Key Points
Lenalidomide augments nanoscale rearrangements in cortical actin at the human NK-cell immune synapse.
Lenalidomide lowers the threshold for NK-cell activation, allowing activation by low levels of ligands on tumor cells.
Abstract
As multiple myeloma (MM) progresses, natural killer (NK)-cell responses decline against malignant plasma cells. The immunomodulatory drug lenalidomide is widely used for treatment of MM but its influence on NK-cell biology is unclear. Here, we report that lenalidomide lowers the threshold for NK-cell activation, causing a 66% decrease in the 50% effective concentration (EC50) for activation through CD16, and a 38% decrease in EC50 for NK group 2 member D (NKG2D)–mediated activation, allowing NK cells to respond to lower doses of ligand. In addition, lenalidomide augments NK-cell responses, causing a twofold increase in the proportion of primary NK cells producing interferon-γ (IFN-γ), and a 20-fold increase in the amount of IFN-γ produced per cell. Importantly, lenalidomide did not trigger IFN-γ production in unstimulated NK cells. Thus, lenalidomide enhances the NK-cell arm of the immune response, without activating NK cells inappropriately. Of particular clinical importance, lenalidomide also allowed NK cells to be activated by lower doses of rituximab, an anti-CD20 monoclonal antibody (mAb) widely used to treat B-cell malignancies. This supports combined use of lenalidomide and rituximab in a clinical setting. Finally, superresolution microscopy revealed that lenalidomide increased the periodicity of cortical actin at immune synapses, resulting in an increase in the area of the actin mesh predicted to be penetrable to vesicles containing IFN-γ. NK cells from MM patients also responded to lenalidomide in this way. This indicates that nanometer-scale rearrangements in cortical actin, a recently discovered step in immune synapse assembly, are a potential new target for therapeutic compounds.
Introduction
Natural killer (NK) cells contribute to defense against cancer by lysis of diseased or stressed cells and secretion of inflammatory cytokines including interferon-γ (IFN-γ).1,2 NK-cell responses are triggered through germline-encoded activating receptors, including NK group 2 member D (NKG2D), which recognizes stress-inducible ligands such as major histocompatibility complex class I chain-related protein A (MICA), and the Fc receptor CD16, which mediates antibody-dependent cellular cytotoxicity (ADCC).3-8 Superresolution microscopy revealed that activating receptor ligation triggers remodeling of cortical actin in specific domains within the NK-cell immune synapse where lytic granules and vesicles containing IFN-γ accumulate.9-13
Multiple myeloma (MM) is a hematologic malignancy characterized by a clonal proliferation of plasma cells in bone marrow and is associated with progressive dysregulation of the immune system.14 NK cells may initially contribute to the control of malignant cells15-17 and evidence suggests that NKG2D is involved in NK-cell recognition of MM cells.18 However, NK-cell surveillance and cytotoxicity against MM decreases as the disease progresses.19-23 There is some evidence that lenalidomide, used for the treatment of MM, can increase NK cell-mediated lysis.24 One study showed that prolonged treatment with lenalidomide enhanced NK-cell cytotoxicity through a mechanism that is partially dependent on the tumor necrosis factor–related apoptosis-inducing ligand system.25 Other research indicates that lenalidomide overcomes the effects of suppressive cytokines on NK-cell responses.26 However, studies also report that lenalidomide does not directly affect NK-cell effector functions27,28 but rather helps via CD4+ T-cell activation.28
Here, we establish that lenalidomide augments NK-cell responses directly on both a population level and a single-cell level. Crucially, lenalidomide lowers the threshold for NK-cell activation through both CD16 and NKG2D, indicating that NK cells could respond to lower densities of activating ligand. Also, superresolution stimulated emission depletion (STED) microscopy revealed that lenalidomide works to augment actin remodeling at the NK-immune synapse.
Methods
Cells and antibodies
Primary human NK (pNK) cells were obtained from healthy donor peripheral blood by negative magnetic selection and cultured as previously described.29 NK cells were used 6 days later. Daudi and Raji were cultured in RPMI 1640 (Sigma-Aldrich), 10% fetal calf serum (FCS; Gibco), 2 mM l-glutamine (Gibco), and 1 mM penicillin and streptomycin (Sigma-Aldrich). NK cells were treated with lenalidomide (Celgene Corporation; 30 mM stock in dimethylsulfoxide [DMSO]) at a final concentration of 0.001 µM to 10 µM in culture medium. For most experiments, lenalidomide was used at a clinically relevant dose of 1 µM lenalidomide as in previous studies.30,31 Human recombinant interleukin-2 (hrIL-2; 150 U/mL) was added alongside lenalidomide or vehicle control (DMSO), unless otherwise indicated. Where indicated, cells were also treated with brefeldin A (5 µg/mL; Sigma-Aldrich).
NK cells from MM patients were isolated from 40 mL of peripheral blood (Manchester Cancer Research Centre [MCRC] Biobank Research Tissue Bank Ethics [ref. 07/H1003/161+5]) in accordance with the Declaration of Helsinki, and used immediately after isolation.
Antibodies against CD16 (clone 3G8, 1 µg/mL; BD Biosciences), NKG2D (clone 149810, 3 µg/mL; R&D Systems), 2B4 (clone 2-69, 3 µg/mL; BD Biosciences), and murine immunoglobulin G1 (IgG1) isotype control (BD Biosciences) were used. Recombinant proteins used were MICA-Fc (R&D Systems; 2 µg/mL unless otherwise stated), intercellular adhesion molecule 1 (ICAM-1, 2.5 µg/mL; R&D Systems), and human IgG (1-500 µg/mL; Sigma-Aldrich). Rituximab (Invivogen) was also used (10 µg/mL).
Cytotoxicity assay
NK-cell cytotoxicity was assessed against Daudi in a standard 5-hour 35S-methionine release assay. pNK cells were added to the target cells at an effector-to-target (E:T) ratio of 10:1 (5 × 104 pNK cells per well). After 5 hours, 35S activity in the supernatant was quantified (MicroBeta; PerkinElmer) and results expressed as percent lysis: (experimental release − spontaneous release)/(maximal release − spontaneous release).
ELISA
NK cells were treated with 0.1 to 10 µM lenalidomide or DMSO, plus 150 U/mL IL-2 and were plated on antibody- or ligand-coated 96-well plates (100 000 pNK cells per well) and incubated for 24 hours at 37°C (5% CO2). For conjugate enzyme-linked immunosorbent assays (ELISAs), pNK cells were co-incubated with target cells at an E:T ratio of 10:1 (20 000 target cells and 200 000 pNK cells) for 24 hours in the presence of 0.001 to 10 µM lenalidomide ± 150 U/mL IL-2.
ELISA plates were coated with anti-IFN-γ monoclonal antibody (mAb) (clone NIB42, 1 µg/mL; BD Biosciences) in binding buffer (carbonate bicarbonate; Sigma), blocked with 1% bovina serum albumin (BSA)/0.05% Tween-20/phosphate-buffered saline (PBS), and supernatants were added in triplicate for 1 hour. Plates were incubated with biotinylated IFN-γ mAb (clone 4S.B3, 1 µg/mL; BD Biosciences) then streptavidin horseradish peroxidase (HRP; BD Biosciences), followed by 3,3′,5,5′-Tetramethylbenzidine (TMB) ELISA substrate (Sigma-Aldrich) and the reaction was halted with 0.5 M H2SO4. Absorbance was measured at 450 nm.
Quantitative reverse transcription polymerase chain reaction
Cells were treated with 1 µM lenalidomide or DMSO plus 150 U/mL IL-2 and were plated on antibody-coated glass slides for 4 hours at 37°C. Cells were then lysed and RNA extracted (RNeasy kit; Qiagen). RNA was reverse transcribed, and subjected to quantitative polymerase chain reaction (qPCR; Applied Biosystems) using Sybr Green detection for IFN-γ using the following primer pair: forward primer, 5′-AAAAATAATGCAGAGCCAAATTG-3′; reverse primer, 5′-TAGCTGCTGGCGACAGTTCA-3′. Data were analyzed by δδ cycle threshold (CT) method and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (detected using the following primer pair: forward primer, 5′-GAAGGTGAAGGTCGGAGT-3′ and reverse primer, 5′-CATGGGTGGAATCATATTGGAA-3′).
Microscopy
Cells were incubated in 8-well borosilicate coverglass chambers (Laboratory-Tek; Nunc) coated with 0.01% poly-l-lysine then mAb or recombinant proteins. Cells were then fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton-X-100. For NKG2D and LFA-1 costimulation, cells were incubated for 90 minutes before fixation, as previously described.13 To visualize F-actin, cells were stained with phalloidin–Alexa Fluor 488 (Invitrogen) or phalloidin–Atto-590 (Atto-tec). To visualize IFN-γ, cells were stained with Alexa Fluor 488– or Alexa Fluor 647–conjugated anti-IFN-γ mAb (clone B27; BD Biosciences). Brightfield, fluorescence, and STED images were obtained (Leica TCS SP8 STED CW) using a 100× oil immersion lens (NA 1.4) at room temperature. STED of Alexa Fluor 488 was achieved using 592-nm continuous-wave fiber laser. For confocal images, 35 to 40 optical slices were collected at 0.2-µm intervals.
Image analysis
For the single-cell IFN-γ analysis, confocal images were imported into Imaris (Bitplane). Individual cells were segmented based on cell body staining (phalloidin–Atto-590) and nucleus staining (NucBlue). IFN-γ vesicles were identified based on Alexa 488–IFN-γ mAb staining within each cell. STED images were deconvolved (Huygens; Scientific Volume Imaging), exported in tagged image file format, and analyzed using MATLAB (Mathworks) as previously described.9
Statistical analyses
Mean values and SD are shown. Data were analyzed by 1-way analysis of variance (ANOVA) and Tukey posttest (Prism version 6; GraphPad). For threshold analysis, IFN-γ production was normalized to the maximum value seen with each receptor and 50% effective concentration (EC50) was determined by nonlinear regression (Prism version 6; GraphPad).
Results
Lenalidomide directly affects NK-cell IFN-γ secretion after activation through different receptors
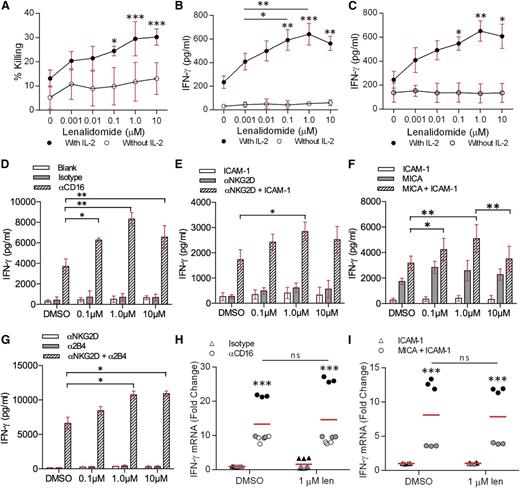
To test whether lenalidomide affects NK cells directly, human pNK cells were co-incubated with Daudi cells (a B-cell lymphoma line susceptible to NK-cell cytotoxicity) in the presence of 0.001 to 10 µM lenalidomide. Lysis of Daudi cells was significantly enhanced with 0.1 to 10 µM lenalidomide in the presence of IL-2 (Figure 1A). Treatment with 10 µM lenalidomide increased the specific lysis from 13% ± 2% to 30% ± 1% (Figure 1A).
Lenalidomide treatment increases IFN-γ secretion from NK cells. (A) Healthy donor pNK cells and Daudi target cells were pretreated with DMSO or 0.001 to 10 µM lenalidomide for 24 hours then the Daudi cells were tested for susceptibility to NK-cell–mediated lysis. Graph shows mean ± SD from 3 independent donors. E:T ratio was 10:1. (B-C) pNK cells and (B) Daudi cells or (C) Raji cells were cocultured for 24 hours with DMSO or 0.001 to 10 µM lenalidomide (±150 U/mL IL-2). IFN-γ release was measured by ELISA. E:T ratio was 10:1 in all experiments. Data shows mean ± SD from 3 donors. Significance compared with DMSO (‘0 µM’) condition. (D-G) Primary NK cells were treated with DMSO or 0.1 to 10 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with (D) anti-CD16 mAb or IgG1 isotype control mAb, (E) recombinant ICAM-1, anti-NKG2D mAb, or both, (F) recombinant ICAM-1, recombinant MICA, or both, (G) anti-NKG2D mAb, anti-2B4, or both. IFN-γ release was assessed by ELISA. Data show mean ± SD from 3 donors. (H-I) Primary NK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 4 hours in wells coated with (H) anti-CD16 mAb or IgG1 isotype control mAb, or (I) recombinant ICAM-1 or recombinant MICA and ICAM-1. IFN-γ mRNA was assessed by qRT-PCR and is normalized to GAPDH. Data shows triplicate data from (H) 3 donors and (I) 2 donors. Data points for each donor are shaded the same. Red line shows the mean. ns, not significant; qRT-PCR, quantitative reverse transcription polymerase chain reaction. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide treatment increases IFN-γ secretion from NK cells. (A) Healthy donor pNK cells and Daudi target cells were pretreated with DMSO or 0.001 to 10 µM lenalidomide for 24 hours then the Daudi cells were tested for susceptibility to NK-cell–mediated lysis. Graph shows mean ± SD from 3 independent donors. E:T ratio was 10:1. (B-C) pNK cells and (B) Daudi cells or (C) Raji cells were cocultured for 24 hours with DMSO or 0.001 to 10 µM lenalidomide (±150 U/mL IL-2). IFN-γ release was measured by ELISA. E:T ratio was 10:1 in all experiments. Data shows mean ± SD from 3 donors. Significance compared with DMSO (‘0 µM’) condition. (D-G) Primary NK cells were treated with DMSO or 0.1 to 10 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with (D) anti-CD16 mAb or IgG1 isotype control mAb, (E) recombinant ICAM-1, anti-NKG2D mAb, or both, (F) recombinant ICAM-1, recombinant MICA, or both, (G) anti-NKG2D mAb, anti-2B4, or both. IFN-γ release was assessed by ELISA. Data show mean ± SD from 3 donors. (H-I) Primary NK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 4 hours in wells coated with (H) anti-CD16 mAb or IgG1 isotype control mAb, or (I) recombinant ICAM-1 or recombinant MICA and ICAM-1. IFN-γ mRNA was assessed by qRT-PCR and is normalized to GAPDH. Data shows triplicate data from (H) 3 donors and (I) 2 donors. Data points for each donor are shaded the same. Red line shows the mean. ns, not significant; qRT-PCR, quantitative reverse transcription polymerase chain reaction. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Secretion of IFN-γ is another important NK-cell function and here, production of IFN-γ by pNK cells in conjugate with Daudi increased threefold in the presence of 1 µM lenalidomide (215 ± 30 pg/mL without lenalidomide; 640 ± 50 pg/mL with 1 µM lenalidomide; Figure 1B). Similarly, IFN-γ secretion by pNK cells in conjugates with the Epstein-Barr virus–transformed B-cell line Raji increased 2.5-fold in the presence of 1 µM lenalidomide (from 245 ± 40 pg/mL without lenalidomide to 650 ± 50 pg/mL with 1 µM lenalidomide) (Figure 1C). Thus, lenalidomide, in the presence of IL-2, has a direct enhancing effect on NK-cell cytotoxicity and IFN-γ secretion.
To test whether the lenalidomide-induced increase in IFN-γ secretion occurs when pNK cells are activated via specific receptors, NK cells were stimulated on surfaces coated with anti-CD16 mAb in the presence of 0.1 to 10 µM lenalidomide. NK cells treated with lenalidomide in uncoated wells or wells coated with isotype-matched control mAb did not secrete IFN-γ (Figure 1D). In contrast, lenalidomide resulted in a twofold increase in the amount of IFN-γ secreted by pNK cells after ligation of CD16 (3700 ± 420 pg/mL without lenalidomide; 8320 ± 370 pg/mL with 1.0 µM lenalidomide) (Figure 1D).
We then tested the effect of lenalidomide on the stress-inducible activating receptor NKG2D, which requires coligation of the integrin LFA-1 to elicit a full NK-cell response. Lenalidomide significantly increased IFN-γ secretion from NK cells costimulated through NKG2D and LFA-1 (via anti-NKG2D mAb or MICA, and ICAM-1, respectively) (Figure 1E-F). However, no significant enhancement was observed when NK cells were treated in the presence of anti-NKG2D mAb, MICA, or ICAM-1 alone (Figure 1E-F), suggesting that full NK-cell activation is required for lenalidomide to have an effect.
To test the effect of lenalidomide on synergistic NK-cell activation,6 pNK cells were treated with 0.1 to 10 µM lenalidomide in the presence of immobilized anti-NKG2D and anti-2B4 mAbs. As expected, IFN-γ secretion was negligible when either receptor was ligated separately (ie, in the absence of full NK-cell activation) whereas coligation of both receptors led to a substantial release of IFN-γ. IFN-γ secretion was further increased in the presence of lenalidomide (6610 ± 620 pg/mL without lenalidomide; 10 740 ± 380 pg/mL with 1.0 µM lenalidomide) (Figure 1G). This establishes that lenalidomide increases IFN-γ secretion irrespective of which activating receptor (or synergistic combination) is ligated.
Although stimulatory conditions elicited a strong induction of IFN-γ messenger RNA (mRNA), no differences were observed in the presence of lenalidomide (Figure 1H-I). Thus, lenalidomide augments IFN-γ secretion, but not IFN-γ transcription.
These effects are not due to lenalidomide altering the surface expression of LFA-1, NKG2D, or CD16 as the levels of all 3 receptors remained the same (supplemental Figure 1, available on the Blood Web site). In addition, no changes in the levels of phosphorylated membrane-proximal signaling proteins could be detected after lenalidomide treatment (supplemental Figure 2).
Overall, lenalidomide increases IFN-γ production via multiple triggers for NK-cell activation. The fact that lenalidomide does not elicit NK-cell effector functions in the absence of activating receptor ligation indicates that NK cells retain their specificity and would not activate without appropriate triggers while in the presence of the drug.
Lenalidomide increases the proportion of cells secreting IFN-γ as well as the amount of cytokine produced per cell
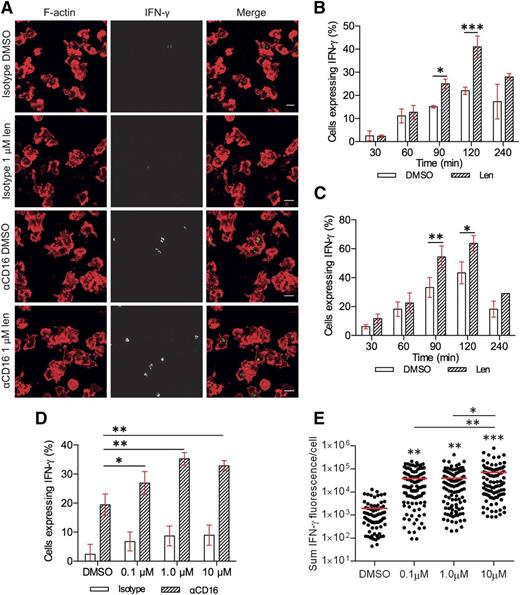
The enhancement in IFN-γ secretion with lenalidomide treatment could involve an increase in the number of NK cells secreting IFN-γ and/or an increase IFN-γ production from individual cells. To test this, lenalidomide-treated pNK cells were incubated on slides coated with anti-CD16 mAb and then imaged by confocal microscopy (Figure 2A). In the absence of lenalidomide, expression of IFN-γ protein could be detected within 30 minutes in a small proportion of primary NK cells (2.5% ± 1.5%) and peaked after 120 minutes’ stimulation, when 22% ± 1% pNK cells expressed IFN-γ (Figure 2B). The percentage of IFN-γ–positive cells decreased by 240 minutes, suggesting that the majority of IFN-γ had been secreted by this time (Figure 2B, white bars). The effect of 1 µM lenalidomide also peaked 120 minutes after stimulation, almost doubling the proportion of NK cells expressing IFN-γ from 22% ± 1% to 41% ± 3% (Figure 2B striped bars). In a separate experiment, to control for lenalidomide treatment time, cells were treated at the start of the experiment and added to anti-CD16 mAb-coated surfaces in reverse order for the time course. This meant, for example, that cells undergoing 30-minute stimulation had 210 minutes’ incubation with lenalidomide prior to plating (Figure 2C). This confirmed that the effect of lenalidomide on pNK-cell IFN-γ production occurs after 90 minutes of stimulation.
Lenalidomide increases the proportion of pNK cells expressing IFN-γ as well as the amount produced per cell. (A) Representative microscopy images of F-actin (red) and IFN-γ (shown in grayscale in middle column, and then green in merged image) in pNK cells stimulated for 120 minutes on surfaces coated with IgG1 isotype control mAb or anti-CD16 mAb in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2). Scale bars, 10 µm. (B) The proportion of cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2) for 30 to 240 minutes. Graph shows mean ± SD, n > 200 from 3 donors. (C) The proportion of cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2) for 30 to 240 minutes. All cells treated with DMSO or 1 µM lenalidomide for a total of 240 minutes and added to stimulating surfaces in the reverse order for the time course (for example, for 30-minute stimulation, pNK cells were treated with DMSO or 1 µM lenalidomide for 210 minutes before plating). Graph shows mean ± SD, n > 100 from 2 donors. (D) The proportion of pNK cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces with 0.1 to 10 µM lenalidomide. Graph shows mean ± SD, n > 100 per donor from 3 donors. (E) Sum IFN-γ fluorescence per cell in pNK cells stimulated as in panel D. Each data point represents a single cell and red line shows the mean. n = 75 to 111 from 3 donors. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide increases the proportion of pNK cells expressing IFN-γ as well as the amount produced per cell. (A) Representative microscopy images of F-actin (red) and IFN-γ (shown in grayscale in middle column, and then green in merged image) in pNK cells stimulated for 120 minutes on surfaces coated with IgG1 isotype control mAb or anti-CD16 mAb in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2). Scale bars, 10 µm. (B) The proportion of cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2) for 30 to 240 minutes. Graph shows mean ± SD, n > 200 from 3 donors. (C) The proportion of cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1 µM lenalidomide (+150 U/mL IL-2) for 30 to 240 minutes. All cells treated with DMSO or 1 µM lenalidomide for a total of 240 minutes and added to stimulating surfaces in the reverse order for the time course (for example, for 30-minute stimulation, pNK cells were treated with DMSO or 1 µM lenalidomide for 210 minutes before plating). Graph shows mean ± SD, n > 100 from 2 donors. (D) The proportion of pNK cells expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces with 0.1 to 10 µM lenalidomide. Graph shows mean ± SD, n > 100 per donor from 3 donors. (E) Sum IFN-γ fluorescence per cell in pNK cells stimulated as in panel D. Each data point represents a single cell and red line shows the mean. n = 75 to 111 from 3 donors. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide (1.0 μM or 10 μM) increased the proportion of cells expressing IFN-γ from 19% ± 2% to 35% ± 1% or 33% ± 1%, respectively (Figure 2D). In addition, the total fluorescence intensity per cell increased with lenalidomide treatment 20-fold (1930 ± 310 [arbitrary units] without lenalidomide; 39 100 ± 5900 with 10 μM lenalidomide; Fig. 2E). These observations were confirmed by flow cytometry (supplemental Figure 3).
These data establish that (1) lenalidomide influences the NK-cell population, almost doubling the proportion of NK cells expressing IFN-γ and (2) lenalidomide affects NK cells on a single-cell level, with a 20-fold increase in the amount of cytokine produced per cell.
Lenalidomide lowers the threshold for NK-cell activation
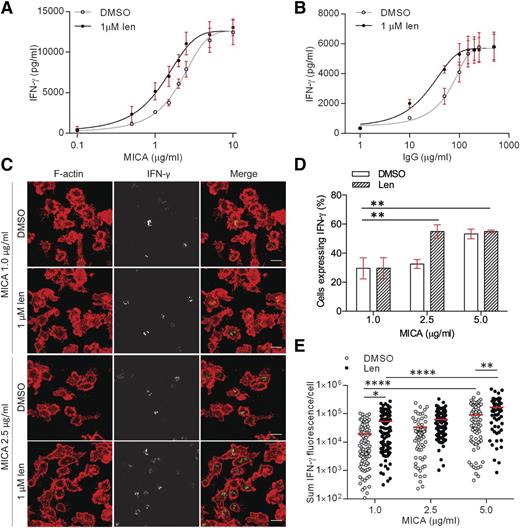
Functionally, a threshold concentration of MICA is required for NKG2D-mediated cytolysis of target cells.32 To test whether lenalidomide influenced the threshold density of MICA proteins needed to activate pNK cells, cells were incubated on surfaces coated with increasing concentrations of MICA (0.1-10 µg/mL) and ICAM-1 (2.5 µg/mL). We then determined the effective concentration of activating receptor that elicited half-maximal responses (EC50) (Figure 3A). Lenalidomide treatment resulted in 38% decrease in the MICA EC50, from 2.1 µg/mL without lenalidomide to 1.3 µg/mL with lenalidomide (Figure 3A). A threshold level of CD16 ligation needed to activate NK cells has also been observed.33 Lenalidomide caused a 66% decrease in the EC50 of immobilized human IgG (hIgG; the cognate ligand for CD16), from 62 µg/mL without lenalidomide to 21 µg/mL with lenalidomide (Figure 3B). Together, these data establish that lenalidomide lowers the threshold for NK-cell activation, even when NK cells are activated via different receptors.
Lenalidomide lowers the threshold for NK-cell activation through NKG2D and CD16. (A-B) pNK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with (A) 0.1 to 10 µg/mL MICA plus 2.5 µg/mL ICAM-1 or (B) 1 to 500 µg/mL human IgG. IFN-γ release was measured by ELISA. Data show mean ± SD from 3 donors. Nonlinear regression fit was applied to data. EC50 values were calculated to be: 2.1 µg/mL (MICA DMSO), 1.3 µg/mL (MICA 1.0µM Len), 62 µg/mL (hIgG DMSO), 21 µg/mL (hIgG 1.0µM Len). (C) Representative microscopy images of F-actin (red) and IFN-γ (shown in grayscale in middle column, and then green in merged image) in pNK cells stimulated for 90 minutes on surfaces coated with 1 µg/mL or 2.5 µg/mL MICA (+2.5 µg/mL ICAM-1) in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 10 µm. (D) The proportion of pNK cells expressing IFN-γ after stimulation with 3 different concentrations of MICA. Graph shows mean ± SD, n > 100 per donor from 3 donors. (E) Sum fluorescence staining for IFN-γ per cell in the same cells as in panel D. Each data point represents a single cell and red line shows the mean. *P < .05, **P < .01, ****P < .0001, 1-way ANOVA with Tukey posttest.
Lenalidomide lowers the threshold for NK-cell activation through NKG2D and CD16. (A-B) pNK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with (A) 0.1 to 10 µg/mL MICA plus 2.5 µg/mL ICAM-1 or (B) 1 to 500 µg/mL human IgG. IFN-γ release was measured by ELISA. Data show mean ± SD from 3 donors. Nonlinear regression fit was applied to data. EC50 values were calculated to be: 2.1 µg/mL (MICA DMSO), 1.3 µg/mL (MICA 1.0µM Len), 62 µg/mL (hIgG DMSO), 21 µg/mL (hIgG 1.0µM Len). (C) Representative microscopy images of F-actin (red) and IFN-γ (shown in grayscale in middle column, and then green in merged image) in pNK cells stimulated for 90 minutes on surfaces coated with 1 µg/mL or 2.5 µg/mL MICA (+2.5 µg/mL ICAM-1) in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 10 µm. (D) The proportion of pNK cells expressing IFN-γ after stimulation with 3 different concentrations of MICA. Graph shows mean ± SD, n > 100 per donor from 3 donors. (E) Sum fluorescence staining for IFN-γ per cell in the same cells as in panel D. Each data point represents a single cell and red line shows the mean. *P < .05, **P < .01, ****P < .0001, 1-way ANOVA with Tukey posttest.
To observe the effect of activating ligand concentration on IFN-γ production at the single-cell level, pNK cells were imaged by confocal microscopy when stimulated by surfaces coated in 1 to 5 µg/mL MICA (Figure 3C). Lenalidomide treatment significantly increased the proportion of cells secreting IFN-γ when stimulated by 2.5 µg/mL MICA (33% ± 1.7% without lenalidomide; 55% ± 2.6% with lenalidomide), but not for 1 µg/mL MICA (Figure 3D). At 5 µg/mL MICA, both conditions showed a similar proportion of cells with IFN-γ protein expression (Figure 3D). This shows that there is a threshold concentration of MICA above which lenalidomide treatment increases the proportion of cells expressing IFN-γ. Interestingly, lenalidomide treatment resulted in a significant increase in the amount of IFN-γ expression per cell in all concentrations of MICA tested (Figure 3E). This establishes that lenalidomide has 2 effects on cells: at any level of NK-cell stimulation, lenalidomide increases the amount of IFN-γ produced per cell and, in addition, lenalidomide lowers the threshold for activation so that a greater proportion of NK cells are involved in the response.
Lenalidomide alters the periodicity of the actin mesh at the NK-cell ADCC synapse
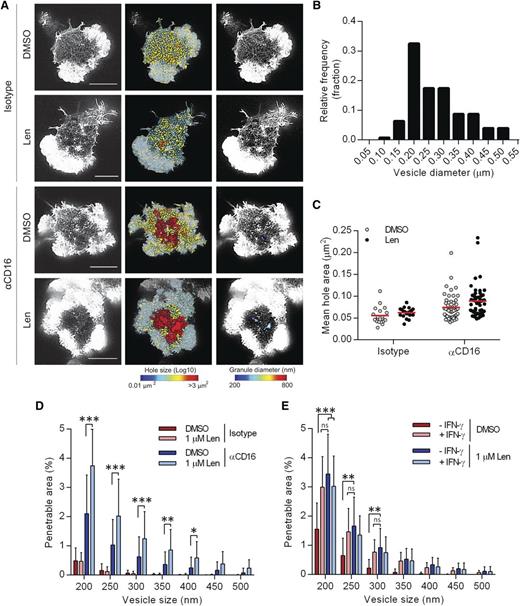
A nanoscale reorganization of synaptic actin has been observed prior to IFN-γ secretion.13 To test the effect of lenalidomide on actin remodeling, pNK cells were treated with 1 µM lenalidomide and stimulated on surfaces coated with anti-CD16 or an isotype-matched control mAb for 120 minutes, and F-actin imaged by STED microscopy (Figure 4A). Areas between individual actin filaments within the central region of the synapse, “holes,”9 were calculated and false-color heat maps were created to display the variation (Figure 4A). The “penetrable area” that would allow passage of vesicles of 200 to 500 nm diameter, the size of the majority of vesicles containing IFN-γ (Figure 4B), was also calculated and displayed as a false-color heat map (Figure 4A).
Lenalidomide treatment augments opening of cortical actin mesh after CD16 stimulation. (A) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 120 minutes on coverslips coated with isotype-matched control antibody or anti-CD16 mAb (both 3 µg/mL) in the presence of DMSO vehicle control or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments in the central region of the synapse shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown within the actin mesh through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (B) Histogram of measured vesicle sizes from 10 cells from 2 independent donors. (C) Average size of holes in the actin mesh at the pNK synapse for cells stimulated as in panel A. Each data point represents a single cell; red lines shows mean from 3 donors, n = 18 to 59 per condition. (D) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel C. (E) Analysis of STED microscopy of membrane proximal actin in pNK cell stimulated as in panel A plus 5 µg/mL brefeldin A and costained with anti-IFN-γ conjugated to Alexa 647. The proportion of the synapse predicted to be penetrable by a particle of 200- to 500-nm diameter, stratified by whether or not the cells stained positive for IFN-γ. Graph shows mean from 3 donors, n = 50. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide treatment augments opening of cortical actin mesh after CD16 stimulation. (A) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 120 minutes on coverslips coated with isotype-matched control antibody or anti-CD16 mAb (both 3 µg/mL) in the presence of DMSO vehicle control or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments in the central region of the synapse shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown within the actin mesh through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (B) Histogram of measured vesicle sizes from 10 cells from 2 independent donors. (C) Average size of holes in the actin mesh at the pNK synapse for cells stimulated as in panel A. Each data point represents a single cell; red lines shows mean from 3 donors, n = 18 to 59 per condition. (D) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel C. (E) Analysis of STED microscopy of membrane proximal actin in pNK cell stimulated as in panel A plus 5 µg/mL brefeldin A and costained with anti-IFN-γ conjugated to Alexa 647. The proportion of the synapse predicted to be penetrable by a particle of 200- to 500-nm diameter, stratified by whether or not the cells stained positive for IFN-γ. Graph shows mean from 3 donors, n = 50. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
For control-treated cells, the mean area of holes between actin filaments was 0.055 ± 0.004 µm2, and did not change significantly in the presence of lenalidomide (Figure 4C). The penetrable area also did not significantly change with lenalidomide (Figure 4D), consistent with the results from Figure 1, where lenalidomide only has an effect when accompanied by ligation of activating receptors. When cells were stimulated by anti-CD16 mAb, the mean hole area significantly increased (Figure 4C) and lenalidomide caused a small, but not significant, further increase, from 0.74 ± 0.004 µm2 to 0.89 ± 0.005 µm2 (Figure 4C). However, lenalidomide increased the penetrable area of the synapse nearly twofold for a wide range of vesicle sizes (Figure 4D). This indicates that, after lenalidomide treatment, a higher proportion of the actin mesh has opened up to become permissive to IFN-γ vesicles. Lenalidomide increased the penetrable area within the actin mesh, irrespective of whether or not the NK cells stained for IFN-γ (in the presence of brefeldin A to block IFN-γ release; Figure 4E), and, unlike the enhancement of IFN-γ production after lenalidomide treatment, the increase in cortical actin remodeling by lenalidomide was independent of IL-2 (supplemental Figure 4). Interestingly, the enhancement of cortical actin remodeling during cellular activation is not restricted to NK cells, as it was also observed in activated T cells after lenalidomide treatment (supplemental Figure 5). Overall, these data establish that lenalidomide influences the nanoscale organization of cortical actin at the NK-cell synapse.
For pNK cells stimulated on anti-CD16 mAb for only 6 minutes, the proportion of the synapse predicted to be penetrable by lytic granules (of at least 250-nm diameter; as defined previously9 ) was 1.5% ± 0.15% in DMSO-treated cells and increased to 1.9% ± 0.2% in lenalidomide-treated cells (supplemental Figure 6A-B). The penetrable area of the cortical actin mesh increased significantly after lenalidomide treatment in cells that were activated (determined by a dense ring of F-actin at the synapse periphery; supplemental Figure 6C). Thus, lenalidomide treatment also augments cortical actin remodeling at an early time point, relevant for lytic granule secretion, although to a lesser extent than observed later, at the time point relevant for cytokine secretion.
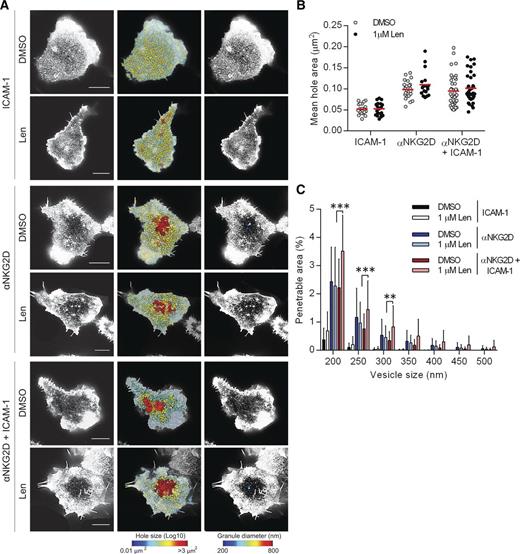
Lenalidomide alters the periodicity of the actin mesh after NKG2D and LFA-1 coligation
NKG2D is a major NK-cell receptor responsible for the recognition of MM cells. We therefore tested the effect of lenalidomide treatment following NKG2D ligation (Figure 5A). When pNK cells were in contact with slides coated with ICAM-1 alone, the mean hole area both with and without lenalidomide treatment was <0.053 µm2 (Figure 5B) and the percentage area of the synapse predicted to be penetrable by IFN-γ vesicles was <0.7% (Figure 5C). Ligation of NKG2D resulted in an opening of the cortical actin mesh (Figure 5B-C) but crucially, lenalidomide enhanced actin remodeling only when NKG2D and LFA-1 were coligated (Figure 5C). NKG2D engagement does not lead to NK-cell effector functions without LFA-1 coligation7 and hence this is further evidence that lenalidomide augments rather than triggers cellular activation.
Lenalidomide treatment augments opening of cortical actin mesh after NKG2D and LFA-1 ligation. (A) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 120 minutes on coverslips coated with recombinant human ICAM-1 (2.5 µg/mL), anti-NKG2D mAb (3 µg/mL), or both in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments in the central region of the synapse shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown within the actin mesh through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (B) Average size of holes in the actin mesh at the pNK synapse for cells stimulated as in panel A. Each data point represents a single cell; red lines shows the mean from 3 donors, n = 18 to 59 per condition. (C) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel B. Graph shows mean ± SD. **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide treatment augments opening of cortical actin mesh after NKG2D and LFA-1 ligation. (A) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 120 minutes on coverslips coated with recombinant human ICAM-1 (2.5 µg/mL), anti-NKG2D mAb (3 µg/mL), or both in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments in the central region of the synapse shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown within the actin mesh through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (B) Average size of holes in the actin mesh at the pNK synapse for cells stimulated as in panel A. Each data point represents a single cell; red lines shows the mean from 3 donors, n = 18 to 59 per condition. (C) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel B. Graph shows mean ± SD. **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
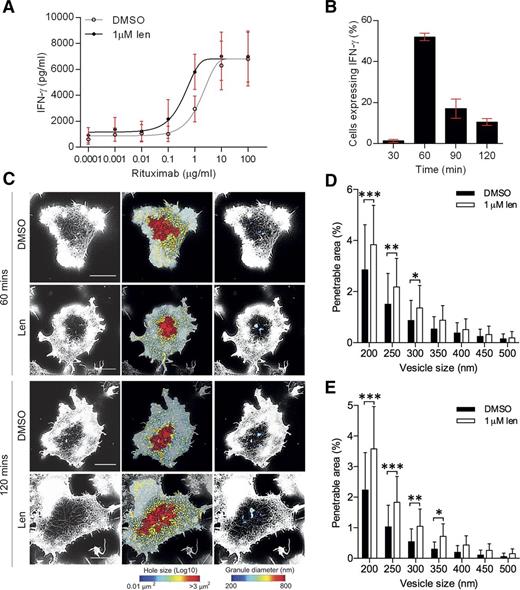
Lenalidomide augments NK-cell activation after rituximab ligation
Lenalidomide in combination with anti-CD20 mAb rituximab is currently in clinical trials for some lymphomas.34,35 To test whether lenalidomide influenced NK-cell activation by rituximab, lenalidomide-treated pNK cells were incubated on surfaces coated with increasing concentrations of rituximab (0.0001-100 µg/mL). A distinct threshold for NK-cell activation was observed and there was a 74% decrease in the EC50 in lenalidomide-treated cells (1.7 µg/mL to 0.4 µg/mL; Figure 6A). This indicates that lenalidomide lowers the minimum concentration of rituximab required to activate NK cells.
Lenalidomide treatment increases NK-cell activation through rituximab. (A) pNK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with 0.0001 to 100 µg/mL rituximab. IFN-γ release was measured by ELISA. Data shows mean ± SD from 3 donors. Nonlinear regression fit was applied to data. EC50 values calculated to be: 1.6 µg/mL without lenalidomide and 0.4 µg/mL with lenalidomide. (B) Proportion of DMSO-treated cells expressing IFN-γ after stimulation on rituximab-coated surfaces in the presence of DMSO or 1 µM lenalidomide for 30 to 120 minutes. Graph shows mean ± SD, n > 200 from 3 donors. (C) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 60 or 120 minutes on coverslips coated with rituximab (10 µg/mL) in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (D) Proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for cells stimulated on 10 µg/mL rituximab for 60 minutes. (E) Proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for cells stimulated on 10 µg/mL rituximab for 120 minutes. Graph shows mean ± SD, n = 45 from 3 donors. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide treatment increases NK-cell activation through rituximab. (A) pNK cells were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with 0.0001 to 100 µg/mL rituximab. IFN-γ release was measured by ELISA. Data shows mean ± SD from 3 donors. Nonlinear regression fit was applied to data. EC50 values calculated to be: 1.6 µg/mL without lenalidomide and 0.4 µg/mL with lenalidomide. (B) Proportion of DMSO-treated cells expressing IFN-γ after stimulation on rituximab-coated surfaces in the presence of DMSO or 1 µM lenalidomide for 30 to 120 minutes. Graph shows mean ± SD, n > 200 from 3 donors. (C) Superresolution images obtained by STED microscopy of membrane proximal F-actin in pNK cells incubated for 60 or 120 minutes on coverslips coated with rituximab (10 µg/mL) in the presence of DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2). Scale bars, 5 µm. Second column: Holes between actin filaments shown as heat maps, with the smallest holes shown in blue (0.01 µm2) and largest holes shown in red (>3 µm2). Third column: Regions are shown through which a particle (such as an IFN-γ vesicle) of diameter 200 nm (blue) to 800 nm (red) could fit. (D) Proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for cells stimulated on 10 µg/mL rituximab for 60 minutes. (E) Proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for cells stimulated on 10 µg/mL rituximab for 120 minutes. Graph shows mean ± SD, n = 45 from 3 donors. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Rituximab-activated pNK cells produced IFN-γ at an earlier time point, 60 minutes, than seen when pNK cells were activated with an anti-CD16 mAb (Figures 6B and 2B, respectively). Thus, pNK cells were incubated on glass slides coated with 10 µg/mL rituximab for 60 minutes and imaged (Figure 6C). Lenalidomide treatment led to a significant increase in the percentage area of the synapse predicted to be penetrable to a vesicle of 200- to 350-nm diameter (Figure 6D). This effect was also observed at 120 minutes (Figure 6E). Thus, lenalidomide increases the sensitivity of NK cells to rituximab, supporting the combined use of lenalidomide and rituximab in the treatment of B-cell malignancies.
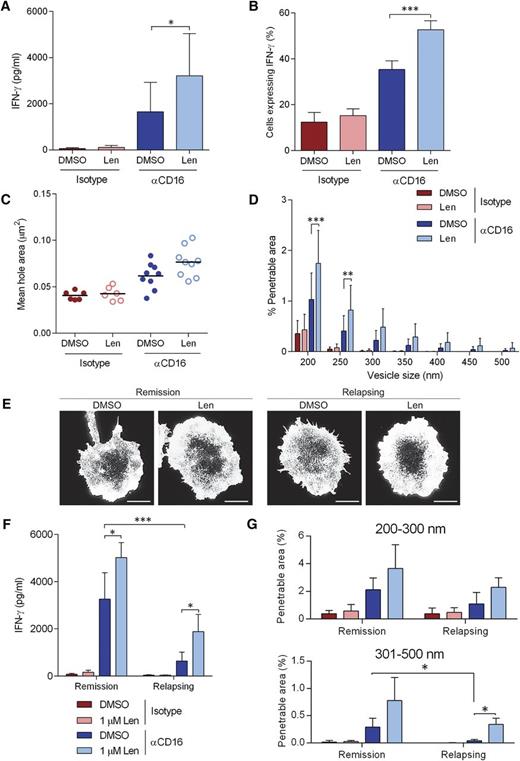
Lenalidomide augments NK-cell activation in NK cells from MM patients
Secretion of IFN-γ from NK-cells isolated from the peripheral blood of MM patients was significantly increased with 1 µM lenalidomide treatment after stimulation though CD16 (Figure 7A) and so was the proportion of cells producing IFN-γ (Figure 7B). In addition, NK cells from MM patients open up the cortical actin mesh after ligation of CD16 (Figure 7C-D), although to a slightly lesser extent than observed in healthy donor NK cells (Figure 4D). Treatment with lenalidomide significantly increased the proportion of the synapse predicted to be penetrable to IFN-γ vesicles (Figure 7D).
Lenalidomide augments NK-cell IFN-γ production and cortical actin rearrangements in NK cells isolated from MM patients. (A) Primary NK cells isolated from MM patients were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with anti-CD16 mAb or IgG1 isotype-matched control mAb. IFN-γ release was assessed by ELISA. E:T ratio was 10:1. Data shows mean ± SD from 9 MM patients. (B) Proportion of primary NK cells from MM patients expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1.0 µM lenalidomide for 120 minutes. Graph shows mean ± SD, n > 100 from 9 MM patients. (C) Average size of holes in the actin mesh at the pNK synapse for NK cells from MM patients stimulated on anti-CD16-coated surfaces and then imaged by STED microscopy. Data show the average per MM patient (20 cells analyzed per patient); black lines show the mean. (D) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel C. (E) Superresolution imaged obtained by STED microscopy of membrane proximal actin in NK cells from MM patient who are either in remission or relapsing, treated with DMSO vehicle control or 1.0 µM lenalidomide. Scale bars, 5 µm. (F) IFN-γ release as shown in panel A separated by whether the patients are in remission or relapsing. (G) The proportion of the synapse area predicted to be penetrable by a vesicle of either 200- to 300-nm diameter or 301- to 500-nm diameter in NK cells from MM patients who are in remission or relapsing. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
Lenalidomide augments NK-cell IFN-γ production and cortical actin rearrangements in NK cells isolated from MM patients. (A) Primary NK cells isolated from MM patients were treated with DMSO or 1.0 µM lenalidomide (+150 U/mL IL-2) for 24 hours in wells coated with anti-CD16 mAb or IgG1 isotype-matched control mAb. IFN-γ release was assessed by ELISA. E:T ratio was 10:1. Data shows mean ± SD from 9 MM patients. (B) Proportion of primary NK cells from MM patients expressing IFN-γ after stimulation on anti-CD16 mAb-coated surfaces in the presence of DMSO or 1.0 µM lenalidomide for 120 minutes. Graph shows mean ± SD, n > 100 from 9 MM patients. (C) Average size of holes in the actin mesh at the pNK synapse for NK cells from MM patients stimulated on anti-CD16-coated surfaces and then imaged by STED microscopy. Data show the average per MM patient (20 cells analyzed per patient); black lines show the mean. (D) The proportion of the synapse area predicted to be penetrable by a vesicle of 200- to 500-nm diameter for same cells as in panel C. (E) Superresolution imaged obtained by STED microscopy of membrane proximal actin in NK cells from MM patient who are either in remission or relapsing, treated with DMSO vehicle control or 1.0 µM lenalidomide. Scale bars, 5 µm. (F) IFN-γ release as shown in panel A separated by whether the patients are in remission or relapsing. (G) The proportion of the synapse area predicted to be penetrable by a vesicle of either 200- to 300-nm diameter or 301- to 500-nm diameter in NK cells from MM patients who are in remission or relapsing. *P < .05, **P < .01, ***P < .001, 1-way ANOVA with Tukey posttest.
The 9 patients we received peripheral blood from were clinically classified as either being in remission or relapsing. NK cells from MM patients classified as relapsing displayed markedly reduced CD16-induced IFN-γ production compared with NK cells from MM patients in remission and this was partially restored with lenalidomide treatment (Figure 7F). The reduced activation was not due to low CD16 expression as NK cells from MM patients expressed CD16 comparably to healthy NK cells (supplemental Figure 7). When cortical actin remodeling was observed by STED microscopy (Figure 7E), the proportion of the synapse predicted to be penetrable to vesicles of 200 to 300 nm diameter was slightly reduced in NK cells from patients who are relapsing and more dramatically reduced for larger vesicles (301-500 nm diameter) (Figure 7G). Treatment with lenalidomide restored actin rearrangements to the level observed in NK cells from MM patients in remission (Figure 7G). These data suggest a significant impairment in cortical actin remodeling in NK cells from MM patients, especially patients who are relapsing, which can be at least partially rescued with lenalidomide.
Discussion
Lenalidomide has significantly improved overall survival in myeloma patients, but recent studies have focused on the mechanism associated with T-cell activation.36-39 Here, we establish that lenalidomide augments NK-cell effector functions triggered through different activating receptors. This implies that lenalidomide acts downstream of receptor proximal signaling, as the signal pathways for CD16- and NKG2D-mediated activation are distinct and converge later. Lenalidomide increased both the proportion of cells producing IFN-γ and the amount of IFN-γ produced per cell but did not activate NK cells without appropriate triggers, indicating that the drug preserves NK-cell tolerance to prevent killing of healthy cells. Importantly, lenalidomide lowered the threshold for activation, suggesting that lenalidomide-treated NK cells would respond to lower concentrations of ligand on tumor cells; perhaps important for recognizing tumor cells which have partially downregulated activating ligands as a mechanism of immune evasion.
Recently, nanoscale remodeling of actin at the NK-cell immune synapse has been recognized as important for directed secretion.9,10,13 Actin remodeling has been considered a binary “all-or-nothing” response but here we found that lenalidomide augments the extent of actin remodeling at the synapse, causing a twofold increase in the area of the synapse predicted to be penetrable by a vesicle of 200- to 500-nm diameter. Lenalidomide-enhanced actin remodeling in NK cells may be linked to the mechanism by which this drug restores synapse assembly in T cells from chronic lymphocytic leukemia and lymphoma patients.30,40,41 Importantly, our data also provide the first evidence that synaptic actin remodeling is druggable, indicating that new compounds could be screened for an effect on cortical actin to augment NK-cell responses.
We have also shown that lenalidomide affects NK-cell activation after ligation of CD16 with rituximab, which is currently in clinical trials in combination with lenalidomide.34,35 Here, we establish that lenalidomide lowers the threshold for NK-cell activation through rituximab, consistent with increased ADCC.24,42,43
Finally, we have shown that lenalidomide augments NK-cell activation in NK cells from MM patients. Overall, our results demonstrate a direct and significant effect of lenalidomide on NK-cell effector functions without activating NK cells inappropriately. The fact that lenalidomide lowers the thresholds for activation of NK cells implies that this drug may be useful in other medical conditions where NK-cell immune responses are beneficial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Stacey for isolation of primary human NK cells. Research samples were obtained from the Manchester Cancer Research Centre (MCRC) Biobank, United Kingdom, with assistance from the Myeloma Unit at The Christie NHS Foundation Trust (Lead: Dr Jim Cavet, Consultant, Haematologist, and Hon. Senior Lecturer, University of Manchester). The authors also thank Deepti Wilks for collection of the samples. Although the MCRC Biobank provides the samples, it cannot endorse studies performed or the interpretation of results.
This work was supported by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, the Manchester Collaborative Centre for Inflammation Research, and Celgene Corporation.
Authorship
Contribution: K.L., R.C., and D.M.D. conceived the project; K.L. and D.M.D. designed experiments and wrote the manuscript; K.L. and D.J.M. performed experiments; and K.L. and A.C. analyzed the data.
Conflict-of-interest disclosure: R.C. is an employee of Celgene Corporation. The remaining authors declare no competing financial interests.
Correspondence: Daniel M. Davis, Manchester Collaborative Center for Inflammation Research, University of Manchester, 46 Grafton St, Manchester, M13 9NT, United Kingdom; e-mail: daniel.davis@manchester.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal