Key Points
Hypermorphic PLCγ2 is independent of BTK activation.
SYK or LYN inhibition antagonizes mutant PLCγ2-mediated signaling events.
Abstract
Ibrutinib has significantly improved the outcome of patients with relapsed chronic lymphocytic leukemia (CLL). Recent reports attribute ibrutinib resistance to acquired mutations in Bruton agammaglobulinemia tyrosine kinase (BTK), the target of ibrutinib, as well as the immediate downstream effector phospholipase C, γ2 (PLCG2). Although the C481S mutation found in BTK has been shown to disable ibrutinib’s capacity to irreversibly bind this primary target, the detailed mechanisms of mutations in PLCG2 have yet to be established. Herein, we characterize the enhanced signaling competence, BTK independence, and surface immunoglobulin dependence of the PLCG2 mutation at R665W, which has been documented in ibrutinib-resistant CLL. Our data demonstrate that this missense alteration elicits BTK-independent activation after B-cell receptor engagement, implying the formation of a novel BTK-bypass pathway. Consistent with previous results, PLCG2R665W confers hypermorphic induction of downstream signaling events. Our studies reveal that proximal kinases SYK and LYN are critical for the activation of mutant PLCG2 and that therapeutics targeting SYK and LYN can combat molecular resistance in cell line models and primary CLL cells from ibrutinib-resistant patients. Altogether, our results engender a molecular understanding of the identified aberration at PLCG2 and explore its functional dependency on BTK, SYK, and LYN, suggesting alternative strategies to combat acquired ibrutinib resistance.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonally derived mature B-cell malignancy characterized by microenvironment dependence and constitutive activation of the B-cell receptor (BCR) pathway.1-4 Conventional therapy for fit, young patients includes chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab.5,6 Outside of allogeneic stem cell transplantation, CLL is considered an incurable disease, and patients will eventually relapse following front-line therapy.
Recent advances in CLL disease biology confirm that the malignant cells receive heterogenic multicellular stimuli within the lymphoid tissues that subvert apoptosis and promote proliferation.3,7 Despite profound interpatient heterogeneity, the BCR pathway is a critical axis of CLL survival signaling, given its dominant role in nearly all cases of CLL.4,8 Mutations of the immunoglobulin heavy-chain variable region (IGVH) gene in CLL are associated with favorable responses, whereas unmutated subsets of CLL predict poor prognosis.9,10 Current studies demonstrate anergic features in mutated CLL leading to downmodulation of surface immunoglobulin M (IgM), whereas unmutated CLL could transmit positive signaling and promote disease progression.11 Hence, novel targeted therapies aimed to abrogate BCR-integral kinases have demonstrated marked clinical activity.12 Ibrutinib is one such BCR-targeted therapy that covalently binds Bruton agammaglobulinemia tyrosine kinase (BTK) at cysteine 481. This prevents the critical LYN and SYK directed autophosphorylation of its Src homology 3 (SH3) domain; thereby preventing downstream activation of PLCγ2 and, in turn, blocking downstream BCR signaling. Nevertheless, ibrutinib resistance due to acquired mutations in BTK and its direct downstream effector PLCγ2 has been documented in a small number of cases.13 Whereas the C481S mutation in BTK prevents ibrutinib covalent binding and thereby converts this agent to a less effective reversible inhibitor,13 the previously identified R665W and L845F in PLCγ2 have been only partially characterized and it remains unclear how these mutations confer molecular resistance. Although it is clear that these mutations are hypermorphic and augment BCR downstream signaling events regardless of BTK activity, we have shown previously that they do not induce autonomous PLCγ2 signaling and still rely on BCR signaling events. Given that conventional activation of PLCγ2 requires the cooperation of BTK,14 the underlying molecular mechanisms that allow these PLCγ2 mutations to bypass BTK inhibition remain an important question when considering new therapeutic strategies to combat ibrutinib resistance.
The BCR is composed of a tetrameric complex of Ig heavy and light chains, including Igα (CD79α) and Igβ (CD79β), in which immunoreceptor tyrosine-based activation motifs are harbored. Upon BCR engagement, Src-family protein tyrosine kinases, primarily LYN, as well as SYK and BTK are activated by tyrosine phosphorylation upon receptor aggregation,15 thereby transducing downstream signals. PLCγ2 is responsible for hydrolyzing phosphatidylinositol to generate diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) upon activation. Whereas IP3 mediates Ca2+ mobilization, DAG activates protein kinase C family members.16 Previous evidence indicates defective responses to α-IgM in Plcg2 knockout mice,17 a similar phenotype observed in BTK-deficient B cells,14 suggesting that BTK and PLCγ2 are prerequisite molecules within the BCR activation pathway. In addition, defective IP3 production and calcium mobilization have been described in SYK-deficient DT40 cells,18 showing that SYK activation is indispensable for coupling BCR signaling to PLCγ2 activation. The adaptor function of B-cell linker protein (BLNK) for recruiting both BTK and PLCγ2 to the membrane compartment has been illustrated,19 indicating a scenario in which SYK activates BTK leading to downstream PLCγ2 activation. Until recently, the functional requirement of LYN in PLCγ2 activation was still unclear. In regard to BCR activation, LYN-mediated Igα-Igβ phosphorylation is essential for SYK activation.20 The direct association between LYN and PLCγ2 has been reported,21 however, LYN-null DT40 cells reveal a differential response in calcium release and IP3 production,18 suggesting that LYN may jointly activate PLCγ2 in coordination with SYK, BTK, and BLNK.
Given the direct clinical need, we sought to investigate the molecular pathogenesis of the acquired PLCγ2 mutation in ibrutinib resistance. Ion torrent sequencing analysis from additional relapsed patients (in some patients without the coexistence of BTK mutations) confirmed the previously identified R665W mutation at PLCγ2. The R665W mutation in PLCG2, which confers BTK independence and molecular resistance to ibrutinib was evaluated for its hypermorphic capacity to impel enhanced downstream signaling and Ca2+ flux in the setting of BCR stimulation. Furthermore, we observed the functional dependence of the R665W mutant on SYK and LYN, which cooperate with mutant PLCγ2 to form a BTK bypass pathway. Together, our findings provide additional insight into the underlying mechanisms of ibrutinib resistance, and identify possible alternative therapies that target this aberration.
Materials and methods
Ion torrent analysis
DNA was extracted from cryopreserved isolated CLL cells using a DNA extraction kit (QIAamp DNA Mini Kit, Qiagen) according to the manufacturer’s recommendations. DNA was quantified using a spectrophotometric method (NanoDrop 2000, Thermo Scientific) using the standard 260/280 optical density ratio. Analysis of the PLCG2 gene was performed using next-generation sequencing Ion Torrent platform and reagents from Life Technologies (Carlsbad, CA). Library was prepared with Ion AmpliSeq Library Kit 2.0 with a custom designed panel of AmpliSeq primers (panel design IAD48992, pipeline version 3.0, 87 amplicons in 2 pools, 17 kb panel size, 99.68% coverage and/or IAD35546 panel, pipeline version 1.2 amplicons in 2 pools, 48 kb panel size, 98.9% coverage). Both panels cover the entire coding sequence and intronic splice acceptor and donor sites for PLCG2. For multiple samples, we used IonExpress barcode adapters. DNA was amplified on the GeneAmp PCR system 9700 Dual 96-well Thermal Cycler from Applied Biosystems. Polymerase chain reaction (PCR) product was purified with the Agencourt AMPure XP Kit (Beckman Coulter, Indianapolis, IN). Library was quantified using real-time PCR with an Ion Library TAQMAN Quantitation Kit on Applied Biosystems ViiA 7 Real-Time PCR System to allow optimal final dilution of library for template preparation on OneTouch OT2 version instrument with Ion PGM Template OT2 200. The ion sphere particles enrichment and purification was performed on the Ion OneTouch 2 enrichment system. Purified iron sphere particles were analyzed on the Ion Torrent Personal Genome Machine using IonPGM Sequencing 200 version 2 Kit and 316/318 chips version 2. Data were collected and analyzed using the Torrent Server with Torrent Suite 4.0.2 version. Final analysis of sequence data was performed using a combination of software: VariantCaller version 4.0-r76860, Ion Torrent IGV3.6.033, and Ion Reporter version 4.0. The NM002661.3 reference sequence was used for analysis. The entire length of sequences was reviewed manually using these programs to assess for deviation from reference sequence, and to evaluate the quality of sequence and the depth of coverage. The depth of coverage ranged from 1000 to 15 000 for different amplicons.
Reagents and antibodies
Antibodies against phospho-AKT (Ser473), AKT, phospho-extracellular signal-regulated kinase (p-ERK)1/2 (Thr202/Tyr204), ERK1/2, phospho-PLCγ2 (Tyr1217), PLCγ2, phospho-SYK (Tyr525/526), BTK, phospho-Src/LYN, and LYN were obtained from Cell Signaling Technologies (Danvers, MA). Phospho-BTK (Tyr223) was obtained from Abcam (Cambridge, MA), SYK (N-19) from Santa Cruz Biotechnology (Santa Cruz, CA), and glyceraldehyde-3-phosphate dehydrogenase from Millipore (Billerica, MA).
PLCG2 cloning and cell culture
The chicken DT40 cell lines were provided by the RIKEN BioResource Center through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Cells were maintained in RPMI 1640 (Life Technologies, Grand Island, NY) with 2 mM l-glutamine, 10% fetal bovine serum, and 56 U/mL penicillin-streptomycin (Life Technologies). Human PLCG2 (BC007565.1) was cloned into a pBABE-Puro vector. Mutation in PLCG2 at R665W was derived using site-directed mutagenesis (QuikChange Lightning, Stratagene-Agilent Technologies, Santa Clara, CA). The pBABE-PLCG2 vectors were retrovirally introduced into cells. Cells stably expressing variant PLCγ2 were selected out in the presence of 1 μg/mL puromycin for 2 weeks.
Immunoblot assays
Total lysate was extracted in M-PER Mammalian Protein Extraction Reagent (Pierce). Equivalent amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The blots were probed with the appropriate primary and horseradish peroxidase-conjugated secondary antibodies and developed with chemiluminescent substrate (Pierce), followed by detection using radiograph film and quantification using ImageJ software.
Calcium flux assays
Intracellular calcium change was measured by Calcium Assay Kit (BD Biosciences, San Diego, CA). Briefly, 5 × 104 cells in 50 μL complete RPMI medium were harvested in 96 wells half-area microplates. An equal volume of 1X Dye-loading Solution was added to each well and incubated for 1 hour at 37°C. After incubation at room temperature for another hour, calcium release was measured with the Beckman Coulter DTX880 Microplate Reader. After the acquisition to determine the baseline, 3 μg/mL anti-chicken IgM (SouthernBiotech, Birmingham, AL) was added to stimulate the cells. The area-under-curve (AUC) was calculated and normalized to samples treated with dimethylsulfoxide (DMSO) alone; 1 μM ibrutinib for 1 hour followed by washout, 2.5 μM R406, 0.5 μM GS-9973, and 0.1 μM dasatinib were used.
Statistical methods
IP-One and calcium flux (AUC) comparisons between relevant conditions (eg, R665W vs wild-type [WT], ibrutinib vs DMSO) were performed using linear mixed effects models to account for correlations among replicates from the same subject/experiment; data from calcium flux experiments were log-transformed. For experiments with multiple comparisons, P values were adjusted using the Holm procedure to control overall type I error at .05. All analyses were performed using SAS/STAT software version 9.3 (SAS Institute Inc., Cary, NC).
Results
Missense point-mutation at R665W in PLCG2 was identified in relapsed patients
We have previously reported that relapse during ibrutinib therapy is associated with the acquisition of specific gene mutations.13,22 Besides C481S at BTK that directly impairs the targeting effect of ibrutinib, our previous studies identified somatically acquired PLCG2 variants and illustrated how those mutations augment downstream signaling in response to BCR engagement. Remarkably, R665W was recognized in 3 individual relapsed patients (Table 1). In all patients, the aberration was acquired during therapy, as deep sequencing of baseline samples did not reveal this mutation. Moreover, in 2 resistant patients, PLCG2 R665W exists without the BTK C481S mutation, indicating that this mutation can lead to resistance without cooperating BTK or other PLCG2 mutations. The detailed characteristics of patients who acquired the R665W mutation are summarized in Table 1.
Relapsed patient information with R665W mutation in PLCG2
| . | . | . | . | Baseline . | Relapse . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Number . | Patient ID . | Days on ibrutinib . | AA change . | Coverage . | Variant frequency (%) . | Coverage . | Variant frequency (%) . | IgVH . | Del(17p) . |
| R-1 | 0002 | 511 | R665W | 870 | 0 | 9977 | 5.5 | U | NA |
| R-2 | 1140* | 505 | R665W | 3465 | 0 | 2781 | 2 | U | Positive |
| R-3 | 0605 | 673 | R665W | 3230 | 0 | 9252 | 45 | M | Positive |
| . | . | . | . | Baseline . | Relapse . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Number . | Patient ID . | Days on ibrutinib . | AA change . | Coverage . | Variant frequency (%) . | Coverage . | Variant frequency (%) . | IgVH . | Del(17p) . |
| R-1 | 0002 | 511 | R665W | 870 | 0 | 9977 | 5.5 | U | NA |
| R-2 | 1140* | 505 | R665W | 3465 | 0 | 2781 | 2 | U | Positive |
| R-3 | 0605 | 673 | R665W | 3230 | 0 | 9252 | 45 | M | Positive |
AA, amino acid; M, mutated status of IgVH; NA, non-detected, U, unmutated status of IgVH.
Also had acquired C481S mutation in BTK at relapse.
Hypermorphic PLCγ2R665W mutation is characterized by the ability to amplify BCR downstream signaling
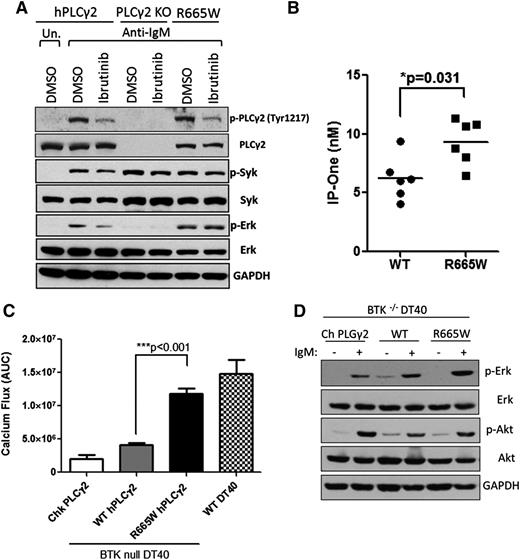
The S707Y variant in human PLCG2 has been documented as an inherited mutation resulting in pathologic autoimmunity, suggesting that aberrations within SH2 loci of the auto-inhibitory domain may reinforce the enzymatic activity of PLCγ2.23 Given that R665W and S707Y are harbored at the same region (Figure 1), a similar capability was hypothesized. To evaluate the functional consequence of R665W, a chicken-derived PLCγ2-deficient DT40 cell line was stably introduced with either WT or mutant human PLCγ2 and stimulated by α-IgM. PLCγ2-deficient DT40 fails to respond to anti-IgM stimulation, whereas the introduction of human PLCγ2 can restore the signaling event (see supplemental Figure 1 on the Blood Web site). Consistent with previous results,13 we found that PLCγ2R665W augments calcium flux, and this effect is resistant to ibrutinib treatment compared with WT (supplemental Figure 2). Also, the R665W variant elicited robust downstream p-ERK activation after α-IgM stimulation (Figure 2A), whereas the WT was sufficiently blocked by ibrutinib. PLCγ2 cleaves its membrane-bound substrate to generate IP3 and DAG. IP3 causes calcium influx from the endoplasmic reticulum and triggers protein kinase C signaling. To examine whether PLCG2R665W potentiates IP3 production, 293T cells were stably introduced with either WT or PLCG2R665W (supplemental Figure 3). IP3 production measured by the accumulation of IP1 was elevated in PLCG2R665W (Figure 2B), implicating that R665W mutation acquires hyperactivity by enhancing downstream signaling. These data suggest that the R665W, similar to the previously identified S707Y mutation,24 confers hypermorphic activity upon BCR activation despite upstream BTK inhibition. Notably, the hypermorphic PLCγ2 mutant fails to retain phosphorylation of Tyr 1217 after ibrutinib treatment despite preserving the capacity to elicit downstream signaling (Figure 2A).
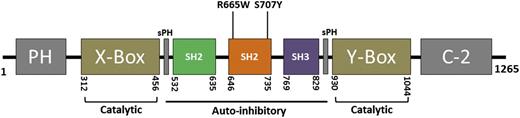
A diagram of the human PLCG2 gene. Depicts encompassed domains of human PLCG2. R665W and S707Y mutations identified in relapse CLL are harbored within the SH2 domain. PH, Pleckstrin homology. C-2, calcium binding motif; SH2, Src homology 2; SH3, Src homology 3; X-box, phosphatidylinositol-specific phospholipase C X domain; Y-box, phosphatidylinositol-specific phospholipase C Y domain.
A diagram of the human PLCG2 gene. Depicts encompassed domains of human PLCG2. R665W and S707Y mutations identified in relapse CLL are harbored within the SH2 domain. PH, Pleckstrin homology. C-2, calcium binding motif; SH2, Src homology 2; SH3, Src homology 3; X-box, phosphatidylinositol-specific phospholipase C X domain; Y-box, phosphatidylinositol-specific phospholipase C Y domain.
Hypermorphic mutation R665W acquires resistance to ibrutinib and functions independently to BTK. (A) Downstream phospho-protein activation upon 0.5 μg/ml α-IgM stimulation for 15 minutes was examined by western blots in comparison with WT PLCγ2 to R665W mutation in DT40 cells. (B) 293T cells were retrovirally transduced with WT PLCγ2 or R665W mutation. The production of IP3 upon 150 ng/ml epidermal growth factor stimulation was measured by IP1 (surrogate of IP3) accumulation using IP-One ELISA Kit (n = 3 repeated experiments). (C) Calcium flux in BTK-deficient DT40 lines introduced with WT PLCγ2 or R665W mutant were examined. Data represents the AUC from 6 replicates upon 3 μg/ml α-IgM stimulation, and (D) downstream phospho-protein activation was examined by western blots. DMSO, dimethylsulfoxide; KO, knockout; Un., untreated.
Hypermorphic mutation R665W acquires resistance to ibrutinib and functions independently to BTK. (A) Downstream phospho-protein activation upon 0.5 μg/ml α-IgM stimulation for 15 minutes was examined by western blots in comparison with WT PLCγ2 to R665W mutation in DT40 cells. (B) 293T cells were retrovirally transduced with WT PLCγ2 or R665W mutation. The production of IP3 upon 150 ng/ml epidermal growth factor stimulation was measured by IP1 (surrogate of IP3) accumulation using IP-One ELISA Kit (n = 3 repeated experiments). (C) Calcium flux in BTK-deficient DT40 lines introduced with WT PLCγ2 or R665W mutant were examined. Data represents the AUC from 6 replicates upon 3 μg/ml α-IgM stimulation, and (D) downstream phospho-protein activation was examined by western blots. DMSO, dimethylsulfoxide; KO, knockout; Un., untreated.
Hyperactivated PLCγ2 no longer requires BTK in the BCR signaling pathway
Given that PLCγ2 is the immediate downstream effector of BTK upon BCR activation, and our results indicate that ibrutinib does not inhibit downstream BCR signaling in the presence of mutated PLCγ2, we sought to investigate the functional role of BTK in hypermorphic PLCγ2. BTK-deficient DT40 cells were introduced with either WT or R665W PLCG2 followed by α-IgM stimulation. Consistent with previous results that BTK−/− DT40 cells were nonresponsive to BCR engagement,14 overexpression of WT human PLCG2 in BTK−/− DT40 cells triggered only modest calcium flux, and PLCG2R665W significantly enhanced BTK-independent calcium release (Figure 2C) as well as downstream ERK activation (Figure 2D), suggesting that this hypermorphic PLCγ2 mutant functionally bypasses BTK and promotes downstream activation of the BCR pathway.
Pharmacologically targeting SYK and LYN can abrogate hypermorphic PLCγ2
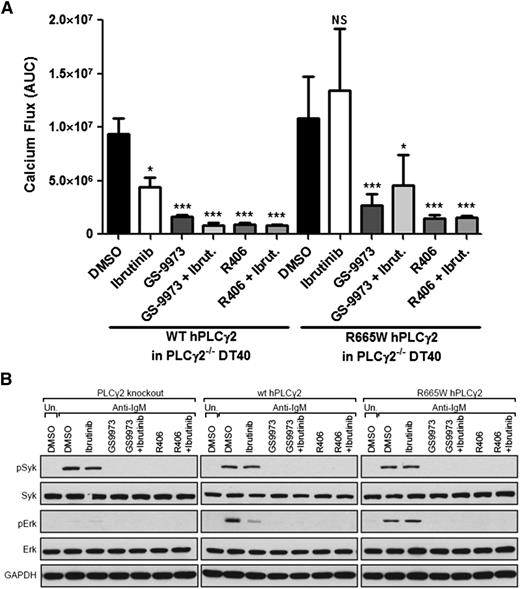
Since BTK is not required for triggering activation of PLCG2R665W, we hypothesized that specific upstream kinases, which are attributed to PLCγ2 activation may still be required, and that targeting these kinases could represent an alternative strategy for the treatment of acquired ibrutinib resistance. Previous studies confirm that SYK-deficient DT40 cells display abolished IP3 or Ca2+ production after BCR engagement.18 Furthermore, SYK has been shown to directly participate in PLCγ2 activation via interaction with BLNK,8 implicating SYK as a possible therapeutic target. To assess this hypothesis, SYK inhibitors were tested against the PLCγ2 mutant. GS-9973 is one of the SYK inhibitors undergoing clinical evaluation for patients with relapsed or refractory hematologic malignancies. R406 is the active derivative of the prodrug fostamatinib that has been assessed in patients with lymphoma or rheumatoid arthritis. Optimal doses of these agents were assessed in DT40 cells to sufficiently abrogate BCR activation without inducing cytotoxicity (supplemental Figure 4). Remarkably, single agent treatment of either GS-9973 or R406 sufficiently impeded the hypermorphic calcium release and downstream ERK activation in PLCγ2-deficient DT40 cells expressed PLCG2R665W, in contrast to ibrutinib, which is only effective in WT (Figure 3A-B). Similar results using combinational treatments compared with the SYK inhibitor alone was observed, demonstrating that SYK proximately functions to BTK/PLCγ2. These data suggest a new therapeutic role for SYK-targeting in CLL patients with acquired hypermorphic PLCγ2R665W.
SYK inhibition abrogates PLCG2R665W induced downstream activation. (A) Calcium influx induced by 3 μg/ml α-IgM stimulation was measured in PLCγ2−/− DT40 stably expressed with either WT human PLCG2 or R665W mutant. The data represents AUC by 6 replicates. In the treatment settings, 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.5 μM GS-9973 or 2.5 μM R406 was treated continuously. *P < .05; ***P < .001; NS = P > .05. (B) Downstream signaling was examined in PLCγ2−/− DT40 expressing either WT PLCG2 or R665W mutant. Cells were treated with 0.5 μg/ml α-IgM for 15 minutes; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.5 μM GS-9973 or 2.5 μM R406 was treated.
SYK inhibition abrogates PLCG2R665W induced downstream activation. (A) Calcium influx induced by 3 μg/ml α-IgM stimulation was measured in PLCγ2−/− DT40 stably expressed with either WT human PLCG2 or R665W mutant. The data represents AUC by 6 replicates. In the treatment settings, 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.5 μM GS-9973 or 2.5 μM R406 was treated continuously. *P < .05; ***P < .001; NS = P > .05. (B) Downstream signaling was examined in PLCγ2−/− DT40 expressing either WT PLCG2 or R665W mutant. Cells were treated with 0.5 μg/ml α-IgM for 15 minutes; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.5 μM GS-9973 or 2.5 μM R406 was treated.
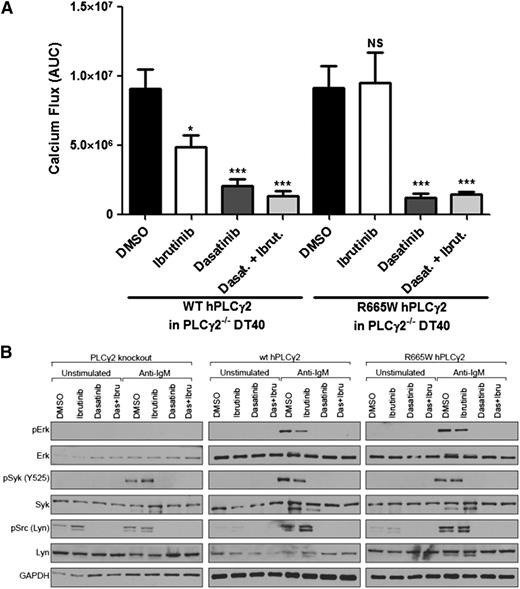
The BCR also utilizes Src-family protein tyrosine kinases like LYN to regulate downstream effectors. Although the functional correlation between LYN and PLCγ2 has not been fully established, reduced activation of PLCγ2 and Ca2+ production has been characterized in LYN-deficient B cells leading to the assumption that hypermorphic PLCγ2 may be dependent upon LYN activation.18 To test this hypothesis, we used dasatinib, a known Src and LYN inhibitor. Our data reveal that PLCG2R665W-induced calcium release and downstream activation could be attenuated by dasatinib (Figure 4A-B). These findings demonstrate the therapeutic potential of LYN inhibition in patients with ibrutinib-resistant PLCG2 mutations, as well as LYN’s involvement in regulating PLCγ2 activation.
LYN suppression abrogates PLCG2R665W-mediated downstream activation. (A) Calcium flux induced by 3 μg/ml α-IgM stimulation was measured in PLCγ2−/− DT40 expressing WT human PLCG2 or R665W mutant. The data represents AUC by 6 replicates. In the treatment settings, 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; 0.1 μM dasatinib was used. *P < .05; ***P < .001; NS = P > .05. (B) Downstream signaling was accessed in PLCγ2−/− DT40 expressing either WT human PLCγ2 or R665W mutant. Cells were treated with 0.5 μg/ml α-IgM; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.1 μM dasatinib was used.
LYN suppression abrogates PLCG2R665W-mediated downstream activation. (A) Calcium flux induced by 3 μg/ml α-IgM stimulation was measured in PLCγ2−/− DT40 expressing WT human PLCG2 or R665W mutant. The data represents AUC by 6 replicates. In the treatment settings, 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; 0.1 μM dasatinib was used. *P < .05; ***P < .001; NS = P > .05. (B) Downstream signaling was accessed in PLCγ2−/− DT40 expressing either WT human PLCγ2 or R665W mutant. Cells were treated with 0.5 μg/ml α-IgM; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout; and 0.1 μM dasatinib was used.
SYK and LYN targeting blocks BCR signaling in ibrutinib-resistant CLL samples
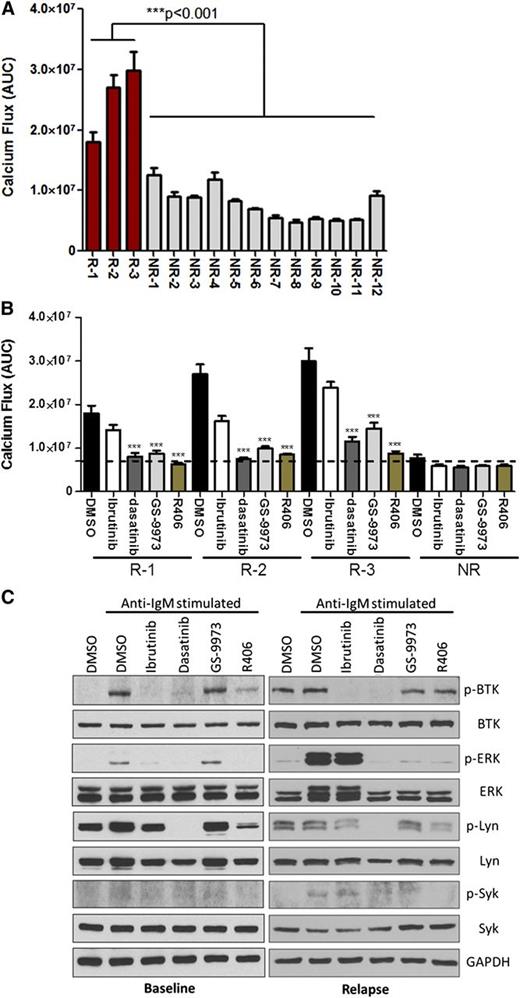
To investigate the effects of targeting SYK and LYN, CLL cells from 3 ibrutinib resistant patients bearing PLCG2R665W were tested. Although modest elevation of Ca2+ influx in nonresistant samples was measured, all 3 ibrutinib-resistant CLL samples induced robust Ca2+ release upon stimulation (Figure 5A), and this effect was significantly inhibited by dasatinib, GS-9973, or R406 treatment (Figure 5B). Moreover, SYK or LYN inhibition reduced Ca2+ flux more effectively than ibrutinib in these resistant samples, reducing overall activation to levels within the range of nonresistant samples (Figure 5B). To gain primary molecular insight, we examined downstream activation upon BCR engagement in baseline or relapse setting in samples obtained from a single patient who developed ibrutinib resistance (patient #2 in Table 1). Although stronger induction of p-ERK is ineffectively suppressed by ibrutinib in the relapse sample, dasatinib, GS-9973, or R406 successfully reversed downstream signaling activation (Figure 5C). Together, our results verify both SYK and LYN as therapeutic targets in patients with acquired PLCγ2 hypermorphic mutations.
Hyperactive downstream signaling in relapse CLL acquired PLCγ2 variants can be blocked by targeting SYK or LYN. (A) Calcium flux from 3 individual relapse CLL (R) bearing mutated PLCγ2 or 12 nonresistant CLL samples (NR) were measured, and 5E6 cells per well in 96-half well microplates were stimulated with 10 μg/ml α-IgM at 37°C for 15 minutes, and then measured by BD Calcium Assay Kit. The data represent AUC from 7 and 12 replicates in resistant and nonresistant samples, respectively; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout. (B) The data represents calcium release by 3 individual relapse CLL (R) or the mean of 12 nonresistant samples (NR) stimulated with 10 μg/ml α-IgM in the presence of 0.5 μM GS-9973, 2.5 μM R406, or 0.1 μM dasatinib; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout. ***P < .001. (C) The downstream phospho-protein activation was accessed in CLL cells from a single patient (patient #2 in Table 1) in the baseline or relapse setting. The detailed information of the 3 relapsed CLL patient samples analyzed here are listed in Table 1.
Hyperactive downstream signaling in relapse CLL acquired PLCγ2 variants can be blocked by targeting SYK or LYN. (A) Calcium flux from 3 individual relapse CLL (R) bearing mutated PLCγ2 or 12 nonresistant CLL samples (NR) were measured, and 5E6 cells per well in 96-half well microplates were stimulated with 10 μg/ml α-IgM at 37°C for 15 minutes, and then measured by BD Calcium Assay Kit. The data represent AUC from 7 and 12 replicates in resistant and nonresistant samples, respectively; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout. (B) The data represents calcium release by 3 individual relapse CLL (R) or the mean of 12 nonresistant samples (NR) stimulated with 10 μg/ml α-IgM in the presence of 0.5 μM GS-9973, 2.5 μM R406, or 0.1 μM dasatinib; 1.0 μM ibrutinib was pretreated for 1 hour followed by washout. ***P < .001. (C) The downstream phospho-protein activation was accessed in CLL cells from a single patient (patient #2 in Table 1) in the baseline or relapse setting. The detailed information of the 3 relapsed CLL patient samples analyzed here are listed in Table 1.
Discussion
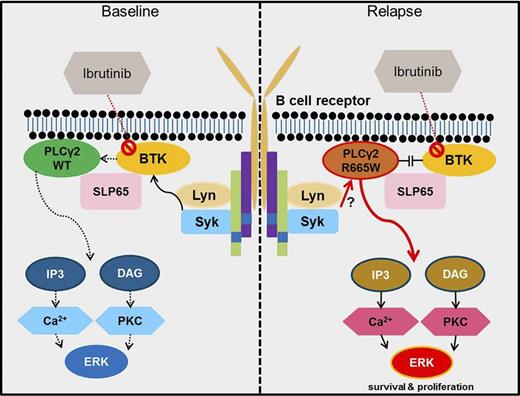
Although microenvironment-derived CLL survival and proliferation stimuli are multifactorial, the BCR plays a critical role in driving disease progression; hence, ibrutinib has demonstrated exceptional clinical results in CLL. Nonetheless, relapses have been observed and understanding the molecular pathways that confer ibrutinib resistance is of the utmost priority in determining the treatment scheme in these patients. Given the predominant effect of ibrutinib in suppressing BCR signaling, the resistant mechanisms are speculated to emerge within this signaling pathway. In this study, extended from our previous report, genetic aberrations at PLCG2 are further elucidated in relapsed patients. Our data thoroughly characterize the competency of the R665W mutation to trigger downstream BCR signaling independent of BTK upon anti-IgM stimulation and explains why these patients are resistant to ibrutinib. We show here, that the proximal effectors SYK and LYN collaborate to activate mutated PLCγ2 regardless of BTK activation. Moreover, pharmacologically targeting either SYK or LYN can overcome persistent survival signaling (Figure 6). Together, these data augment the understanding of mutated PLCγ2-mediated ibrutinib resistance, and suggest alternative approaches to overcome the genetic abnormalities.
The diagram illustrates PLCG2R665W-mediated ibrutinib resistance. In treatment naïve CLL, proximal BCR signaling triggers downstream BTK activation. PLCγ2 is consequently activated in a BTK-dependent manner. Targeting BTK by ibrutinib can abrogate PLCγ2-initiated downstream survival signal (left panel); in contrast, mutant PLCγ2 (R665W) can be activated via SYK or LYN, bypassing BTK dependency in the resistant CLL, thereby propagating downstream survival signals despite ibrutinib treatment (right panel). PKC, protein kinase C.
The diagram illustrates PLCG2R665W-mediated ibrutinib resistance. In treatment naïve CLL, proximal BCR signaling triggers downstream BTK activation. PLCγ2 is consequently activated in a BTK-dependent manner. Targeting BTK by ibrutinib can abrogate PLCγ2-initiated downstream survival signal (left panel); in contrast, mutant PLCγ2 (R665W) can be activated via SYK or LYN, bypassing BTK dependency in the resistant CLL, thereby propagating downstream survival signals despite ibrutinib treatment (right panel). PKC, protein kinase C.
Similar to drug resistances that result from hampered affinity, mutations in BTK have been recognized in patients who relapsed on ibrutinib. These findings further support the prominent role of BCR-mediated CLL progression. Intriguingly, BTK remains normal in a subset of relapsed patients with PLCG2R665W, suggesting that PLCG2R665W is sufficient to induce ibrutinib resistance. Remarkably, the aberration in PLCγ2 appears to be somatically acquired only after long-term ibrutinib treatment, suggesting that this clone evolved under continuous pressure from this irreversible agent. Indeed, although subgroups of CLL drive toward anergy, differential capacity of BCR signaling has been demonstrated to correlate with surface IgM expression.25 Although comparable sIgM was detected in DT40 expressing PLCγ2R665W, CLL with this hypermorphic aberration may enhance the survival advantage and gain access to tissue environment.25 PLCγ2 activity is correlated with tyrosine phosphorylation at 753, 759, 1197, and 1217.26,27 On a molecular level, the phospholipase activity is repressed by the auto-inhibitory region encompassed within X- and Y-box domains. Recent studies indicate the crucial function of carboxyl-terminal SH2 in the regulatory element in suppressing PLCγ2 activity.26 The inherited S707Y mutation at this region has been reported to confer a hypermorphic effect and leads to an auto-inflammatory response.24 Consistent with this notion, R665W residing within this region appears to elicit an analogous mechanism to impose enzymatic activity via releasing the catalytic domain of PLCγ2 through a peptide conformation change. Nevertheless, direct phosphorylation sites triggered by BTK, such as Tyr1217, remains functionally inhibited in the presence of ibrutinib despite hyperactive downstream signaling events induced by mutated PLCγ2 (Figure 2A), affirming the activation of mutant PLCγ2 is discrete from BTK activity. Although previous studies have confirmed the indispensable role of BTK for PLCγ2 activation,14 mutated PLCγ2 may shift the functional dependency to other proximal kinases. Moreover, PLCγ2 activation also requires membrane docking; a process recruited by BLNK (SLP-65),28 suggesting the possibility that mutated PLCγ2 may be retained at the plasma membrane through positive modulation by BLNK. In regard to proximal kinases that initiate PLCγ2 activation, our data shows that SYK and LYN are essential for the activation of mutated PLCγ2. Considering membrane localization requires interactions between Pleckstrin homology domain at PLCγ2 and phosphatidylinositol lipids within the plasma membrane,29,30 PI3K inhibitors were also evaluated as potential alternatives. Nevertheless, our data indicates neither idelalisib nor IPI-145, inhibitors of PI3K p110δ and/or p110γ subunits in clinical use, could abrogate PLCG2R665W-mediated downstream signaling despite AKT inhibition (supplemental Figure 5).
R665W aberration at PLCγ2 is in close proximity to identified phosphorylation sites, Tyr753 and Tyr 759, raising the possibility that the mutation may lead to conformational change of PLCγ2, amplifying its catalytic function upon stimulation regardless of the phosphorylation status at these tyrosine residues. However, it is also unknown whether the hypermorphic mutation could acquire higher affinity to SYK, LYN, or BLNK, leading to sustained activity after stimulation.
It is important to note that at most 45% of the variant frequencies are at R665W; however, the frequency of this aberration is variable when measured in peripheral blood samples from relapsed CLL patients, denoting that resistant clones bearing PLCG2R665W might enrich within bone marrow or lymphoid organs. Likewise, advanced sequencing technologies are in demand to certify if the aberration existed with extremely low frequency at baseline. Alternatively, cooperative scenarios leading to resistance may also contribute to relapse settings. Conforming to this, BTK or other PLCG2 mutations were concurrently observed in relapsed patients with R665W (data not shown), suggesting additional aberrations at this signaling axis may synergistically contribute to CLL progression. Further studies will be required to fully elucidate this. Finally, despite the elusive origins of these abnormalities, work is underway to scrutinize the functional roles of diverse aberrations in PLCγ2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported with grants by the Specialized Center of Research from the Leukemia and Lymphoma Society, the National Institutes of Health’s National Cancer Institute (P50-CA140158, R01 CA177292, R01 CA183444, K23 CA178183, and P01 CA95426), the D. Warren Brown Foundation, Four Winds Foundation, the Sullivan Chronic Lymphocytic Leukemia Research Fund, Mr and Mrs Michael Thomas, Al and Midge Lipkin, and the Harry T. Mangurian Foundation.
Authorship
Contribution: T.-M.L., J.A.W., and Y.Z. designed the research, performed experiments, analyzed data, generated figures, and wrote the manuscript; E.S. and S.D. were involved in planning the research and performed experiments; A. Lozanski and G.L. performed ion torrent sequencing and analyzed data; A. Lehman and X.Z. performed statistical analysis; J.A.J., J.F., L.A.A., K.M., S.M.J., and K.A.B. provided clinical practice, reviewed drafts, and approved the final version of the manuscript; J.C.B., J.A.D., and A.J.J. planned the proposal, supervised the research, reviewed and modified drafts, obtained funding for the research work, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy J. Johnson, The Ohio State University, OSUCCC Bldg, Room 455C, 410 W. 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.
References
Author notes
T.-M.L., J.A.W., Y.Z., J.A.D., and A.J.J. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal