). Interestingly, plasma TAT levels were affected by platelet depletion only at 3 hours post-APAP, suggesting an early and transient contribution of platelets to the generation of thrombin after APAP overdose. Platelet depletion significantly reduced APAP-induced liver injury at both 6 and 24 hours (Figure 4D-E,H). Hepatic RBC accumulation apparent in mice given APAP and control IgG was reduced in platelet-depleted mice (Figure 4F,H). Moreover, hepatic neutrophil accumulation was attenuated in livers of platelet-depleted mice given APAP (supplemental Figure 3). Hepatic GSH concentration 30 minutes after APAP treatment was not affected by platelet depletion (supplemental Figure 2B). It is possible that antibody pretreatment triggers induction of heme oxygenase-1 and metallothionein, which are known to reduce APAP hepatotoxicity35 ; however, treatment with either control IgG or anti-CD41 IgG did not affect expression of these genes (supplemental Figure 2C-D). As observed with lepirudin pretreatment, the nitrotyrosine signal in liver sections was significantly reduced in platelet-depleted mice compared with control mice at 6 hours after APAP administration (Figure 4G).
Effect of platelet depletion on APAP-induced hepatotoxicity. Mice were treated with 300 mg/kg APAP or vehicle IP at time 0 hour. Anti-CD41 antibody or isotype control antibody (2 mg/kg) was given IP 12 and 2 hours before administration of APAP. (A) Blood platelet concentration. (B) Quantification of CD41 signal in the liver at 3 hours after APAP injection. Signal intensity of CD41 from different groups was normalized to saline-pretreated group at time 0 hour. (C) Concentration of TAT complexes in plasma over time. (D) ALT activity in plasma. (E) Area of liver necrosis and degeneration. (F) RBC to total liver tissue (%). (G) Nitrotyrosine label at 6 hours at after APAP injection quantified as described in “Materials and methods.” (H) Representative liver sections from each group at 6 and 24 hours after APAP injection (×20 objective). *Significantly different from control IgG group at the same time. For mice given control IgG, the number of mice per group was 5 (0 hour), 3 (2 hours), 7 (3 hours), 13 (6 hours), and 13 (24 hours). For mice given anti-CD41 IgG, the number of mice per group was 5 (0 hour), 4 (2 hours), 7 (3 hours), 15 (6 hours), and 15 (24 hours).
Effect of platelet depletion on APAP-induced hepatotoxicity. Mice were treated with 300 mg/kg APAP or vehicle IP at time 0 hour. Anti-CD41 antibody or isotype control antibody (2 mg/kg) was given IP 12 and 2 hours before administration of APAP. (A) Blood platelet concentration. (B) Quantification of CD41 signal in the liver at 3 hours after APAP injection. Signal intensity of CD41 from different groups was normalized to saline-pretreated group at time 0 hour. (C) Concentration of TAT complexes in plasma over time. (D) ALT activity in plasma. (E) Area of liver necrosis and degeneration. (F) RBC to total liver tissue (%). (G) Nitrotyrosine label at 6 hours at after APAP injection quantified as described in “Materials and methods.” (H) Representative liver sections from each group at 6 and 24 hours after APAP injection (×20 objective). *Significantly different from control IgG group at the same time. For mice given control IgG, the number of mice per group was 5 (0 hour), 3 (2 hours), 7 (3 hours), 13 (6 hours), and 13 (24 hours). For mice given anti-CD41 IgG, the number of mice per group was 5 (0 hour), 4 (2 hours), 7 (3 hours), 15 (6 hours), and 15 (24 hours).
APAP-induced hepatic platelet accumulation and injury are PAR-4-dependent
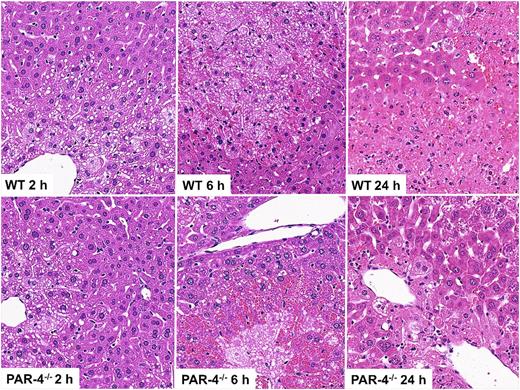
Because we found that thrombin activity contributed to hepatic platelet accumulation after APAP overdose and that depletion of platelets attenuated APAP-induced liver damage, we examined the role of the platelet thrombin receptor, PAR-4.36 Early hepatic platelet accumulation (2 hours) was significantly reduced in APAP-treated PAR-4−/− mice compared with APAP-treated WT mice (Figure 5A), and the increase in plasma TAT concentration was attenuated (Figure 5B). The reduction in hepatic platelet accumulation in PAR-4−/− mice was accompanied by a significant reduction in APAP-induced liver injury, indicated by reduced plasma ALT activity and liver necrosis at both 6 and 24 hours (Figures 5C-D and 6). Additionally, PAR-4 deficiency significantly attenuated plasma HA levels after APAP administration (Figure 5G), suggesting a reduction in liver sinusoidal endothelial cell (LSEC) damage. Qualitative histologic assessment suggested that accumulation of RBCs in hepatic lesions at 6 hours was mainly related to sinusoidal congestion, and this was more pronounced in PAR-4−/− mice (Figures 5E and 6). In contrast, RBC accumulation at 24 hours was predominantly due to hemorrhage and was less severe in PAR-4−/− mice than in WT mice treated with APAP. As with lepirudin pretreatment (Figure 2E) and platelet depletion (Figure 4G), there was a significant decrease in nitrotyrosine labeling in liver tissue collected at 6 hours from PAR-4−/− mice (Figure 5F). The protection afforded by PAR-4 deficiency could not be ascribed to an effect on hepatic GSH depletion after APAP administration (supplemental Figure 2E).
APAP-induced liver injury in WT and PAR-4−/− mice. PAR-4−/− mice and matching WT mice were treated with 300 mg/kg APAP IP. (A) Quantification of CD41 signal in the liver at 2 hours. Signal intensity of CD41 was normalized to that of WT mice not treated with APAP. (B) TAT concentration in plasma at 2 hours after APAP injection. (C) ALT activity in plasma. (D) Area of necrosis and degeneration in the liver. (E) RBC to total liver tissue (%). (F) Nitrotyrosine label quantified in liver sections as described in “Materials and methods” at 6 hours after APAP injection. (G) Plasma hyaluronic acid was determined 24 hours after APAP administration (n = 6-7 mice per group). &Significantly different from all the other groups. *Significantly different from the WT group at same time. #Significantly different from APAP-treated WT mice. For WT mice, the number of mice per group was 4 (0 hour), 13 (2 hours), 10 (6 hours), and 13 (24 hours). For PAR-4−/− mice, the number of mice per group was 3 (0 hour), 10 (2 hours), 9 (6 hours), and 14 (24 hours).
APAP-induced liver injury in WT and PAR-4−/− mice. PAR-4−/− mice and matching WT mice were treated with 300 mg/kg APAP IP. (A) Quantification of CD41 signal in the liver at 2 hours. Signal intensity of CD41 was normalized to that of WT mice not treated with APAP. (B) TAT concentration in plasma at 2 hours after APAP injection. (C) ALT activity in plasma. (D) Area of necrosis and degeneration in the liver. (E) RBC to total liver tissue (%). (F) Nitrotyrosine label quantified in liver sections as described in “Materials and methods” at 6 hours after APAP injection. (G) Plasma hyaluronic acid was determined 24 hours after APAP administration (n = 6-7 mice per group). &Significantly different from all the other groups. *Significantly different from the WT group at same time. #Significantly different from APAP-treated WT mice. For WT mice, the number of mice per group was 4 (0 hour), 13 (2 hours), 10 (6 hours), and 13 (24 hours). For PAR-4−/− mice, the number of mice per group was 3 (0 hour), 10 (2 hours), 9 (6 hours), and 14 (24 hours).
APAP-induced liver injury in WT and PAR-4−/− mice. Mice were treated as mentioned in the legend to Figure 5. Representative sections from WT and PAR-4−/− mice taken 2, 6, or 24 hours after treatment with APAP (original magnification ×200).
APAP-induced liver injury in WT and PAR-4−/− mice. Mice were treated as mentioned in the legend to Figure 5. Representative sections from WT and PAR-4−/− mice taken 2, 6, or 24 hours after treatment with APAP (original magnification ×200).
Hematopoietic cell PAR-4 does not contribute to APAP-induced coagulation or hepatotoxicity
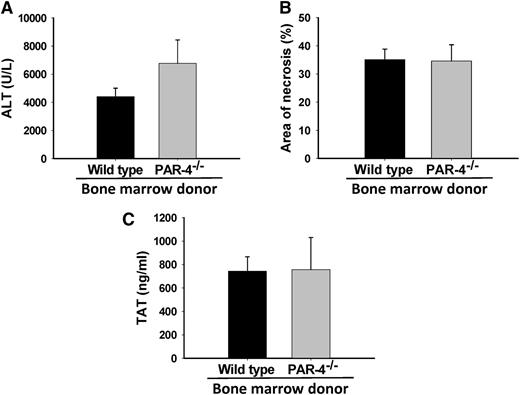
The impact of hematopoietic cell PAR-4 deficiency on bleeding and thrombosis is recognized to occur due to a significant reduction in thrombin-mediated platelet activation.36 To determine the effect of selective hematopoietic cell PAR-4 deficiency on APAP-induced liver injury, we generated chimeric WT mice with either WT or PAR-4−/− bone marrow. Eight weeks after transplantation, whole blood genotyping confirmed efficient reconstitution of cells from each donor genotype (data not shown). To our surprise, 24 hours after administration of APAP, hematopoietic cell PAR-4 deficiency did not impact APAP-induced liver injury, as estimated by serum ALT activity and hepatic necrosis (Figure 7A-B). Likewise, hematopoietic cell PAR-4 deficiency had no effect on plasma TAT levels 3 hours after APAP administration (Figure 7C). Importantly, hematopoietic cell PAR-4 deficiency had no effect on APAP metabolism, as indicated by similar levels of protein-derived APAP-Cys adducts in livers of mice with WT and PAR-4 bone marrow (0.60 ± 0.03 nmol/mg liver vs 0.75 ± 0.13 nmol/mg liver, respectively).
Effect of hematopoietic cell PAR-4 deficiency on APAP-induced liver injury in mice. WT mice were irradiated and reconstituted with either WT or PAR-4−/− bone marrow, as described in “Materials and methods.” Mice were given 250 mg/kg APAP IP. (A) ALT activity in plasma at 24 hours. (B) Area of hepatocellular necrosis at 24 hours, and (C) TAT concentration in plasma at 3 hours. No significant differences were observed. For ALT and necrosis, N = 13 mice with WT bone marrow and 9 with PAR-4−/− bone marrow. For TAT, N = 5 mice with WT bone marrow and 4 with PAR-4−/− bone marrow.
Effect of hematopoietic cell PAR-4 deficiency on APAP-induced liver injury in mice. WT mice were irradiated and reconstituted with either WT or PAR-4−/− bone marrow, as described in “Materials and methods.” Mice were given 250 mg/kg APAP IP. (A) ALT activity in plasma at 24 hours. (B) Area of hepatocellular necrosis at 24 hours, and (C) TAT concentration in plasma at 3 hours. No significant differences were observed. For ALT and necrosis, N = 13 mice with WT bone marrow and 9 with PAR-4−/− bone marrow. For TAT, N = 5 mice with WT bone marrow and 4 with PAR-4−/− bone marrow.
Discussion
Strong evidence continues to emerge indicating a contribution of hemostatic system components to APAP hepatotoxicity. We reported previously that PAR-1 deficiency or administration of anticoagulant heparin reduced APAP-induced liver damage.14 Here we further delineated the role of thrombin in APAP-induced hepatotoxicity using the specific inhibitor, lepirudin. Building on our previous findings with heparin, lepirudin attenuated APAP-induced liver damage irrespective of whether it was given before or after APAP bioactivation. We demonstrated that platelets accumulate in the liver before the onset of liver damage and that platelets contributed to APAP-induced liver damage. The accumulation of platelets was attenuated by administration of lepirudin. Moreover, depletion of platelets blunted the early increase in plasma TAT in APAP-treated mice. Collectively, these results suggest that platelets contribute to APAP-induced liver damage and that there is an interdependence of early coagulation cascade activation and platelet activity after APAP overdose.
Thrombin’s proteolytic activity is fundamentally linked to platelet activation through PARs, and in the mouse, thrombin-mediated platelet activation occurs via PAR-4.36 Global loss of PAR-4 and hematopoietic cell-selective PAR-4 deficiency are each associated with prolonged bleeding time in mouse models of vascular injury because platelets no longer respond to thrombin.36 Thus, we considered the possibility that PAR-4 activation was required for platelet accumulation and contributed to the progression of liver injury in APAP-treated mice. Indeed, early coagulation cascade activation and hepatic platelet accumulation were reduced in APAP-treated PAR-4−/− mice, and this was associated with a reduction in hepatocellular necrosis. This protection could not be explained by a reduction in APAP bioactivation in the PAR-4−/− mice. These results suggest that PAR-4 plays an important role in the progression of APAP hepatotoxicity.
Surprisingly, hematopoietic cell-specific PAR-4 deficiency had no effect on coagulation cascade activation or hepatic injury in APAP-treated mice. These results indicate that hematopoietic cell PAR-4 is dispensable for APAP-induced liver damage. Coupled with the injury reduction afforded by global PAR-4 knockout, this result suggests that PAR-4 on cells other than platelets contributes to APAP hepatotoxicity. Although widely appreciated for its role in platelet activation, PAR-4 expression has been reported on several cell types residing in the liver, including endothelial cells.37-39 However, additional studies are required to identify the responsible PAR-4–expressing cell(s) that contribute(s) to APAP-induced liver damage. A potential candidate is the LSEC. Endothelial injury occurs in mice as well as humans during APAP toxicity.40-42 The mechanism whereby LSEC dysfunction occurs during APAP hepatotoxicity is not entirely clear. Here, we found that LSECs were injured in APAP-treated WT mice, as noted by an increase in plasma HA concentration. This increase was blunted in APAP-treated PAR-4-deficient mice, suggesting that PAR-4 activation drives endothelial dysfunction. This thrombin-dependent LSEC dysfunction could be responsible for provoking hepatic platelet accumulation and exacerbation of hepatic injury. PAR-4 and PAR-1 have each been shown to elicit intracellular signaling and transcriptional changes in endothelial cells, including increased NO production.37-39,43 Increased NO production and the presence of peroxynitrite is observed in LSECs after APAP overdose28 and is postulated to exacerbate the subsequent hepatocellular injury.44,45 Consistent with this, we found that nitrotyrosine labeling was significantly attenuated in APAP-treated PAR-4-deficient mice. Of importance, a higher plasma HA concentration has been associated with poor outcome in APAP overdose patients.40 Thus, uncovering the cellular mechanism whereby PAR-4 exacerbates APAP-induced endothelial dysfunction has the potential to result in novel therapies to reduce APAP-induced liver damage.
Our results definitively link platelets to the progression of APAP-induced liver damage in mice, although the mechanisms whereby platelets accumulate and contribute to early APAP-induced liver damage remain to be elucidated. It is conceivable that platelet-mediated, early amplification of thrombin generation promotes liver injury through either PAR-4 or PAR-1 signaling. Alternatively, platelets could contribute to APAP hepatotoxicity by exacerbating the accumulation of potentially damaging inflammatory cells. Indeed, in experimental settings where neutrophils are definitively linked to hepatotoxicity, the interaction of platelets and neutrophils worsens liver damage.46-48 We found that platelet depletion tended to reduce accumulation of neutrophils within necrotic foci in APAP-treated mice. It is worth noting, however, that the role of neutrophils in APAP hepatotoxicity continues to be debated.8,49-51 Although we cannot exclude the possibility that activated platelets promote neutrophil-driven exacerbation of injury, it is also possible that reduced neutrophil accumulation in platelet-depleted mice reflects reduced necrosis (ie, a diminished recruitment of neutrophils secondary to necrosis). Additional studies are required to elucidate the exact mechanism, whereby platelets drive early APAP-induced liver damage.
The lack of effect of hematopoietic PAR-4 deficiency on APAP-induced liver damage suggests that direct thrombin-platelet interaction is not essential. Similarly, administration of the P2Y12 inhibitor clopidogrel did not affect APAP-induced mortality in mice,52 suggesting that ADP-mediated activation of platelets plays a minimal role. Administration of the cyclooxygenase (COX)-1 inhibitor aspirin has been shown to reduce APAP-induced liver injury in mice,52,53 suggesting involvement of COX-derived mediators in APAP hepatotoxicity. Indeed, inhibitors of thromboxane A2 synthesis significantly reduced APAP hepatotoxicity54,55 and elevated the levels of prostacyclin, the administration of which protected mice from APAP hepatotoxicity.55 In contrast, other studies found no effect of the COX inhibitors, indomethacin and SC560, on APAP-induced liver injury and mortality.52,55 Interpretation of these various results is complicated by lack of confirmation of selectivity of these agents for COX-1 or COX-2 at the administered dose, which is important because selective COX-2 deficiency exacerbates APAP hepatotoxicity.56 Finally, recent clinical studies identified an imbalance in von Willebrand factor and its cleaving protease ADAMTS13 in patients with acute liver failure.16 Accordingly, it was suggested that platelet-induced microthrombus formation could contribute to liver damage.16 Notably, the standard therapy for APAP overdose in patients (N-acetylcysteine) was found to reduce the levels of von Willebrand factor multimers in plasma.57 These connections highlight the need for additional studies to define which agonists of platelet activation and aggregation contribute to APAP-induced liver injury.
In conclusion, evidence exists for concurrent activation of the coagulation system and platelets in humans after APAP overdose. This is supported by our findings of platelet sequestration in livers of mice given a hepatotoxic dose of APAP. The mechanisms whereby platelets contribute to APAP-induced liver injury are not entirely understood. The present studies coupled with our previous findings suggest that platelet-dependent amplification of thrombin activity could promote liver injury through PAR-4 and/or PAR-1–dependent mechanisms. This forms the basis for additional studies defining the intricacies whereby these various mediators promote APAP-induced liver damage. Such studies have potential to direct new therapies for APAP hepatotoxicity given the spectrum of drugs approved by the Food and Drug Administration that target platelets, thrombin, and PARs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK087886 and R01 DK105099).
Authorship
Contribution: K.M., N.J., B.P.S., C.B., H.J., M.R.M., M.A.S., P.E.G., J.P.L., and R.A.R. designed research; K.M., N.J., B.P.S., R.A., H.J., M.R.M., J.P.L., and M.A.S. performed research; B.P.S. collected data; K.M., N.J., C.B., P.E.G., J.P.L., and R.A.R. analyzed data; K.M., N.J., B.P.S., C.B., M.A.S., P.E.G., J.P.L., and R.A.R. interpreted data; K.M. performed statistical analysis; K.M. N.J., P.E.G., J.P.L., and R.A.R wrote the manuscript; and B.P.S., R.A., C.B., H.J., M.R.M., and M.A.S. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.B. is Institute of Functional and Applied Anatomy, Hannover Medical School, Hannover, Germany.
Correspondence: Robert A. Roth, 1129 Farm Lane, 221 Food Safety and Toxicology Building, Michigan State University, East Lansing, MI 48824; e-mail: rothr@msu.edu.

![Figure 1. Platelet accumulation during development of APAP-induced liver injury in mice. C57BL/6J mice were treated with 300 mg/kg APAP or with saline vehicle (0 hr) IP. (A) ALT activity in plasma. (B) Concentration of platelets in blood. (C) Quantification of CD41 signal in the liver at 0.5, 2, 3, and 6 hours. Signal intensity of CD41 was normalized to the CD41 signal at time 0. (D) Representative photomicrographs of frozen liver sections labeled with anti-CD41 antibody to identify platelets; 4′,6 diamidino-2-phenylindole stain was used to identify nuclei. Platelets are pseudocolored red; nuclei are pseudocolored blue. (Original magnification ×100) #Significantly different from the saline-treated group (n = 5 mice [0 hour], 5 mice [0.5 hours], 3 mice [2 hours], 7 mice [3 hours], and 5 mice [6 hours]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/15/10.1182_blood-2014-09-598656/4/m_1835f1.jpeg?Expires=1769796780&Signature=UAj0SoEGwQIPK-ijbY2zur~bIGIoOopcALL1zgDktL~66F0YZpB8mveQ~ggPJBvvkf9iV93Z-qtGBUqLScfYqWIzeriGHszoPREpi3KBmAqSGUgeaATH9Xev3x-JKjIXkjN~Kn3wi7UrX0QAcNPrYnIUEFRDIkqys8jCxNkaAZIV3mONxXG-eZHEMAQig69V~ZjAOrk8hV31lSQAQJLulG6QKVOZwO-gp4kB8IXGrMa4pyr~nN2oXQI3VNOtQg~elb95QqXh-oAbRCK~ASFZeKdNztR-wNUE6DUjULwzPG6frVT9x2-~sNJOYOD18Kb6i8eRI0xATJSwoypnwwc~nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal