Key Points
GVHD induction is dependent on functional miR-155 in DCs of the allo-HCT recipient.
MiR-155 deficiency reduces ATP-mediated cell migration, ERK and inflammasome activation, and IL-1β production of DCs.
Abstract
The successful treatment of acute leukemias with allogeneic hematopoietic cell transplantation (allo-HCT) is limited by acute graft-versus-host disease (GVHD). Because microRNA-155 (miR-155) regulates activation of the innate immune system, we aimed to determine its function in dendritic cells (DCs) during GVHD in an experimental model. We observed that miR-155 deficiency of the recipient led to improved survival, reduced serum levels of proinflammatory cytokines, and lower GVHD histopathology scores. In addition, miR-155−/− bone marrow chimeric mice receiving allo-HCT and miR-155−/− DCs showed that miR-155 deficiency in the DC compartment was responsible for protection from GVHD. Activated miR-155−/− DCs displayed lower expression of various purinergic receptors and impaired migration toward adenosine triphosphate (ATP). Microarray analysis of lipopolysaccharide/ATP-stimulated miR-155−/− DCs revealed mitogen-activated protein kinase pathway dysregulation and reduced inflammasome-associated gene expression. Consistent with this gene expression data, we observed reduced ERK activation, caspase-1 cleavage, and IL-1β production in miR-155−/− DCs. The connection between miR-155 and inflammasome activation was supported by the fact that Nlrp3/miR-155 double-knockout allo-HCT recipient mice had no increased protection from GVHD compared with Nlrp3−/− recipients. This study indicates that during GVHD, miR-155 promotes DC migration toward sites of ATP release accompanied by inflammasome activation. Inhibiting proinflammatory miR-155 by antagomir treatment could help reduce this complication of allo-HCT.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is increasingly performed because it is the only curative treatment for high-risk acute leukemias and aggressive lymphomas. Unfortunately, its benefits are limited by the occurrence of acute graft-versus-host disease (GVHD) grades II-IV, which occurs in 30% to 40% of patients.1 Despite numerous clinical studies, the standard immunosuppressive regimens for the prevention and treatment of acute GVHD have changed little in the last 2 decades. The most frequently applied prophylaxis for GVHD are tacrolimus, cyclosporine A, methotrexate, mycophenolic acid, antithymocyte globulin, and alemtuzumab.2 First-line treatment of the clinical manifestation of acute GVHD is typically corticosteroids, whereas second-line therapies include tacrolimus, cyclosporine A, extracorporeal photopheresis, mycophenolic acid, mammalian target of rapamycin (mTOR) inhibitors, tumor necrosis factor (TNF) antagonists, pentostatin, or others.2 A better understanding of the pathophysiology of GVHD may thus help to improve patient outcomes after allo-HCT.
MicroRNAs (miRs) are small, endogenous noncoding RNAs that posttranscriptionally regulate gene expression by base pairing with sequences typically located in the 3′ untranslated region (3′ UTR) of target messenger RNAs (mRNAs) and subsequently repressing translation or inducing mRNA degradation.3 MiRs act on the expression of multiple target genes and may thereby influence the immune system at different cellular and functional levels. In light of their myriad roles, miRs are potentially attractive targets for the modification of allogeneic immune responses using miR mimics and inhibitors (antagomirs).
A central cytokine-promoting acute GVHD is interleukin (IL)-1β,4,5 and previous work has revealed that the Toll-like receptor/IL-1β inflammatory pathway is a target of miR-155.6 Recently, it was shown that miR-155 is required in the donor T-cell compartment for the development of acute GVHD.7 Compatible with a role in T-cell immune responses, miR-155 was also shown to positively regulate the function and lineage commitment of pathogenic T cells in experimental autoimmune encephalomyelitis.8 In addition to its role in adaptive immunity, miR-155 is involved in innate immune responses.6,9 We therefore studied its function in the activation process of recipient-type dendritic cells (DCs) after allo-HCT.
We observed that the transfer of miR-155−/− DCs induced less severe GVHD in a murine model of allo-HCT. Unbiased gene expression analysis of miR-155−/− DCs stimulated by adenosine-5′-triphosphate (ATP) and lipopolysaccharide (LPS) suggested that miR-155 deficiency induced defective DC activation during GVHD, a topic we had investigated previously in reports examining the ATP/P2X7 pathway,10 Toll-like receptor stimulation,11 and inflammasome activation.4 Functional studies guided by the gene expression data subsequently revealed that miR-155 was required in the DC compartment for migration up an ATP gradient and for inflammasome-mediated IL-1β production. The in vivo relevance of these findings was supported by the GVHD phenotype of newly generated Nlrp3/miR-155 double knockout (DKO) mice used as allo-HCT recipients.
Materials and methods
Mice
C57BL/6 (H-2Kb, Thy-1.2) and BALB/c (H-2Kd, Thy-1.2) mice were purchased from the local stock of the animal facility at Freiburg University Medical Center. MiR-155−/− C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). P2rx7−/−, P2ry2−/−, and P2ry12−/− mice on the C57BL/6 background were kindly provided by M. Idzko (Freiburg University Medical Center, Freiburg, Germany). Nlrp3−/− mice were originally generated by J. Tschopp (University of Lausanne, Lausanne, Switzerland) and kindly provided by S. Martin (Freiburg University Medical Center, Freiburg, Germany). CD45.1+ and CD45.2+ C57BL/6 mice were kindly provided by C. Klein (Freiburg University Medical Center, Freiburg, Germany). Nlrp3−/− and miR-155−/− mice were crossed to generate Nlrp3/miR-155 DKO mice on the C57BL/6 background. All mice were raised under specific pathogen-free conditions. Genotypes were confirmed by polymerase chain reaction (PCR). Mice were used between 6 and 25 weeks of age and only gender-matched combinations were used in transplant experiments. All animal protocols (G-12/34, G-13/114) were approved by the University Committee on the Use and Care of Laboratory Animals at Albert-Ludwigs-University, Freiburg, Germany.
Allo-HCT and induction of GVHD
Allo-HCT experiments were performed as previously described.12 Briefly, recipients received myeloablative total body irradiation (TBI) in 2 equal split doses (9-10 Gy in total), followed by IV injection of 5 × 106 allogeneic bone marrow (BM) cells. For induction of acute GVHD, CD4+ and CD8+ splenic T cells were enriched with CD4/CD8 MACS MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and the following quantities were injected: 3 × 105 T cells for BALB/c → wild-type (WT) and miR-155−/− C57BL/6, 8 × 105 T cells for BALB/c → WT and Nlrp3−/− C57BL/6.
Generation of BM chimeric recipients and additional transfer of DCs or macrophages in allo-HCT
In a first syngeneic transplantation, WT C57BL/6 recipients were given 5 × 106miR-155−/−, Nlrp3−/−, or Nlrp3/miR-155 DKO BM cells IV after TBI with 10 Gy. In an allo-HCT 30 days later, the BM chimeric mice received 5 × 106 allogeneic BM cells and 8 × 105 allogeneic T cells from a BALB/c donor. Where indicated, groups additionally received 2 × 106 C57BL/6 DCs or macrophages on the day of allo-HCT to reconstitute the BM chimeric mice with recipient-type DCs. To assess reconstitution of the recipients with transferred DCs, CD45.2+ C57BL/6 mice received a syngeneic transplantation with CD45.1+ C57BL/6 BM cells, followed by an allo-HCT (>30 days post auto-HCT) with BM and T cells from a BALB/c donor, along with CD45.1+ C57BL/6 DCs. Analysis of the spleen of the recipient mice 24 hours after the allo-HCT showed that 97% of the CD11c+ cells were replaced by the transferred DCs, which were CD45.1+.
Cytokine measurements in the serum of mice with GVHD
Serum was collected from the recipients 7 days after allo-HCT and the levels of the cytokines, interferon-γ (IFN-γ), IL-12, and monocyte chemotactic protein-1 (MCP-1) were measured using the Cytometric Bead Array Inflammation kit (BD Biosciences, San Jose, CA).
Histopathology scoring of acute GVHD
Sections of the small intestine, large intestine, and liver were collected from the recipient 7 days after allo-HCT, stained with hematoxylin and eosin (H&E), and scored by a pathologist blinded to the treatment groups according to a previously published histopathology scoring system.13
In vivo antagomir treatment
Antagomirs were custom synthesized (Biozym, Hessisch Oldendorf, Germany) with the following sequences: antagomir-control: 5′-AAGGCAAGCUGACCCUGAAGUU-3′; antagomir-155: 5′-CCCCUAUCACAAUUAGCAUUAA-3′. Mice received a tail vein injection of 8 mg/kg antagomir on days 0, 3, and 9 after allo-HCT.
T-cell proliferation assay
Lethally irradiated WT or miR-155−/− mice were transplanted with 5 × 106 allogeneic BM cells and 1 × 106 CD4+/CD8+ allogeneic T cells that had been labeled with 5 µm carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA), as previously described.14 On day 1 after allo-HCT, axillary, inguinal, and mesenteric lymph nodes of the recipient mice were isolated and CFSE fluorescence in donor T cells was analyzed by flow cytometry.
Generation of BM-derived DCs
BM-derived DCs were prepared as previously described,15 except that IL-4 was not added to the cultures. Cells were used on days 7 to 12 of culture.
Generation of BM-derived macrophages
After erythrocyte lysis, BM cells were plated at a concentration of 8 × 106 cells per 8 mL of medium. For differentiation of BM cells into macrophages, 20 ng/mL macrophage colony-stimulating factor (M-CSF) were added. On day 3 of the culture, 8 mL medium and 20 ng/mL M-CSF were added to every dish. On day 5, 8 mL of every dish were replaced with 8 mL of fresh medium supplemented with 20 ng/mL M-CSF. On day 7, the purity of the macrophages characterized by CD11b and F4/80 reached 98.7% of all CD11b+ Ly-6Clow cells.
Flow cytometry
The following monoclonal antibodies were used: CD3 (Alexa Fluor 647, 17A2; BioLegend, San Diego, CA), CD4 (peripheral blood [PB], RM4-5; BioLegend), CD11b (PB, M1/70; BioLegend), CD11c (Alexa Fluor 647, N418; BioLegend), CD25 (phycoerythrin [PE], PC61; BioLegend), CD45.1 (fluorescein isothiocyanate [FITC], A20; BioLegend), CD45.2 (PB, 104; BioLegend), CD69 (PE, H1.2F3; BioLegend), CD80 (PE, 16-10A1; BioLegend), CD86 (PB, GL-1; BioLegend), CD107a (PE, 1D4B; BioLegend), F4/80 (Alexa Fluor 647, CI:A3-1; AbD Serotec, Kidlington, UK), H-2Kd (PB, SF1-1.1; BioLegend), Ly-6C (FITC, HK1.4; BioLegend), P2X7 (PE, Hano43; Santa Cruz Biotechnology, Dallas, TX), IFN-γ (PE, XMG1.2; eBioscience, San Diego, CA), IL-4 (antigen-presenting cell [APC], 11B11; eBioscience), IL-6 (APC, MP5-20F3; BioLegend), IL-12/IL-23 p40 (APC, C15.6; BioLegend), IL-17A (FITC, TC11-18H10.1; BD Biosciences), and TNF-α (PB, MP6-XT22; BioLegend). Intracellular staining for cytokines was performed using BD Cytofix/Cytoperm (BD Biosciences). Samples were acquired on a CyAn ADP flow cytometer (Beckman Coulter, Brea, CA) and analyzed with FlowJo v7.6.5 (TreeStar, Ashland, OR).
DC migration assay
DC migration was analyzed as previously described.16 Briefly, 5 × 105 DCs were allowed to migrate through a polycarbonate filter (membrane pore size 3.0 µm) of a 24-well transwell migration plate (Corning Inc., Corning, NY) over 5 hours at 37°C. Where indicated, 0.1 mM ATP (Sigma-Aldrich, St. Louis, MO) was added to the medium.
Western blot
DCs were, where indicated, stimulated with 50 ng/mL LPS (Sigma-Aldrich) and 5 mM ATP (Sigma-Aldrich), and lysed using radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology) supplemented with Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich). The protein concentration was determined using a bicinchoninic acid assay (Thermo Scientific, Waltham, MA). Total cell protein was separated in a 4% to 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Invitrogen) and transferred onto a polyvinylidene fluoride membrane (GE Healthcare, Chalfont St Giles, UK). The membrane was incubated with primary antibodies against extracellular signal–regulated kinase (ERK; #9102, Cell Signaling, Cambridge, UK), phospho-ERK (#9101, Cell Signaling), and caspase-1 p10 (sc-514, Santa Cruz Biotechnology). Horseradish peroxidase–conjugated anti-rabbit IgG was used as a secondary antibody (#7074, Cell Signaling). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (G9295, Sigma-Aldrich) was used as a loading control. Chemiluminescent substrates were purchased from Thermo Scientific and GE Healthcare.
Enzyme-linked immunosorbent assay for IL-1β
On day 7 or 12 of DC culture, the cells were stimulated for 15 or 30 minutes with 50 ng/mL LPS and 5 mM ATP. IL-1β concentrations were measured in the supernatant using the Mouse IL-1β ELISA Set (BD Biosciences).
Quantitative reverse-transcription PCR
For analysis of miR-155 expression in DCs, BM-derived DCs from BALB/c mice were treated with 10 ng/mL LPS for 24, 48, and 72 hours. For analysis of P2rx7 expression, DCs were stimulated with 10 ng/mL LPS overnight. Total RNA was isolated using the miRNeasy Mini Kit (QIAGEN, Venlo, Netherlands). 2 ng/µL total RNA was transcribed into complementary DNA (cDNA) using the MultiScribe Reverse Transcriptase (Life Technologies, Carlsbad, CA) or the First Strand cDNA synthesis kit (Thermo Scientific). Quantitative reverse-transcription PCR (qRT-PCR) was performed on a MyIQ (Bio-Rad Laboratories, Hercules, CA) or a LightCyler 480 (Roche, Rotkreuz, Switzerland). TaqMan primers for mmu-miR-155 (assay ID 001806; TaqMan MicroRNA Assay Kit, Life Technologies) were used to monitor miR-155 expression. P2rx7 expression was determined using FAST BLUE qRT-PCR MasterMix, and primers and dual-labeled probes that were designed as previously described (Eurogentec, Seraing, Belgium).17 MiR-155 or P2rx7 gene expression was normalized to the reference genes Rnu19 (assay ID 001003) or β2-microglobulin, and expressed relative to the untreated or WT group, respectively, using the 2–ΔΔCt method.18
Microarray analysis
DCs were stimulated with 50 ng/mL LPS (Sigma-Aldrich) and 5 mM ATP (Sigma-Aldrich) for 15 minutes. RNA was extracted with the microRNeasy Mini Kit (QIAGEN) and used to generate a microarray-based gene expression profile. Total RNA quality was verified with an Agilent BioAnalyzer 2100. cDNA was generated from the RNA samples using the Ambion WT Expression kit and then fragmented and labeled with the Affymetrix Terminal Labeling kit before hybridization on Affymetrix GeneChip Mouse Gene 1.0 ST arrays for 16 hours at 45°C and 60 rpm in an Affymetrix Hybridization Oven 645. After washing and staining, the arrays were scanned with the Affymetrix GeneChip Scanner 3000 7G. CEL-files were produced from the raw data with Affymetrix GeneChip Command Console Software Version 3.0.1, and Genedata Expressionist software was used to perform preprocessing steps including GC background subtraction, quantile normalization, and probe summarization.19 Differentially expressed genes were identified by performing an analysis of variance and calculating the Benjamini-Hochberg q-value.20 Only genes from the categories “main” and “unmapped” (see Affymetrix transcript annotation NA32) were included, thereby omitting control probes from further analysis. Gene Set Enrichment Analysis software and the Molecular Signature Database were used for further analysis.21 Gene sets were self-assembled or curated from the Kyoto Encyclopedia of Genes and Genomes.22 The enrichment ratio used in the heat maps was calculated as the sample expression divided by the mean expression of the other group for each respective gene. The complete gene expression data are available at ArrayExpress (www.ebi.ac.uk/arrayexpress), accession #E-MTAB-2608.
Statistical analysis
Normally distributed data were compared using a 2-sided unpaired Student t test. If the data did not meet the criteria of normality, the Mann-Whitney U test was performed. Data are presented as mean ± standard error of the mean. Power analysis was performed to assess the sample size in the mouse GVHD survival experiments. A sample size of at least n = 10 per group was determined capable of detecting, with 80% power, an effect size of at least 1.06 with P < .05. Differences in animal survival (Kaplan-Meier survival curves) were analyzed by a log-rank test. A P value < .05 was considered significant.
Results
MiR-155 expression is upregulated in LPS-stimulated DCs and miR-155 deficiency of the recipient decreases GVHD
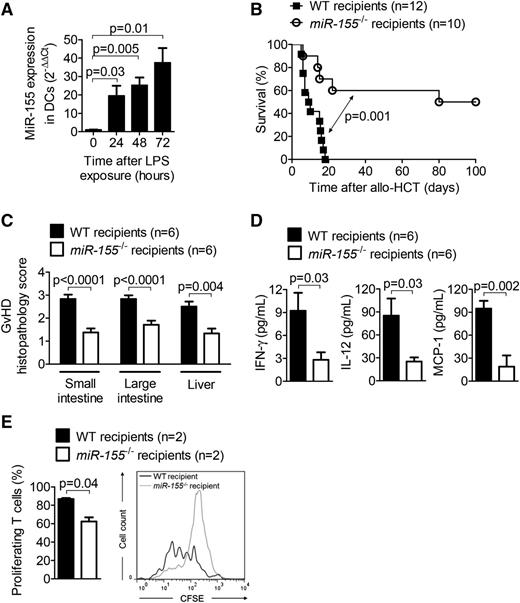
Because LPS is known to exacerbate GVHD severity by leaking through damaged intestinal and skin barriers,23 we studied the impact of LPS on miR-155 expression and observed that LPS induced miR-155 in DCs in proportion to the duration of LPS exposure (Figure 1A).
MiR-155 expression is increased in LPS-stimulated DCs, and recipient miR-155 deficiency decreases GVHD. (A) RNA was isolated from DCs stimulated with 10 ng/mL LPS for the indicated time intervals. MiR-155 expression was analyzed by qRT-PCR. Data are pooled from 3 independent experiments. (B) Allo-HCT was performed as described for the BALB/c into WT (n = 12) and miR-155−/− (n = 10) C57BL/6 combination. Survival was monitored for 100 days. (C) On day 7 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), organs were isolated and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described. (D) On day 7 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), serum was isolated from the recipients, and the concentrations of INF-γ, IL-12, and MCP-1 were determined. (E) On day 1 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), axillary, inguinal, and mesenteric lymph nodes were isolated and CFSE dilution of CD3+ H-2Kd+ donor T cells was analyzed.
MiR-155 expression is increased in LPS-stimulated DCs, and recipient miR-155 deficiency decreases GVHD. (A) RNA was isolated from DCs stimulated with 10 ng/mL LPS for the indicated time intervals. MiR-155 expression was analyzed by qRT-PCR. Data are pooled from 3 independent experiments. (B) Allo-HCT was performed as described for the BALB/c into WT (n = 12) and miR-155−/− (n = 10) C57BL/6 combination. Survival was monitored for 100 days. (C) On day 7 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), organs were isolated and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described. (D) On day 7 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), serum was isolated from the recipients, and the concentrations of INF-γ, IL-12, and MCP-1 were determined. (E) On day 1 after allo-HCT (BALB/c → WT and miR-155−/− C57BL/6), axillary, inguinal, and mesenteric lymph nodes were isolated and CFSE dilution of CD3+ H-2Kd+ donor T cells was analyzed.
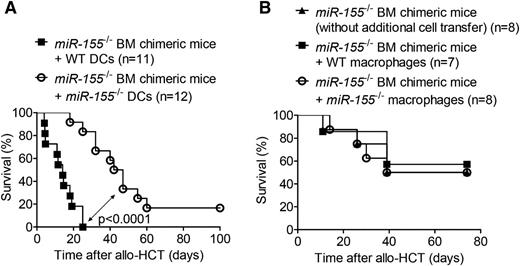
We further assessed the role of miR-155 in GVHD by performing allo-HCT on WT and miR-155−/− recipients. The increased survival of the miR-155−/− cohort relative to the WT recipients was suggestive of a functional role for miR-155 in GVHD development (Figure 1B). MiR-155−/− mice exhibited lower GVHD histopathology scores of the small intestine, large intestine, and liver compared with WT mice (Figure 1C). The cytokines IFN-γ, IL-12, and MCP-1, which are surrogate parameters for GVHD severity,16,24 were reduced in the serum of miR-155−/− mice compared with WT mice on day 7 after allo-HCT (Figure 1D). Proliferation of donor-derived T cells was reduced in miR-155−/− recipient mice compared with WT recipient mice 24 hours after allo-HCT (Figure 1E). To decipher whether recipient-type DCs were responsible for the observed reduction in GVHD severity, we generated mice lacking miR-155 specifically in the hematopoietic system (miR-155−/− BM chimeric mice) via syngeneic transplantation. After full engraftment, these mice received an allo-HCT comprising BM cells and T cells from a BALB/c donor, and recipient-type WT or miR-155−/− DCs, which reconstituted 97% of the CD11c+ recipient cells by day 1 after allo-HCT (supplemental Figure 1A, available on the Blood Web site). Survival was significantly improved when miR-155−/− DCs were transferred compared with WT DCs (Figure 2A). Conversely, infusion of macrophages characterized by CD11b and F4/80 (supplemental Figure 1B) from either WT or miR-155−/− mice as a non-DC APC population into the miR-155−/− BM chimeric mice was not associated with reduced survival compared with miR-155−/− BM chimeric mice that received no additional cell transfer (Figure 2B). These data therefore support the hypothesis that miR-155 expression in DCs of the recipient plays a key role in GVHD induction.
MiR-155 deficiency in adoptively transferred recipient-type DCs is connected to lower GVHD severity. (A) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic miR-155−/− C57BL/6 donor. After 30 days, the miR-155−/− BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106 WT or miR-155−/− C57BL/6 BM-derived DCs on the day of allo-HCT. Survival data were pooled from 2 independent experiments (n = 11-12/group). (B) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic miR-155−/− C57BL/6 donor. After 30 days, the miR-155−/− BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106 WT or miR-155−/− C57BL/6 BM-derived macrophages on the day of allo-HCT (n = 7-8/group).
MiR-155 deficiency in adoptively transferred recipient-type DCs is connected to lower GVHD severity. (A) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic miR-155−/− C57BL/6 donor. After 30 days, the miR-155−/− BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106 WT or miR-155−/− C57BL/6 BM-derived DCs on the day of allo-HCT. Survival data were pooled from 2 independent experiments (n = 11-12/group). (B) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic miR-155−/− C57BL/6 donor. After 30 days, the miR-155−/− BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106 WT or miR-155−/− C57BL/6 BM-derived macrophages on the day of allo-HCT (n = 7-8/group).
Global gene expression analysis reveals downregulation of multiple proinflammatory purinergic receptors in miR-155−/− DCs
By isolating total RNA from WT or miR-155−/− DCs and performing a microarray-based analysis, we observed alterations in gene expression of DCs activated by LPS and ATP in the absence of miR-155. Several genes that were predicted by TargetScan (http://targetscan.org/) to be regulated by miR-155 were de-repressed in miR-155−/− DCs, including Msk1, Satb1, Nik, Etv3, and Mlk2 (data not shown). Because miR-155 mediates a proinflammatory phenotype in our GVHD model, we next searched for genes exhibiting lower expression in miR-155−/− compared with WT DCs. One group of genes displaying such an expression pattern was the purinergic receptors P2rx7, P2ry12, and P2ry2 (Figure 3A), which have been previously reported to be involved in multiple inflammatory models.10,25,26 The expression of P2rx7 (P = .007) and P2ry12 (P = .01) was significantly lower in miR-155−/− DCs compared with WT DCs. There was a trend toward lower expression of P2ry2 (P = .26) in miR-155−/− DCs compared with WT DCs, although the difference did not reach statistical significance. We first turned toward P2ry12 and P2ry2 because these receptors have been shown to be critical for DC migration,27-29 a central event during the priming phase of GVHD. We used DCs generated from gene-targeted mice lacking P2ry12 or P2ry2 and compared their migration toward ATP in vitro. Migration of P2ry12−/− and P2ry2−/− DCs up an ATP gradient was reduced compared with WT DCs (Figure 3B-C). Consistent with diminished expression in miR-155−/− DCs of these 2 purinergic receptors relevant to motility, migration of miR-155−/− DCs was also reduced compared with WT DCs (Figure 3D). These data indicate a functional connection between miR-155 and P2Y12 and P2Y2 during ATP-mediated migration, a central event during the priming phase of GVHD.
MiR-155 deficiency leads to downregulation of P2ry12, P2ry2, and P2rx7, and diminished migratory ability. (A) Heat map showing the expression of purinergic receptor mRNA in a microarray analysis of WT and miR-155−/− C57BL/6 DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. (B) Migration of BM-derived DCs from WT and P2ry12−/− mice up an ATP gradient. Data were acquired from 3 independent experiments. (C) Migration of BM-derived DCs from WT and P2ry2−/− mice up an ATP gradient. Data were acquired from 4 independent experiments. (D) Migration of BM-derived DCs from WT and miR-155−/− mice up an ATP gradient. The experiment was performed 4 times with 3 technical triplicates each. (E) WT and miR-155−/− DCs were stimulated with 10 ng/mL LPS overnight, and P2rx7 expression on the mRNA level was determined by qRT-PCR (n = 2/group). (F) Expression of P2X7 protein on WT and miR-155−/− DCs was determined by flow cytometry. Data were pooled from 4 independent experiments.
MiR-155 deficiency leads to downregulation of P2ry12, P2ry2, and P2rx7, and diminished migratory ability. (A) Heat map showing the expression of purinergic receptor mRNA in a microarray analysis of WT and miR-155−/− C57BL/6 DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. (B) Migration of BM-derived DCs from WT and P2ry12−/− mice up an ATP gradient. Data were acquired from 3 independent experiments. (C) Migration of BM-derived DCs from WT and P2ry2−/− mice up an ATP gradient. Data were acquired from 4 independent experiments. (D) Migration of BM-derived DCs from WT and miR-155−/− mice up an ATP gradient. The experiment was performed 4 times with 3 technical triplicates each. (E) WT and miR-155−/− DCs were stimulated with 10 ng/mL LPS overnight, and P2rx7 expression on the mRNA level was determined by qRT-PCR (n = 2/group). (F) Expression of P2X7 protein on WT and miR-155−/− DCs was determined by flow cytometry. Data were pooled from 4 independent experiments.
Finally, the significant reduction in P2rx7 expression we observed in miR-155−/− DCs compared with WT DCs (Figure 3A) hinted at potential dysfunction in the ability of miR-155−/− DCs to promote an inflammatory response because the role of P2X7 in inflammation has been well documented.30 Because we had also previously demonstrated the involvement of P2X7 in GVHD,10 we confirmed the reduced expression of P2rx7 in miR-155−/− DCs at the mRNA and protein levels by qRT-PCR and flow cytometry, respectively (Figure 3E-F).
MiR-155−/− DCs display a reduced mitogen-activated protein kinase response to LPS/ATP stimulation
To better understand the defect in miR-155−/− DCs, we analyzed gene sets that have been shown to be relevant in GVHD, including the mitogen-activated protein kinase (MAPK) signaling pathway31 and the inflammasome complex.4 The microarray analysis revealed dysregulated expression of MAPK family genes in miR-155−/− DCs compared with WT DCs (Figure 4A). To determine whether this was functionally relevant, we measured ERK activation, a central signaling event in DC activation and migration.32 We observed significantly lower ERK phosphorylation in miR-155−/− DCs compared with WT DCs in response to LPS and ATP (Figure 4B). However, we detected no impact of miR-155 deficiency on CD80 or CD86 expression (supplemental Figure 2A); T-cell phenotype with respect to IFN-γ, IL-4, and IL-17A production (supplemental Figure 2B-C); or CD69, CD107a, and CD25 expression after allo-stimulation (supplemental Figure 2D).
MiR-155−/− DCs exhibit broad dysregulation of MAPK signaling. (A) Expression of the 70 most highly up- and downregulated genes in the Kyoto Encyclopedia of Genes and Genomes MAPK pathway in microarray data from WT and miR-155−/− DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. Genes contributing to path enrichment were identified and ordered using Gene Set Enrichment Analysis. (B) DCs were stimulated with 50 ng/mL LPS and 5 mM ATP for 15 minutes. Protein expression of phosphorylated ERK (pERK) was determined by Western blot, expressed relative to total ERK (tERK), and normalized to the stimulated WT group. GAPDH was used as a loading control. One representative blot is shown. For the quantification, data were pooled from 3 independent experiments.
MiR-155−/− DCs exhibit broad dysregulation of MAPK signaling. (A) Expression of the 70 most highly up- and downregulated genes in the Kyoto Encyclopedia of Genes and Genomes MAPK pathway in microarray data from WT and miR-155−/− DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. Genes contributing to path enrichment were identified and ordered using Gene Set Enrichment Analysis. (B) DCs were stimulated with 50 ng/mL LPS and 5 mM ATP for 15 minutes. Protein expression of phosphorylated ERK (pERK) was determined by Western blot, expressed relative to total ERK (tERK), and normalized to the stimulated WT group. GAPDH was used as a loading control. One representative blot is shown. For the quantification, data were pooled from 3 independent experiments.
Inflammasome priming has been previously demonstrated to be dependent on ERK activation.33 Therefore, our observation of diminished ERK activation in miR-155−/− DCs implicates defects in inflammasome activation compared with WT DCs.
MiR-155−/− DCs exhibit reduced inflammasome responsiveness to LPS/ATP stimulation
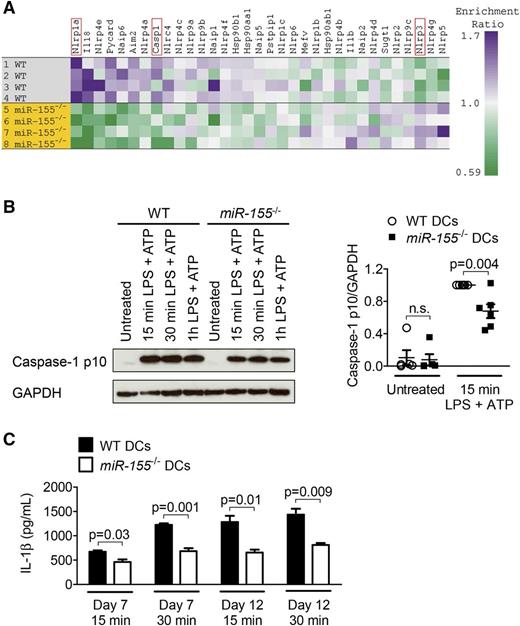
The Nlrp3 inflammasome is activated when ATP binds to P2X7.34 Given the reduced P2X7 levels on miR-155−/− DCs we had observed, we next analyzed the expression of genes associated with the inflammasome in miR-155−/− DCs and found multiple inflammasome-related genes to be downregulated in miR-155−/− DCs compared with the WT DCs. Nlrp1a, which was shown to be involved in inflammation and pyroptosis,35 exhibited the most significantly reduced gene expression of the set in the miR-155−/− DCs (Figure 5A). Activation with LPS and ATP led to lower levels of cleaved caspase-1 in miR-155−/− DCs compared with WT DCs (Figure 5B). Cleaved caspase-1 is an established indicator for the activity of the Nlrp3 inflammasome.36 Importantly, miR-155−/− DCs also displayed a diminished ability to secrete IL-1β after LPS/ATP stimulation compared with WT DCs (Figure 5C). Conversely, we observed that the production of non-inflammasome–related cytokines such as IL-6, IL-23, and TNF-α was preserved in miR-155−/− DCs compared with WT DCs (supplemental Figure 3). Based on these findings, we propose that miR-155 deficiency leads to reduced inflammasome activation, a central event during early GVHD development.4
MiR-155−/− DCs display decreased levels of cleaved caspase-1 and IL-1β. (A) A heat map showing known and potential inflammasome component gene expression in a microarray analysis of WT and miR-155−/− DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. (B) DCs were stimulated with 50 ng/mL LPS and 5 mM ATP for the indicated time intervals. Protein expression of cleaved caspase-1 (caspase-1 p10) was determined by Western blot and normalized to the stimulated WT group. GAPDH was used as a loading control. One representative blot is shown. For the quantification, data were pooled from 6 independent experiments. (C) On day 7 or 12 of DC culture, the cells were stimulated with 50 ng/mL LPS and 5 mM ATP for the indicated time intervals. IL-1β concentrations were measured in the culture supernatant (n = 3/group). Basal IL-1β production was subtracted.
MiR-155−/− DCs display decreased levels of cleaved caspase-1 and IL-1β. (A) A heat map showing known and potential inflammasome component gene expression in a microarray analysis of WT and miR-155−/− DCs after stimulation with 50 ng/mL LPS and 5 mM ATP for 15 minutes. (B) DCs were stimulated with 50 ng/mL LPS and 5 mM ATP for the indicated time intervals. Protein expression of cleaved caspase-1 (caspase-1 p10) was determined by Western blot and normalized to the stimulated WT group. GAPDH was used as a loading control. One representative blot is shown. For the quantification, data were pooled from 6 independent experiments. (C) On day 7 or 12 of DC culture, the cells were stimulated with 50 ng/mL LPS and 5 mM ATP for the indicated time intervals. IL-1β concentrations were measured in the culture supernatant (n = 3/group). Basal IL-1β production was subtracted.
The functional connection of Nlrp3 and miR-155 is supported by the absence of synergistic protection when both factors are lacking
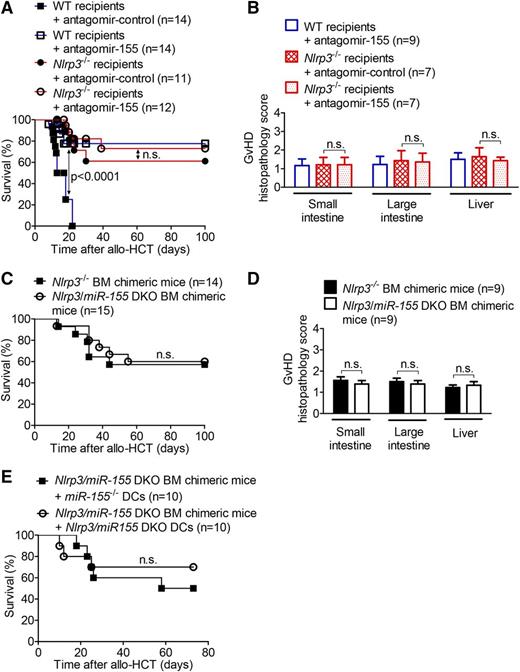
To clarify whether the in vitro defects of miR-155−/− DCs with respect to inflammasome activation were relevant in vivo, we used Nlrp3−/− mice as allo-HCT recipients and administered a course of antagomir-155 treatment with the goal of examining synergistic protection from GVHD in relation to antagomir-control–treated recipient mice. Such synergy, indicative of 2 factors functioning along independent pathways, was not identified in the Nlrp3−/− recipients treated with antagomir-155 with respect to survival (Figure 6A) or histopathology scoring of the small intestine, large intestine, and liver (Figure 6B). To verify this finding via a second approach, we generated Nlrp3/miR-155 DKO BM chimeric mice and used them as allo-HCT recipients. We observed no difference in survival or histopathology scoring between Nlrp3/miR-155 DKO and Nlrp3−/− BM chimeric mice (Figure 6C-D). The observation that GVHD was not further improved in Nlrp3/miR-155 DKO BM chimeric mice compared with Nlrp3−/− BM chimeric mice supports the hypothesis that the miR-155 and Nlrp3 axes are functionally connected in the development of GVHD. To directly compare miR-155−/− and Nlrp3/miR-155 DKO DCs, we generated Nlrp3/miR-155 DKO BM chimeric mice and transplanted them with grafts containing miR-155−/− or Nlrp3/miR-155 DKO DCs. No difference with respect to survival was observed between the 2 groups (Figure 6E), indicating that similar pathways are blocked when DCs lacked miR-155 or Nlrp3.
Nlrp3 deficiency and either miR-155 antagonism or miR-155 deficiency display no synergy in protection from GVHD. (A) Mice received an allo-HCT (BALB/c → WT or Nlrp3−/− C57BL/6) and were treated with nonfunctional antagomir-control or functional antagomir-155 on days 0, 3, and 9 after allo-HCT. Survival was monitored for 100 days. The experiment was performed twice (n = 11-14/group; P < .0001 for WT recipients, no significant difference observed for Nlrp3−/− recipients). (B) Organs of the recipients that had survived in (A) until day 100 were isolated, and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described. The experiment was performed twice (n = 7-9/group, no significant difference observed between Nlrp3−/− recipients treated with antagomir-control and antagomir-155). (C) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic Nlrp3−/− or Nlrp3/miR-155 DKO C57BL/6 donor. After 30 days, the BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Survival data were pooled from 2 independent experiments (n = 14-15/group, no significant difference between the groups). (D) Organs of the recipients that had survived in (C) until day 100 were isolated, and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described (n = 9/group, no significant difference observed between Nlrp3−/− and Nlrp3/miR-155 DKO BM chimeric mice). (E) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic Nlrp3/miR-155 DKO C57BL/6 donor. After 30 days, the Nlrp3/miR-155 DKO BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106miR-155−/− or Nlrp3/miR-155 DKO C57BL/6 BM-derived DCs on the day of allo-HCT (n = 10/group).
Nlrp3 deficiency and either miR-155 antagonism or miR-155 deficiency display no synergy in protection from GVHD. (A) Mice received an allo-HCT (BALB/c → WT or Nlrp3−/− C57BL/6) and were treated with nonfunctional antagomir-control or functional antagomir-155 on days 0, 3, and 9 after allo-HCT. Survival was monitored for 100 days. The experiment was performed twice (n = 11-14/group; P < .0001 for WT recipients, no significant difference observed for Nlrp3−/− recipients). (B) Organs of the recipients that had survived in (A) until day 100 were isolated, and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described. The experiment was performed twice (n = 7-9/group, no significant difference observed between Nlrp3−/− recipients treated with antagomir-control and antagomir-155). (C) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic Nlrp3−/− or Nlrp3/miR-155 DKO C57BL/6 donor. After 30 days, the BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Survival data were pooled from 2 independent experiments (n = 14-15/group, no significant difference between the groups). (D) Organs of the recipients that had survived in (C) until day 100 were isolated, and paraffin sections of the small intestine, large intestine, and liver were stained with hematoxylin and eosin. Histopathology scoring was performed as described (n = 9/group, no significant difference observed between Nlrp3−/− and Nlrp3/miR-155 DKO BM chimeric mice). (E) Lethally irradiated WT C57BL/6 mice were transplanted with 5 × 106 BM cells from a syngeneic Nlrp3/miR-155 DKO C57BL/6 donor. After 30 days, the Nlrp3/miR-155 DKO BM chimeric mice were irradiated with 5 Gy and injected with 5 × 106 BM cells and 8 × 105 CD4+/CD8+ T cells from an allogeneic BALB/c donor. Groups additionally received 2 × 106miR-155−/− or Nlrp3/miR-155 DKO C57BL/6 BM-derived DCs on the day of allo-HCT (n = 10/group).
Importantly, WT allo-HCT recipients treated with antagomir-155 survived significantly longer than WT recipients receiving the antagomir-control, underscoring the potential therapeutic applications of miR-155 inhibition in GVHD.
Discussion
Our understanding of the pathophysiologic role of hematopoietic and nonhematopoietic antigen-presenting cells during GVHD is still incomplete. Because multiple antigen-presenting cell functions, including migration, costimulatory molecule expression, and cytokine production, are required for their full ability to stimulate allogeneic T cells, several layers of immune regulation in this cell type are conceivable.
Here, we present the regulation of DC function through miR-155, a miR that, in GVHD, has thus far only been characterized in T cells as a requirement for disease induction.7 We observed that miR-155 was essential for several proinflammatory events in DCs that contributed to GVHD. An unbiased microarray-based gene expression approach allowed us to detect major changes in the expression of inflammation-related gene signatures including purinergic receptors, MAPK pathway–related genes, and inflammasome-associated genes. These gene expression changes had functional consequences because ATP-mediated DC migration, ERK activation, caspase-1 cleavage, and IL-1β production were all impaired in miR-155−/− DCs. This is consistent with the fact that miR-155 has multiple target genes and is therefore involved in multiple proinflammatory events.
We investigated miR-155−/− DCs in an allo-HCT setting because of the observation that miR-155 deficiency in the recipient reduces GVHD severity and the fact that DCs were shown to survive conditioning for several days and prime incoming donor T cells. Consistent with the crucial role of recipient DCs in GVHD induction, we observed increased disease severity with the adoptive transfer of WT DCs, an effect that was reversed when miR-155−/− DCs were used. This finding is comparable with previous reports indicating that miR-155 is also involved in innate immune responses with respect to cytokine production and costimulatory molecule expression by DCs.6,9,37 Intracellular IFN-γ levels in T cells were not altered after coculture with allogeneic miR-155−/− vs WT DCs and after allo-HCT of miR-155−/− vs WT recipient mice, whereas IFN-γ serum levels were reduced in miR-155−/− recipient mice compared with WT recipient mice. This can be explained by the fact that in addition to T cells, other sources of IFN-γ found in the serum may be relevant, such as plasmacytoid DCs,38 a cell type with known involvement in GVHD.39 The reduced IFN-γ levels in the serum could be caused by an indirect effect. Because GVHD in general is reduced in miR-155−/− recipients, there is less immune activation, which may lead to lower overall IFN-γ production. Furthermore, serum analysis in vivo was performed 7 days after allo-HCT, whereas intracellular staining for IFN-γ was performed after 24 hours or 3 days. In sum, the reduced in vivo IFN-γ levels are likely not specific to miR-155 deficiency, but are rather an indirect effect caused by lower GVHD severity.
We found several major defects in miR-155−/− DCs that could interfere with their ability to promote GVHD. To prime the allogeneic donor T cells, DCs need to migrate toward sites of tissue damage, and ATP released from dying cells was shown to be a major chemo-attractant for DCs and other innate immune cell types.40 MiR-155−/− DCs exhibited a reduced capacity to migrate up an ATP gradient, although no difference in costimulatory molecule expression was detected. We therefore focused on other pathways that promote inflammation in GVHD, such as MAPK signaling.31 This analysis identified large changes in the expression of MAPK family members in miR-155−/− DCs compared with WT DCs. Importantly, reduced ERK activation stood out from variegated alterations in MAPK gene expression, indicative of major defects, in miR-155−/− DCs compared with WT DCs when stimulated with LPS and ATP. This signaling defect links the MAPK pathway to our observations of both reduced DC migration, because ERK activation is a key event in DC motility,41 namely toward sites of inflammation32 ; and impaired inflammasome activation, as ERK phosphorylation leads to inflammasome priming.33 Decreased P2X7 expression in LPS/ATP-stimulated miR-155−/− DCs further suggested reduced inflammasome activation, because ATP stimulation of P2X7 triggers the inflammasome.34 The connection between miR-155 and P2Y12 and P2Y2, however, might be operational during the priming phase of GVHD in vivo. In addition, we consistently observed reduced inflammasome-related gene expression in miR-155−/− DCs compared with WT DCs. Because the inflammasome gene expression pattern was only suggestive and not definitive, we studied cleaved caspase-1 and IL-1β as functional indicators of Nlrp3 inflammasome activation. Levels of both proteins were reduced in miR-155−/− DCs compared with WT DCs, thereby clearly linking miR-155 deficiency to reduced inflammasome activation in cells stimulated with LPS and ATP. Cleaved caspase-1 levels were only significantly decreased 15 minutes after stimulation, which is consistent with the rapid activation of the P2X7/Nlrp3 axis by ATP as reported by others.42,43 A relationship has previously been drawn between downregulated miR-155 levels and increased pro–IL-1β in primary human monocyte–derived DCs.6 On a different level of regulation, however, our data support a role for miR-155 in licensing the inflammasome, as evidenced by our detection of lower concentrations of cleaved caspase-1 and secreted IL-1β in miR-155−/− DCs. The importance during early GVHD development of Nlrp3 inflammasome activation via the ATP/P2X7 axis and the response to bacterial components such as LPS has been reported.4,10 To test for the connection between miR-155 and inflammasome activation in vivo, we compared newly generated Nlrp3/miR-155 DKO mice with Nlrp3−/− mice and found no difference with respect to survival or GVHD histopathology scores. Furthermore, when Nlrp3 deficiency was accompanied by simultaneous antagomir-mediated blocking of miR-155 function, we did not observe increased protection from GVHD. Consistent with our data, elevated expression of miR-155 was shown to correlate with IL-1β production in human peripheral blood mononuclear cells.44 The severity of GVHD in Nlrp3−/− recipients is dependent on the donor T-cell dose, and the dosage we used induces lethal GVHD.4 Although a role for miR-155 in T cells had been described,7 we extend these findings by showing that miR-155 plays an important role in APCs as well during GVHD.
Similar to our findings in the mouse model, Xie et al demonstrated that miR-155 can serve as a biomarker for human GVHD,45 and an ongoing clinical trial is assessing the role of miR-155 in acute GVHD (ClinicalTrials.gov #NCT01521039).
In summary, this study is the first to demonstrate that miR-155 is a central miR-regulating inflammatory gene expression in DCs including purinergic receptors, MAPK family members, and inflammasome-associated genes upon activation with the archetypal GVHD danger signals. On a functional level, we connect the gene expression changes to a defect in DC migration toward ATP, impaired ERK activation, and reduced IL-1β production. By linking miR-155 to these different immune activation pathways in DCs, our study provides a rationale for a novel approach to interfering with GVHD by targeting miR-155 to simultaneously prevent multiple proinflammatory events.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to R. Thimme for helpful discussion. They thank C. Klein for providing CD45.1+ and CD45.2+ C57BL/6 mice.
This study was supported by the Deutsche Forschungsgemeinschaft, Germany (Heisenberg Professorship ZE 872/3-1 (R.Z.); DFG individual grant DFG ZE 872/1-2 (R.Z.); Z2 in SFB 850 (R.Z.), Else Kröner-Fresenius-Stiftung individual grants (2013_A04) (S.G., R.Z.), a MOTI-VATE stipend (Else Kröner-Fresenius-Stiftung) (S.C.), and a Fulbright Grant (US) (B.A.H.S.).
Authorship
Contribution: S.C. and B.A.H.S. helped design most of the experiments, performed experiments, analyzed data, and helped to write the manuscript; J.I. and A.P. performed experiments (allo-HCT, T-cell proliferation assay, flow cytometry); D.P. performed microarrays and analyzed the data; S.G. helped to identify target genes and to design experiments; A.S.-G. performed histopathology scoring; M.I. helped to design experiments and provided essential reagents; Y.B. and G.P. helped with the mouse experiments; J.F. and J.D. helped to design the studies and discussed the data; and R.Z. designed the studies, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Zeiser, Department of Hematology and Oncology, Freiburg University Medical Center, Albert-Ludwigs-University, Freiburg, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de.
References
Author notes
S.C. and B.A.H.S. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal