Key Points
Modulation of FX(a) zymogenicity yields proteins with a broad range of half-lives and biologic function yet potent in vivo efficacy.
Zymogen-like variants are attractive molecules for alleviating bleeding in different clinical scenarios such as hemophilia.
Abstract
There is a clinical need to develop safe therapeutic strategies to mitigate bleeding. Previously, we found that a novel zymogen-like factor Xa variant (FXa-I16L) was effective in correcting the coagulation defect in hemophilic mice. Here we expand the mutational framework to tune the FX(a) zymogen-like state. Alteration of FXa zymogenicity yields variants (V17M, I16L, I16M, V17T, V17S, and I16T) with a wide range (≤1000-fold) of reduced function toward physiologic substrates and inhibitors. The extent of zymogen-like character, including resistance to antithrombin III, correlates well with plasma half-life (<2 minutes to >4 hours). Importantly, biologic function, including that of the most zymogen-like variant (FXa-I16T), was greatly enhanced when bound to FVa membranes. This resulted in improvement of clotting times and thrombin generation in hemophilic plasma. The FXa variants were remarkably effective in mouse injury models. In these systems, the data show that the more active the protease, the more difficult it is to overcome the protective mechanism of circulating inhibitors to achieve a therapeutic benefit. Depending on the treatment situation, the more zymogen-like variants (V17S and I16T) were most useful when given before injury whereas variants exhibiting greater activity but shorter half-lives (I16L and I16M) were most effective when administered after injury. This new class of FXa variants provides a useful and flexible platform for selectively bioengineering biologic function and half-life to target different clinical bleeding scenarios.
Introduction
Plasma-derived or recombinant coagulation factors provide an important option for the prevention and treatment of bleeding episodes for patients with coagulation disorders, including hemophilia with or without inhibitors.1 Although current treatment is efficacious, there are limitations prompting the research community to develop new therapeutics, including protein, DNA/RNA, and gene-based approaches. Efforts centered on increasing protein activity or circulating half-life are most advanced and are in clinical development or have just been approved.2-4
Recently, we took a different approach and used information about the allosteric control of coagulation serine proteases to stabilize factor Xa (FXa) in a zymogen-like state by using mutagenesis.5-7 FX circulates as a zymogen and is converted to the protease state after proteolysis at a highly conserved site (R15-I16).8,9 Liberation of the new N-terminus (I16-VGG) drives a conformational change that leads to maturation of the catalytic domain which imparts function.10-12 We generated a novel FXa variant (ie, FXa-I16L) with zymogen-like properties that shows promise in bypassing deficiencies upstream of the common pathway such as hemophilia. FXa-I16L is rendered partially inactive because of a defect in transitioning from zymogen to the protease state. However, its biologic activity is fully rescued when it is bound to FVa.5 Preclinical studies in hemophilic mice indicate that FXa-I16L appears to be safe and is highly efficacious after a single dose in multiple injury models. Further, it is ∼20- to 50-fold more effective than recombinant FVIIa in these models.6,7
Although FXa-I16L corrects the coagulation defect in hemophilic mice, its half-life is relatively short (<15-30 minutes) which could limit its use as a prophylactic for the prevention of bleeds. Because of the general mechanism by which zymogens transition to the protease state, the full range of activity and/or half-life that could be achieved in this new class of FXa variants has yet to be fully explored. On the basis of our prior work, mutations at position 16 or 17 will have an impact on function; yet the extent of the alteration with a given amino acid is not obvious and needs to be empirically determined. This altered function is defined as the enzyme’s zymogenicity—the ratio of the activity of the mature protease to the activity of the zymogen. Like most serine proteases, FX/FXa has a large (>10 000-fold) zymogenicity, which suggests that it should be possible to generate variants with a wide range of activities that reflect, at least in part, a differential shift along the zymogen-to-protease transition pathway. Here, we altered the zymogenicity of FXa and created a repertoire of variants with attractive properties for treatment of bleeding conditions.
Methods
Reagents
Tissue culture reagents were from Invitrogen except for insulin-transferrin-sodium selenite, which was from Roche. The substrate methoxycarbonyl-cyclohexylglycyl-glycyl-arginine-p-nitroanilide (Spectrozyme Xa [Spec Xa]) was from America Diagnostica (Greenwich, CT). H-d-phenylalanyl-pipecolyl-arginine-p-nitroanilide (S-2238) was purchased from Diapharma Group, Inc. (West Chester, OH). Substrate solutions were prepared in water, and concentrations were verified by using E342 = 8270 M−1 cm−1.13 Small, unilamellar phospholipid vesicles (phosphatidylcholine-phosphatidylserine) composed of 75% (w/w) hen egg L-α-phosphatidylcholine and 25% (w/w) porcine brain L-α-phosphatidylserine (Avanti Polar Lipids, Alabaster, AL) were prepared as described.14 Technothrombin thrombin calibrator and thrombin generation assay (TGA) reagent RB were purchased from Diapharma Group Inc. The fluorogenic substrate Z-Gly-Gly-Arg-AMC was purchased from Bachem Bioscience Inc. and prepared in 15 mM CaCl2, and its concentration was verified using E326 = 17 200 M−1 cm−1. Normal human [pooled] plasma and factor-deficient plasmas were purchased from George King Biomedical, Inc. Automated activated partial thromboplastin time (aPTT) reagent (TriniClot) was from Trinity Biotech.
Proteins
Recombinant wild-type (wt) –FXa and FVa were purified as described previously.15,16 Human prothrombin was isolated from plasma as described previously.17 The FX activator from Russell’s viper venom (RVVX-CP) was purified as described before.18 Molecular weights and extinction coefficients (E 0.1% 280 nm) of various proteins used were taken as previously reported.6,16 All functional assays were performed at 25°C in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.15 M NaCl, 0.1% (w/v) polyethylene glycol 8000, and 2 mM CaCl2 at pH 7.4 (assay buffer).
Preparation and functional characterization of FXa enzyme activity
A description of the preparation of recombinant FX/FXa (rFX/FXa) and characterization of enzyme activity, including chromogenic substrate hydrolysis, inhibition by Pefabloc tPa/Xa or antithrombin III (ATIII), and prothrombin activation kinetics can be found in supplemental Methods available at the Blood Web site.
Plasma-based coagulation assays
Thrombin generation assays in human or mouse plasma were determined as described.7,19 The aPTT clotting assay was performed by a modified one-stage assay as described.5 Briefly, 50 µL of FXa variants (final concentration 0.1 nM) in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.15 M NaCl, 0.1% polyethylene glycol 8000 (pH 7.5; dilution buffer) was incubated with 50 µL human FIX-deficient plasma and 50 µL aPTT reagent. A volume of 50 µL of 25 mM CaCl2 was added, and the time to clot formation was measured by using a Stat4 Coagulation Instrument (Diagnostica Stago, Parsippany, NJ). For ex vivo half-life determinations, 20 nM wt-FXa or FXa variants or 60 nM FXa-I16T were incubated in human hemophilia A (HA) or hemophilia B (HB) plasma or murine HB plasma (diluted fivefold) at room temperature, and at various time points, aliquots were further diluted (0.1-3 nM FXa, final) in dilution buffer. Residual FXa activity was assessed by an aPTT-clotting assay as previously described.6,7
Animals
Hemophilia B mice on the Balb/c strain have been previously described and backcrossed for more than 5 generations.20,21 Wild-type mice on the Balb/c strain used as controls were purchased from The Jackson Laboratory (Bar Harbor, ME). For all experiments, mice were 8 to 12 weeks old and weighed 25 to 30 g. Approval for the experiment was obtained from the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Coagulation assays
Blood from mice was collected in 3.8% sodium citrate by sectioning the distal part of the tail and was processed immediately. Plasma was collected from whole blood after centrifugation, and samples were evaluated fresh or snap frozen and stored at −80°C. Thrombin-ATIII complexes (TAT) were measured by Enzygnost TAT (Dade Behring, Marburg, Germany) and D-dimer was measured by using a sandwich-type enzyme-linked immunosorbent assay kit (Asserachrom D-Di; Diagnostica Stago).
Tail clip assay
Two different versions of the tail clip assay were used as previously described.7 In the first version, protein or phosphate-buffered saline (PBS) was injected via the tail vein approximately 5, 15, or 30 minutes before injury. In the second method, protein or PBS was infused 2 minutes after injury via a preinserted jugular vein cannulus. In both cases, the tail was transected at a diameter of 3 mm, and blood was collected for 10 minutes. Quantitative assessment of blood loss was determined by measuring total hemoglobin by absorbance at 575 nm after red cell lysis and was converted to total blood loss (μL) by using a standard curve.
Ferric chloride carotid artery model
Ferric chloride-induced injury was performed according to published procedures.7,22 Briefly, blood flow measurements were performed with a Doppler flow probe (Model 0.5VB; Transonic Systems, Ithaca, NY) positioned around the carotid artery. Mice received protein via tail vein injection 15 minutes before or via a jugular vein cannula 10 minutes after injury. Injury was made with a 2-mm2 piece of Whatman #1 filter paper soaked in 7.5% FeCl3 (0.46 M) to the adventitial surface of the artery for 2 minutes, and blood flow was monitored for 30 minutes. In these experiments, the time to occlusion was defined as the time from infusion of clotting factors until the blood flow decreased by 90%.
Data analysis
Data were analyzed according to nonlinear least squares regression analysis. Estimates of error represent ± 2 standard deviations. The qualities of fits were assessed by criteria previously described.23 Initial velocity measurements were analyzed by fitting data to the Henri-Michaelis-Menten equation to yield values for the Michaelis constant (Km) and maximum rate (Vmax).24 The rate of inhibition of FXa by ATIII was measured under pseudo first-order rate conditions, and the second-order rate constant was calculated by dividing the pseudo first-order rate constant (kobs) by the concentration of ATIII.25 In vivo results are expressed as mean ± standard error of the mean and were analyzed by using analysis of variance followed by a Student-Newman-Keuls test. A P value < .05 was considered statistically significant.
Results
Expansion of zymogen-like FXa mutational framework
Previously we characterized a zymogen-like FXa variant (FXa-I16L), which proved safe and efficacious in bypassing the hemophilic phenotype.5-7 New variants at position 16 or 17 (Table 1) were transiently transfected in HEK293 cells. We selected amino acids that were generally hydrophobic and slightly smaller or larger than Ile or Val. We also avoided charged amino acids because the new N-terminus inserts into a hydrophobic pocket following activation to FXa. After correction for antigen levels, activation with RVV-Xcp yielded proteins that fell into 3 groups relative to wt-FXa. Select variants were chosen for characterization: Group 1 (FXa-V17M; ∼25% activity), Group 2 (FXa-I16M, FXa-V17T; ∼5% activity), and Group 3 (FXa-V17S, FXa-I16T; <1% to 2% activity). Sodium dodecyl sulfate polyacrylamide gel electrophoresis showed that the purified proteases exhibit the characteristic α and β forms of FXa, and γ-carboxyglutamic acid analysis yielded the expected results (data not shown).
Active site function of FXa variants
| Construct . | % wt-antigen . | % Specific activity . |
|---|---|---|
| Group 1 | ||
| Val17→Met (V17M) | 57 | 25 |
| Val17→Leu (V17L) | 52 | 21 |
| Ile16→Leu (I16L) | 60 | 7 |
| Ile16→Met (I16M) | 54 | 6 |
| Group 2 | ||
| Val17→Thr (V17T) | 100 | 5 |
| Val17→Ala (V17A) | 100 | 3 |
| Val17→Ser (V17S) | 60 | 2 |
| Ile16→Thr (I16T) | 57 | 1 |
| Group 3 | ||
| Ile16→Gly (I16G) | 52 | <1 |
| Ile16→Phe (I16F) | 90 | <1 |
| Val17→Gly (V17G) | 88 | <1 |
| Construct . | % wt-antigen . | % Specific activity . |
|---|---|---|
| Group 1 | ||
| Val17→Met (V17M) | 57 | 25 |
| Val17→Leu (V17L) | 52 | 21 |
| Ile16→Leu (I16L) | 60 | 7 |
| Ile16→Met (I16M) | 54 | 6 |
| Group 2 | ||
| Val17→Thr (V17T) | 100 | 5 |
| Val17→Ala (V17A) | 100 | 3 |
| Val17→Ser (V17S) | 60 | 2 |
| Ile16→Thr (I16T) | 57 | 1 |
| Group 3 | ||
| Ile16→Gly (I16G) | 52 | <1 |
| Ile16→Phe (I16F) | 90 | <1 |
| Val17→Gly (V17G) | 88 | <1 |
Antigen levels are calculated from FX-specific enzyme-linked immunosorbent assay; FXa activity levels are based on the rate of peptidyl substrate hydrolysis (100 µM Spec Xa) after activation by RVVX-CP; % specific activity values are based on comparison with wt-FXa antigen and/or activity levels.
Characterization of enzyme function
Maturation of the active site and stabilization of the oxyanion hole are hallmarks of the zymogen-to-protease transition.9 Kinetic studies with the chromogenic substrate Spec Xa (Table 2) or the active site FXa inhibitor Pefabloc tPA/Xa (Table 2) revealed that the variants have an impaired ability to bind these probes relative to wt-FXa. The catalytic efficiency (kcat) for Spec Xa hydrolysis was also reduced to various degrees. These data show that large differences in activity and substrate binding can be achieved depending on which amino acid is at position 16 or 17.
Assessment of active site binding for FXa and prothrombinase
| Probe . | Spec Xa* . | ATIII† . | Pefabloc tPA/Xa† . | |
|---|---|---|---|---|
| Km µM . | kcat s−1 . | k2 ± SD × 103 M–1 s−1 . | KiµM . | |
| Factor Xa | ||||
| wt-FXa | 86 ± 7 | 75 ± 1.5 | 1.67 ± 0.07 | 0.07 ± 0.004 |
| Group 1 | ||||
| FXa-V17M | 600 ± 60 | 62 ± 3.2 | 0.1 ± 0.008 | 1.0 ± 0.09 |
| Group 2 | ||||
| FXa-I16L | 1200 ± 270 | 38 ± 8.0 | 0.03 ± 0.002 | 1.4 ± 0.07 |
| FXa-V17T | >1 mM | >35 | 0.02 ± 0.001 | ND |
| FXa-I16M | >1 mM | >30 | 0.02 ± 0.0008 | 30 ± 1.8 |
| Group 3 | ||||
| FXa-V17S | >1 mM | >15 | 0.01 ± 0.0007 | 100 ± 16 |
| FXa-I16T | >1 mM | >1 | ND | 600 ± 70 |
| Prothrombinase | ||||
| wt-FXa | 160 ± 20 | 115 ± 4.0 | — | 0.3 ± 0.02 |
| Group 1 | ||||
| FXa-V17M | 260 ± 50 | 64 ± 4.9 | — | 0.15 ± 0.006 |
| Group 2 | ||||
| FXa-I16L | 370 ± 30 | 47 ± 1.9 | — | 0.2 ± 0.02 |
| FXa-V17T | 540 ± 60 | 60 ± 3.8 | — | ND |
| FXa-I16M | 500 ± 80 | 55 ± 5.1 | — | 1.15 ± 0.08 |
| Group 3 | ||||
| FXa-V17S | 1100 ± 130 | 41 ± 8.5 | — | 40 ± 4 |
| FXa-I16T | 500 ± 63 | 5.3 ± 0.38 | — | 70 ± 9 |
| Probe . | Spec Xa* . | ATIII† . | Pefabloc tPA/Xa† . | |
|---|---|---|---|---|
| Km µM . | kcat s−1 . | k2 ± SD × 103 M–1 s−1 . | KiµM . | |
| Factor Xa | ||||
| wt-FXa | 86 ± 7 | 75 ± 1.5 | 1.67 ± 0.07 | 0.07 ± 0.004 |
| Group 1 | ||||
| FXa-V17M | 600 ± 60 | 62 ± 3.2 | 0.1 ± 0.008 | 1.0 ± 0.09 |
| Group 2 | ||||
| FXa-I16L | 1200 ± 270 | 38 ± 8.0 | 0.03 ± 0.002 | 1.4 ± 0.07 |
| FXa-V17T | >1 mM | >35 | 0.02 ± 0.001 | ND |
| FXa-I16M | >1 mM | >30 | 0.02 ± 0.0008 | 30 ± 1.8 |
| Group 3 | ||||
| FXa-V17S | >1 mM | >15 | 0.01 ± 0.0007 | 100 ± 16 |
| FXa-I16T | >1 mM | >1 | ND | 600 ± 70 |
| Prothrombinase | ||||
| wt-FXa | 160 ± 20 | 115 ± 4.0 | — | 0.3 ± 0.02 |
| Group 1 | ||||
| FXa-V17M | 260 ± 50 | 64 ± 4.9 | — | 0.15 ± 0.006 |
| Group 2 | ||||
| FXa-I16L | 370 ± 30 | 47 ± 1.9 | — | 0.2 ± 0.02 |
| FXa-V17T | 540 ± 60 | 60 ± 3.8 | — | ND |
| FXa-I16M | 500 ± 80 | 55 ± 5.1 | — | 1.15 ± 0.08 |
| Group 3 | ||||
| FXa-V17S | 1100 ± 130 | 41 ± 8.5 | — | 40 ± 4 |
| FXa-I16T | 500 ± 63 | 5.3 ± 0.38 | — | 70 ± 9 |
The errors in the fitted constants represent ± 2 standard deviations (SDs) for each variant. The data are representative of 2 to 3 independent measurements. Description of the experimental design and reactant concentrations can be found in supplemental Methods.
ND, not determined.
Kinetic parameters for free Xa or prothrombinase were determined from initial velocity studies conducted with Spec Xa or S-2238.
Inhibition kinetics of free Xa were determined from initial velocity studies with Spec Xa or S-2765.
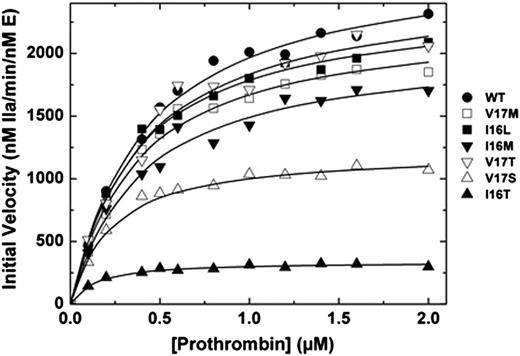
We and others have shown that the FVa binding site, active site, and new N-terminus of FXa are allosterically linked.5,15,26-28 Correspondingly, saturating concentrations of membrane-bound FVa can thermodynamically rescue the activity of zymogen-like variants.5,6 Binding of the different FXa variants to Spec Xa in the presence of saturating amounts of FVa membranes was improved (Table 2). More compelling evidence comes from prothrombin activation experiments. When assembled into prothrombinase, Group 1 and 2 variants exhibit kinetic parameters that were similar to those of wt-FXa (Figure 1). Remarkably, even Group 3 variants (FXa-V17S and FXa-I16T) have second-order rate constants for prothrombin activation that were within a factor of 5 despite exhibiting activity in the absence of FVa that was altered 100 to 1000-fold relative to wt-FXa (Figure 1). Together these data show the remarkable and surprising ability of prothrombinase assembly to rescue FXa zymogen-like function.
Activation of prothrombin. Reaction mixtures containing increasing concentrations of prothrombin (0.1-2 µM) with 20 µM phosphatidylcholine-phosphatidylserine were incubated at 25°C in the presence of 20 nM FVa. The reaction was initiated with 0.1 or 0.2 nM wt-FXa (●), FXa-V17M (□), FXa-I16L (▪), FXa-I16M (▼), FXa-V17T (▿), FXa-V17S (△) and FXa-I16T (▲). Aliquots of the reaction mixture were quenched during initial rate of the reaction (0, 0.5, 1, 1.5, and 2 minutes), and thrombin generation was determined by using the chromogenic substrate S-2238. The solid lines were drawn following analysis of all data sets to a rectangular hyperbola with the following fitted parameters: wt-FXa: Km = 0.4 ± 0.04 µM, kcat = 2700 ± 75 minutes−1; FXa-V17M: Km = 0.36 ± 0.04 µM, kcat = 2300 ± 70 minutes−1; FXa-I16M and FXa-V17T: Km = 0.38 ± 0.05 µM, kcat = 2000 ± 80 and 2500 ± 120 minutes−1; FXa-V17S: Km = 0.2 ± 0.02 µM, kcat = 1200 ± 30 minutes−1; and FXa-I16T: Km = 0.12 ± 0.02 µM, kcat = 340 ± 7 minutes−1. The data are representative of 2 to 3 similar experiments.
Activation of prothrombin. Reaction mixtures containing increasing concentrations of prothrombin (0.1-2 µM) with 20 µM phosphatidylcholine-phosphatidylserine were incubated at 25°C in the presence of 20 nM FVa. The reaction was initiated with 0.1 or 0.2 nM wt-FXa (●), FXa-V17M (□), FXa-I16L (▪), FXa-I16M (▼), FXa-V17T (▿), FXa-V17S (△) and FXa-I16T (▲). Aliquots of the reaction mixture were quenched during initial rate of the reaction (0, 0.5, 1, 1.5, and 2 minutes), and thrombin generation was determined by using the chromogenic substrate S-2238. The solid lines were drawn following analysis of all data sets to a rectangular hyperbola with the following fitted parameters: wt-FXa: Km = 0.4 ± 0.04 µM, kcat = 2700 ± 75 minutes−1; FXa-V17M: Km = 0.36 ± 0.04 µM, kcat = 2300 ± 70 minutes−1; FXa-I16M and FXa-V17T: Km = 0.38 ± 0.05 µM, kcat = 2000 ± 80 and 2500 ± 120 minutes−1; FXa-V17S: Km = 0.2 ± 0.02 µM, kcat = 1200 ± 30 minutes−1; and FXa-I16T: Km = 0.12 ± 0.02 µM, kcat = 340 ± 7 minutes−1. The data are representative of 2 to 3 similar experiments.
Assessment of half-life and ATIII inhibition
Consistent with their altered zymogenicity, Group 3 variants exhibit the longest half-life (>350 minutes) in HB plasma, followed by Group 2 (>80 minutes) and Group 1 variants (∼10 minutes; Table 3 and Figure 2A). Similar results were obtained using by human HA plasma (data not shown) and mouse HB plasma (Figure 2B). ATIII, a serpin, which largely targets the active site of serine proteases, is considered one of the major physiologic inhibitors of FXa.29 Consistent with extended half-lives, the variants have altered second-order rate constants for inhibition by ATIII compared with wt-FXa (Table 2). These data show that the zymogen-like conformation protects the FXa variants from plasma inhibitors to various degrees that correspond to their level of zymogenicity. This has a direct impact on their half-lives because the physiologic inhibitors largely dictate this parameter.
Coagulation and thrombin generation assay parameters
| Group . | Sample . | aPTT (sec) . | aPTT + FVa (sec) . | ETP (nM·min) . | Peak height (nM) . | Time to peak (min) . | ETP + FVa (nM·min) . | Peak height + FVa (nM) . | Time to peak + FVa (min) . | t1/2 (min) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | NHP | 38 ± 0.4 | ND | 5700 | 370 | 29 | — | — | — | — |
| Control | HB | 110 ± 4 | ND | NA | NA | NA | NA | NA | NA | — |
| Control | HB + FIX | 40 ± 0.4 | ND | 4800 | 370 | 14 | — | — | — | — |
| Wild-type | HB + wt-FXa | 32 ± 1 | 25 ± 0.3 | 2700 | 120 | 17 | 3600 | 170 | 17 | 1.15 ± 0.05 |
| Group 1 | HB +FXa-V17M | 41 ± 0.4 | 31 ± 0.9 | 4500 | 270 | 14 | 4500 | 230 | 17 | 11 ± 0.4 |
| Group 2 | HB + FXa-I16L | 54 ± 2 | 33 ± 2 | 3900 | 200 | 17 | 4300 | 200 | 18 | 41 ± 3.5 |
| HB + FXa-V17T | 47 ± 2 | 29 ± 1 | 4000 | 200 | 15 | — | — | — | 79 ± 5.5 | |
| HB + FXa-I16M | 60 ± 4 | 39 ± 2 | 3000 | 160 | 18 | 3900 | 190 | 18 | 115 ± 10 | |
| Group 3 | HB + FXa-V17S | 76 ± 4 | 45 ± 2 | 3800 | 170 | 20 | 3600 | 170 | 17 | 307 ± 12.5 |
| HB + FXa-I16T | 94 ± 1 | 73 ± 2 | 300 | 25 | 24 | 2000 | 100 | 16 | 447 ± 29 |
| Group . | Sample . | aPTT (sec) . | aPTT + FVa (sec) . | ETP (nM·min) . | Peak height (nM) . | Time to peak (min) . | ETP + FVa (nM·min) . | Peak height + FVa (nM) . | Time to peak + FVa (min) . | t1/2 (min) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | NHP | 38 ± 0.4 | ND | 5700 | 370 | 29 | — | — | — | — |
| Control | HB | 110 ± 4 | ND | NA | NA | NA | NA | NA | NA | — |
| Control | HB + FIX | 40 ± 0.4 | ND | 4800 | 370 | 14 | — | — | — | — |
| Wild-type | HB + wt-FXa | 32 ± 1 | 25 ± 0.3 | 2700 | 120 | 17 | 3600 | 170 | 17 | 1.15 ± 0.05 |
| Group 1 | HB +FXa-V17M | 41 ± 0.4 | 31 ± 0.9 | 4500 | 270 | 14 | 4500 | 230 | 17 | 11 ± 0.4 |
| Group 2 | HB + FXa-I16L | 54 ± 2 | 33 ± 2 | 3900 | 200 | 17 | 4300 | 200 | 18 | 41 ± 3.5 |
| HB + FXa-V17T | 47 ± 2 | 29 ± 1 | 4000 | 200 | 15 | — | — | — | 79 ± 5.5 | |
| HB + FXa-I16M | 60 ± 4 | 39 ± 2 | 3000 | 160 | 18 | 3900 | 190 | 18 | 115 ± 10 | |
| Group 3 | HB + FXa-V17S | 76 ± 4 | 45 ± 2 | 3800 | 170 | 20 | 3600 | 170 | 17 | 307 ± 12.5 |
| HB + FXa-I16T | 94 ± 1 | 73 ± 2 | 300 | 25 | 24 | 2000 | 100 | 16 | 447 ± 29 |
Concentrations: FXa, 0.1 nM; FVa, 10-20 nM when added; FIX, 5 µg/mL.
HB, hemophilia B; NA, not applicable; ND, not determined; NHP, normal human plasma.
In vitro half-life studies in HB plasma. Factor Xa (20 nM wt-FXa [●]; zymogen-like 1: FXa-V17M [□]; zymogen-like 2: FXa-I16L [▪], FXa-I16M [▼], FXa-V17T [▿]; zymogen-like 3: FXa-V17S [△] and 60 nM FXa-I16T [▲]) were incubated in (A) human or (B) mouse HB plasma at the indicated time points, and residual activity was assessed by clotting assay. The solid lines were drawn after analysis of data sets to an exponential decay function with the following fitted parameters for half-life. The fitted parameters for the (A) human proteins can be found in Table 2 and those for (B) mouse proteins are as follows: FXa-I16T ∼60 minutes, FXa-V17S ∼17 minutes, FXa-I16M ∼9 minutes, FXa-I16L ∼5 minutes7 , FXa-V17M ∼1.2 minutes, and wt-FXa 0.15 minutes. The data are representative of 3 similar experiments.
In vitro half-life studies in HB plasma. Factor Xa (20 nM wt-FXa [●]; zymogen-like 1: FXa-V17M [□]; zymogen-like 2: FXa-I16L [▪], FXa-I16M [▼], FXa-V17T [▿]; zymogen-like 3: FXa-V17S [△] and 60 nM FXa-I16T [▲]) were incubated in (A) human or (B) mouse HB plasma at the indicated time points, and residual activity was assessed by clotting assay. The solid lines were drawn after analysis of data sets to an exponential decay function with the following fitted parameters for half-life. The fitted parameters for the (A) human proteins can be found in Table 2 and those for (B) mouse proteins are as follows: FXa-I16T ∼60 minutes, FXa-V17S ∼17 minutes, FXa-I16M ∼9 minutes, FXa-I16L ∼5 minutes7 , FXa-V17M ∼1.2 minutes, and wt-FXa 0.15 minutes. The data are representative of 3 similar experiments.
Assessment of zymogen-like variants in hemophilic plasma
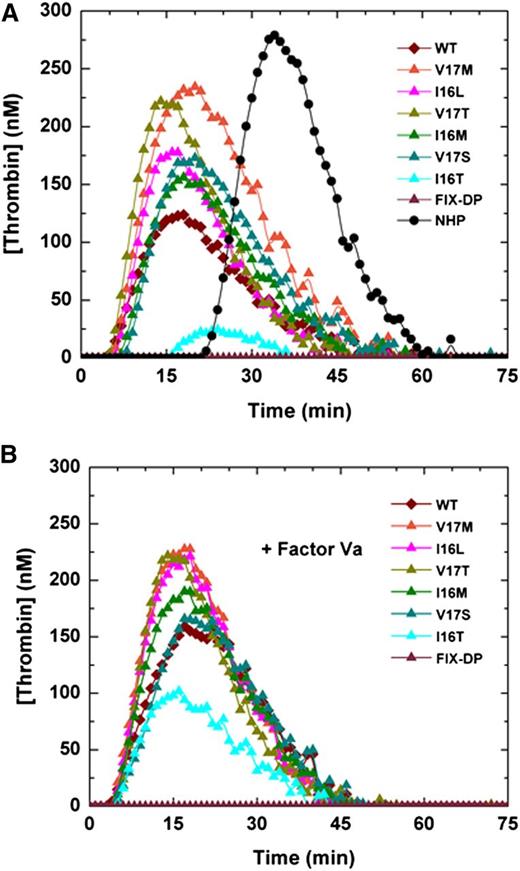
Plasma-based assays were used to determine whether the unique properties of the variants translate into a bypassing approach to mitigate the hemophilic phenotype. By using a modified aPTT clotting assay with human HB plasma, the Group 1 variant (0.1 nM; FXa-V17M) normalized the prolonged clotting time whereas Group 2 variants (0.1 nM; FXa-I16M, FXa-V17T, and FXa-I16L) substantially shortened the clotting time (Table 3). The most zymogenized variants (0.1 nM; FXa-V17S, FXa-I16T) had a modest but significant effect (Table 3). Consistent with the idea that suboptimal amounts of FVa are generated in situ during the clotting assay, saturating amounts of FVa (10 nM) added to the assay further shortened the clotting times (Table 3).
Similarly, FXa variants added to the TGA in HB plasma yielded a robust thrombin signal with endogenous thrombin potential (ETP) and peak heights comparable to wt-FXa (Table 3 and Figure 3A). Only FXa-I16T exhibited a markedly reduced thrombin generation profile. We speculate that this reflects its significant zymogen-like character and the need for higher concentrations of FVa to rescue the variant. Consistent with this, the addition of FVa (20 nM) yielded approximately five- to eightfold improvement in peak thrombin and ETP for FXa-I16T (Figure 3B and Table 3). Because other zymogenic variants had robust TGA profiles, the addition of FVa had only a modest effect (Figure 3B and Table 3). Similar results were obtained by using human HA plasma (data not shown).
Thrombin generation assay. Thrombin generation was measured in (A) human HB plasma supplemented with 0.1 nM of FXa or (B) with FXa supplemented with 20 nM FVa in the presence of 2.0 pM TF/4 µM phospholipid (reagent RB; Technoclone). Thrombin generation was initiated with CaCl2, and a thrombin fluorogenic substrate as detailed in “Methods.” Each curve is representative of 3 independent experiments. NHP, normal human plasma.
Thrombin generation assay. Thrombin generation was measured in (A) human HB plasma supplemented with 0.1 nM of FXa or (B) with FXa supplemented with 20 nM FVa in the presence of 2.0 pM TF/4 µM phospholipid (reagent RB; Technoclone). Thrombin generation was initiated with CaCl2, and a thrombin fluorogenic substrate as detailed in “Methods.” Each curve is representative of 3 independent experiments. NHP, normal human plasma.
In vivo evaluation of zymogen-like FXa variants
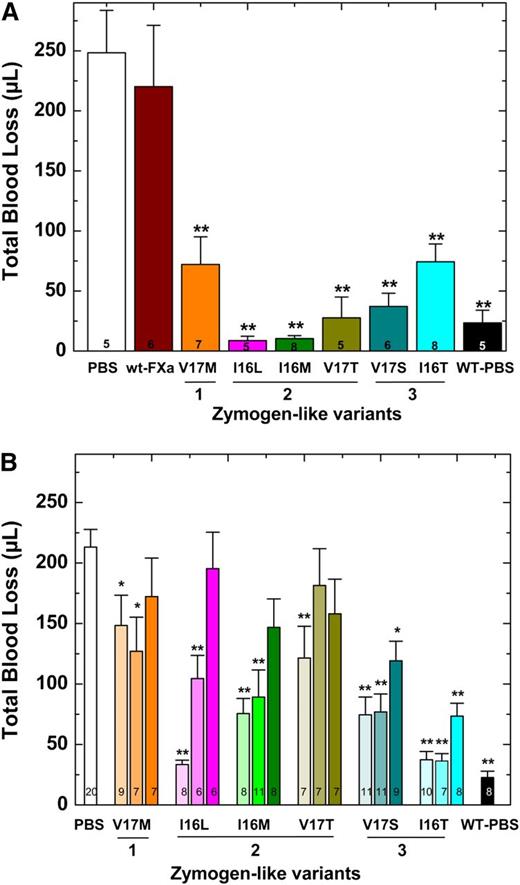
Blood loss was monitored after a hemostatic challenge by using HB mice on the Balb/c background. These experiments were conducted in 2 ways; protein was infused 2 minutes after injury (Figure 4A) or protein was infused before injury (Figure 4B). In either scenario, HB mice infused with PBS exhibit substantial blood loss (>200 μL) compared with hemostatically normal mice (Figure 4). Infusion of Group 2 variants after injury significantly reduced blood loss (Figure 4A). Interestingly and perhaps counterintuitively, the most active zymogen-like variant (FXa-V17M) was partially effective, and wt-FXa was ineffective. These proteins are sensitive to circulating inhibitors and correspondingly have short half-lives that limit their efficacy. We concluded that the Group 2 variants, including FXa-I16L, are the most potent prohemostatic agents for acute bleeding situations because they have a balance of activity and half-life most conducive to rapidly stopping bleeding.
Blood loss following tail clip in HB mice. (A) Tail clip assay was performed on HB mice (n = 5-8) and blood was collected for 2 minutes. FXa (450 μg/kg) or PBS was then administered via a pre-inserted jugular vein cannulus. Blood was collected for additional 10 minutes in a different tube of saline, and subsequent total blood loss (μL) was measured. (B) Five (light shaded column), 15 (medium shaded column), or 30 (dark shaded column) minutes before injury, FXa (450 μg/kg) or PBS was administered to HB mice (n = 6-20) via tail vein and total blood loss (μL) was measured after tail transection. In both panels, HB mice (PBS) or wt-mice infused with PBS (WT-PBS) served as controls. The number of animals per group is indicated in each column, and all measurements are presented as mean ± standard error of the mean (SEM). **P < .001 and *P < .05 represent WT or HB FXa-treated animals vs HB-PBS control.
Blood loss following tail clip in HB mice. (A) Tail clip assay was performed on HB mice (n = 5-8) and blood was collected for 2 minutes. FXa (450 μg/kg) or PBS was then administered via a pre-inserted jugular vein cannulus. Blood was collected for additional 10 minutes in a different tube of saline, and subsequent total blood loss (μL) was measured. (B) Five (light shaded column), 15 (medium shaded column), or 30 (dark shaded column) minutes before injury, FXa (450 μg/kg) or PBS was administered to HB mice (n = 6-20) via tail vein and total blood loss (μL) was measured after tail transection. In both panels, HB mice (PBS) or wt-mice infused with PBS (WT-PBS) served as controls. The number of animals per group is indicated in each column, and all measurements are presented as mean ± standard error of the mean (SEM). **P < .001 and *P < .05 represent WT or HB FXa-treated animals vs HB-PBS control.
The results were different when proteins were administered before injury. In a timed tail transection experiment, Group 3 variants were most effective when the proteins were given 15 or even 30 minutes before injury (Figure 4B). As expected, the Group 1 variant FXa-V17M was essentially ineffective. The Group 2 variants were effective if administered 5 minutes before injury, but they exhibited decreasing efficacy if given 15 or 30 minutes before injury. Together these data suggest that when FXa variants are administered before injury, proteins with a long half-life and acceptable activity profile are the most effective. Overall, the data show that with the administration of a protease, the activity half-life and thus sensitivity toward circulating inhibitors contributes in a major way to efficacy and provides a short-term prohemostatic effect.
In addition to the tail transection model, injury to the carotid artery by using FeCl3 is useful for assessing the efficacy of therapeutic procoagulants. As shown previously,7,22 HB mice do not generally form an occlusive thrombus in this model. Following application of FeCl3 (7.5%) to the carotid artery, hemostatically normal mice displayed full vessel occlusion within 15 minutes (Table 4). Administration of zymogen-like variants (450 μg/kg) 10 minutes after injury led to rapid and complete occlusion (<3 minutes) in nearly all mice (Table 4). Because of its short half-life in mouse plasma (<20 seconds; Figure 2B), wt-FXa was ineffective. When proteins were administrated 15 minutes before injury, FXa-V17M and FXa-I16M variants had poor efficacy but FXa-I16T was highly effective and yielded complete vessel occlusion in 8 of 9 mice (Table 4).
Time to carotid artery occlusion with FeCl3-induced injury
| Group . | Genotype* . | Sample . | Dose (µg/kg) . | No. of mice . | Time to occlusion (min) . | |||
|---|---|---|---|---|---|---|---|---|
| Total . | No occlusion . | Transient occlusion . | Complete occlusion . | |||||
| Control | WT | PBS | – | 7 | 0 | 0 | 7 | 15 ± 0.5 |
| Control | HB | PBS | – | 7 | 7 | 0 | 0 | >30 |
| Experimental, 10 minutes after injury | ||||||||
| Wild-type | HB | wt-FXa | 450 | 5 | 5 | 0 | 0 | >30 |
| Group 1 | HB | FXa-V17M | 450 | 7 | 0 | 1 | 6 | 3.2 ± 0.3 |
| Group 2 | HB | FXa-I16M | 450 | 7 | 0 | 0 | 7 | 2.0 ± 0.1 |
| Group 3 | HB | FXa-I16T | 450 | 9 | 0 | 0 | 9 | 3.3 ± 0.3 |
| Experimental, 15 minutes before injury | ||||||||
| Group 1 | HB | FXa-V17M | 450 | 7 | 7 | 0 | 0 | >30 |
| Group 2 | HB | FXa-I16M | 450 | 7 | 6 | 0 | 1 | 7.1 |
| Group 3 | HB | FXa-I16T | 450 | 9 | 1 | 0 | 8 | 7.6 ± 1.2 |
| HB | FXa-I16T | 225 | 7 | 2 | 0 | 5 | 12 ± 0.7 | |
| HB | FXa-I16T | 113 | 7 | 2 | 0 | 5 | 13 ± 1 | |
| HB | FXa-I16T | 56 | 5 | 4 | 0 | 1 | 12 | |
| Group . | Genotype* . | Sample . | Dose (µg/kg) . | No. of mice . | Time to occlusion (min) . | |||
|---|---|---|---|---|---|---|---|---|
| Total . | No occlusion . | Transient occlusion . | Complete occlusion . | |||||
| Control | WT | PBS | – | 7 | 0 | 0 | 7 | 15 ± 0.5 |
| Control | HB | PBS | – | 7 | 7 | 0 | 0 | >30 |
| Experimental, 10 minutes after injury | ||||||||
| Wild-type | HB | wt-FXa | 450 | 5 | 5 | 0 | 0 | >30 |
| Group 1 | HB | FXa-V17M | 450 | 7 | 0 | 1 | 6 | 3.2 ± 0.3 |
| Group 2 | HB | FXa-I16M | 450 | 7 | 0 | 0 | 7 | 2.0 ± 0.1 |
| Group 3 | HB | FXa-I16T | 450 | 9 | 0 | 0 | 9 | 3.3 ± 0.3 |
| Experimental, 15 minutes before injury | ||||||||
| Group 1 | HB | FXa-V17M | 450 | 7 | 7 | 0 | 0 | >30 |
| Group 2 | HB | FXa-I16M | 450 | 7 | 6 | 0 | 1 | 7.1 |
| Group 3 | HB | FXa-I16T | 450 | 9 | 1 | 0 | 8 | 7.6 ± 1.2 |
| HB | FXa-I16T | 225 | 7 | 2 | 0 | 5 | 12 ± 0.7 | |
| HB | FXa-I16T | 113 | 7 | 2 | 0 | 5 | 13 ± 1 | |
| HB | FXa-I16T | 56 | 5 | 4 | 0 | 1 | 12 | |
In HB mice, protein was infused 10 minutes after or 15 minutes before 7.5% FeCl3 exposure. Data for mice presenting with no occlusion, transient occlusion, or complete occlusion of the carotid artery following FeCl3 exposure are provided. Occlusion time measurements are presented as mean ± SEM and are derived from both transient and complete events.
HB, hemophilia B mice; WT, hemostatically normal mice.
Expanded characterization of FXa-I16T
On the basis of the low activity of FXa-I16T, we were surprised that FVa could nearly rescue its function with respect to prothrombin activation. Additionally, the variant is highly efficacious in both injury models especially when given before injury. In plasma, FXa-I16T has a half-life of more than 6 to 7 hours. (Table 3). These data suggest that FXa-I16T may be ideally suited for prophylaxis situations or to prevent bleeding.
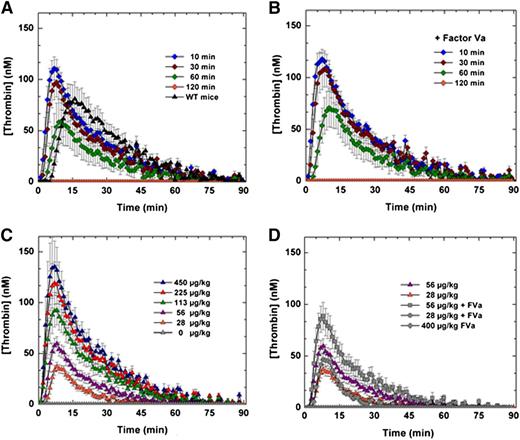
The efficacy and safety of FXa-I16T was examined in additional experiments. HB mice were treated with the variant (450 μg/kg), and blood was collected at various times (10, 30, 60, and 120 minutes) after protein injection. As shown in Figure 5A and supplemental Figure 1, intravenous administration of FXa-I16T yielded a robust thrombin generation profile that persisted for up to 1 hour. Similar results were obtained in peak thrombin and ETP when 20 nM FVa was exogenously added to mouse plasma before initiation of the TGA (Figure 5B). Infusion of FXa-I16T (28-450 µg/kg) in HB mice dose-dependently enhanced thrombin generation with normal peak thrombin levels achieved between 56 and 113 µg/kg (Figure 5C). Co-infusion of FVa (200 µg/kg) with FXa-I16T (28 or 56 µg/kg) enhanced thrombin generation twofold (Figure 5D). Together, these data show that despite the poor activity of FXa-I16T, it is effective at restoring thrombin generation and can do so even when given up to 1 hour before blood collection.
Ex vivo assessment of FXa-I16T-Thrombin generation assay. (A-B) HB mice (n = 5-7 per group) were administered FXa-I16T (450 μg/kg) and at various time points (10, 30, 60, and 120 minutes) and blood was collected and processed for thrombin generation assay. The assay was performed in either (A) the absence or (B) the presence of added FVa (20 nM). (C-D) HB mice (n = 5-7/group) were administered different doses (28-450 μg/kg) of FXa-I16T (C) alone or (D) together with FVa (200 μg/kg). After 10 minutes, blood was collected and processed for thrombin generation assay. For comparison purposes, data from (C) (28 and 56 μg/kg FXa-I16T) are provided in (D). Control experiments include (A) wt-mice (n = 5), (C) HB mice injected with PBS (n = 5), and (D) mice administered only FVa 400 μg/kg (n = 3). Data are presented as mean ± SEM.
Ex vivo assessment of FXa-I16T-Thrombin generation assay. (A-B) HB mice (n = 5-7 per group) were administered FXa-I16T (450 μg/kg) and at various time points (10, 30, 60, and 120 minutes) and blood was collected and processed for thrombin generation assay. The assay was performed in either (A) the absence or (B) the presence of added FVa (20 nM). (C-D) HB mice (n = 5-7/group) were administered different doses (28-450 μg/kg) of FXa-I16T (C) alone or (D) together with FVa (200 μg/kg). After 10 minutes, blood was collected and processed for thrombin generation assay. For comparison purposes, data from (C) (28 and 56 μg/kg FXa-I16T) are provided in (D). Control experiments include (A) wt-mice (n = 5), (C) HB mice injected with PBS (n = 5), and (D) mice administered only FVa 400 μg/kg (n = 3). Data are presented as mean ± SEM.
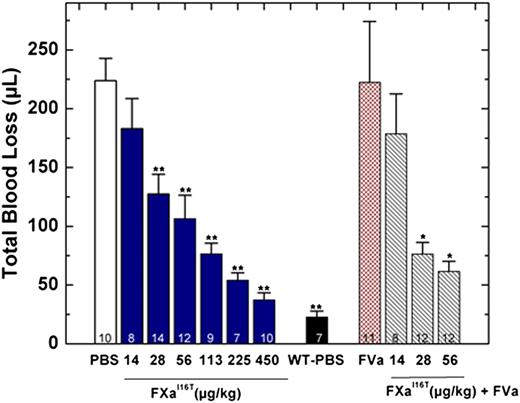
In addition to restoring thrombin generation, FXa-I16T dose-dependently reduced total blood loss in HB mice. When given 5 minutes before injury, the protein showed statistically significant results at 28 µg/kg and normalized blood loss at 450 µg/kg (Figure 6). There was a modest but statistically significant change in total blood loss when FXa-I16T was coadministered with FVa (400 µg/kg) vs the variant alone (28 or 56 µg/kg; P < .05), suggesting that excess FVa can improve efficacy. No significant change in total blood loss was found when HB mice were infused with only FVa (400 µg/kg). In addition to the tail transection model, FXa-I16T showed a dose-dependent response in the FeCl3 injury model (Table 4). Together, these data show that FXa-I16T is highly efficacious and appears to be ideally suited for the prevention of bleeding because of its suitable activity and long half-life.
Blood loss after tail clip in HB mice following FXa-I16T administration. Five minutes before injury, FXa-I16T was given to HB mice via tail vein at the indicated dosage (blue bars). In some experiments, mice received FXa-I16T 14-56 μg/kg with FVa 400 μg/kg (hatched bars). Control experiments include HB mice treated with PBS (white bar), wt-mice (black bar) injected with PBS, and HB mice injected with FVa 400 μg/kg (red bar). Total blood loss (µL) was measured following tail transection. The number of animals per group is indicated, and all measurements are presented as mean ± SEM. For statistical comparisons, HB animals injected with different doses of FXa-I16T and wt-mice are compared with HB-PBS controls: **P < .001; HB animals injected with different doses of FXa-I16T along with FVa are compared with FXa-I16T-injected HB: *P < .05.
Blood loss after tail clip in HB mice following FXa-I16T administration. Five minutes before injury, FXa-I16T was given to HB mice via tail vein at the indicated dosage (blue bars). In some experiments, mice received FXa-I16T 14-56 μg/kg with FVa 400 μg/kg (hatched bars). Control experiments include HB mice treated with PBS (white bar), wt-mice (black bar) injected with PBS, and HB mice injected with FVa 400 μg/kg (red bar). Total blood loss (µL) was measured following tail transection. The number of animals per group is indicated, and all measurements are presented as mean ± SEM. For statistical comparisons, HB animals injected with different doses of FXa-I16T and wt-mice are compared with HB-PBS controls: **P < .001; HB animals injected with different doses of FXa-I16T along with FVa are compared with FXa-I16T-injected HB: *P < .05.
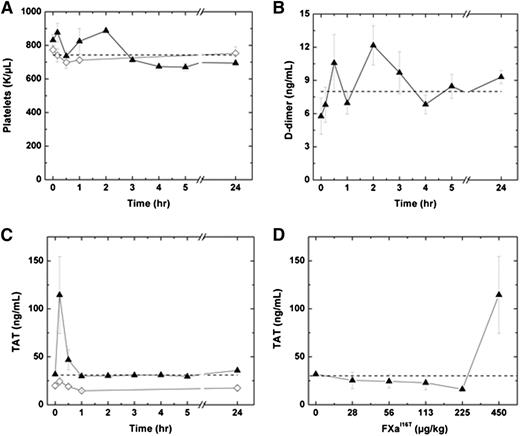
We next determined whether FXa-I16T is associated with an increase in markers of activation of coagulation. Following administration of 450 µg/kg FXa-I16T, no significant change in platelets counts or D-dimer levels were detected in HB mice over a 24-hour period (Figure 7A-B). Similar results were obtained by using a range of doses (28-225 µg/kg; n = 6-7; data not shown). Infusion of FXa-I16T at the highest dose (450 µg/kg) exhibited a transient increase in TAT levels at 10 minutes, which normalized to baseline within 1 hour (Figure 7C). No change in TAT levels was observed in HB mice injected with lower therapeutic doses of the variant (28-225 µg/kg; Figure 7D). Furthermore, there was no statistically significant difference in coagulation markers following infusion with FXa-I16T (450 µg/kg) once per day for 3 consecutive days (data not shown).
Effect of FXa-I16T on safety parameters. (A-C) HB mice (n = 5-12 per group) were injected with PBS (♢) or FXa-I16T 450 µg/kg (▲), and at the indicated time intervals, blood was collected and the following parameters were measured: (A) platelet count, (B) D-dimer, and (C) TAT. (D) HB mice (n = 5-7 per group) were injected with different amounts of FXa-I16T, and blood was collected 10 minutes after injection. In all panels, the dashed line represents values obtained with wt-mice. All values are presented as mean ± SEM.
Effect of FXa-I16T on safety parameters. (A-C) HB mice (n = 5-12 per group) were injected with PBS (♢) or FXa-I16T 450 µg/kg (▲), and at the indicated time intervals, blood was collected and the following parameters were measured: (A) platelet count, (B) D-dimer, and (C) TAT. (D) HB mice (n = 5-7 per group) were injected with different amounts of FXa-I16T, and blood was collected 10 minutes after injection. In all panels, the dashed line represents values obtained with wt-mice. All values are presented as mean ± SEM.
Discussion
Recently, novel FVIII and FIX products have been approved and more are in clinical development.3,4 Enhancements made to these proteins, especially half-life extension, should have a substantial impact on the clinical management of hemophilia patients. However, patients who develop inhibitory antibodies to FVIII or FIX typically rely on bypassing strategies to manage bleeding events and may not immediately benefit from these new advances. Approaches to treat inhibitor patients include recombinant FVIIa and activated prothrombin complex concentrates.30,31 These proteins reconstitute thrombin generation by enhancing cell-surface FXa production and ultimately prothrombinase levels.32 Improvements to FVIIa are being explored and include half-life extension technology as well as higher-activity variants.4 Because of a host of factors, including half-life, effective dose range, cost, and immunogenicity, several novel approaches are being investigated as alternatives to FVIIa and activated prothrombin complex concentrates. These include probes that block tissue factor pathway inhibitor, an RNA interference molecule that targets ATIII, and antibody mimetics of the FXase complex.3 Together, the bypassing approaches either directly enhance FXa production or dampen it by blocking inhibitory pathways. In principle, a more straightforward approach would be to provide FXa directly. Because of the properties inherent in wt-FXa, which are too difficult to overcome, it cannot be used as a bypass product.33,34
As shown in this study, altering FXa to a zymogen-like state circumvents several roadblocks and yields prohemostatic agents that are remarkably effective at enhancing thrombin generation at sites of vascular injury. Overall, this strategy paradoxically diminishes rather than enhances enzyme function by shuttling the enzyme to a zymogen-like state. This, in general, is not an obvious first approach to bioengineering proteins because gains in enzyme activity are generally sought. However, plasma inhibitors suppress the ability of administered FXa to function. A clear example of this is the ineffectiveness of wt-FXa or the most active zymogen-like variant (FXa-V17M) in vivo. Thus, strategies that dampen protease inhibition could generally prove useful to enhance or rather prolong enzyme function. A broad approach being pursued by others is the reduction of specific inhibitory pathways. In essence, this would enhance the prothrombotic response via control of negative regulatory pathways such as the protein C pathway, tissue factor pathway inhibitor, and ATIII.2-4 These targeted approaches have their advantages and disadvantages, and clinical trials investigating their feasibility are ongoing. Our work and these exciting alternative approaches have been buoyed by an expanded understanding of mechanisms that regulate clotting factor function, especially in the context of hemophilia.
Another key aspect of the FXa variants is that they could be rescued via incorporation into the prothrombinase complex. On the basis of our prior studies,5-7 this is the result of FVa preferentially binding the protease conformation of the protein and altering the equilibrium between zymogen and protease. In the models used, enough FVa derived from plasma or platelet stores must be available at the injury site to turn on the variants. In the absence of FVa, the variants have low activity and are at least partially resistant to circulating inhibitors such at ATIII. The extent of this resistance correlates with their half-life. For example, FXa-I16T has a half-life of more than 6 hours and was most resistant to ATIII. It also had markedly (1000-fold vs wt-FXa) reduced activity in the absence of FVa. With this large activity reduction, we anticipated that the variant would be shifted too far to the zymogen-like state and would not be rescued by FVa. However, this was not the case because FXa-I16T incorporated into prothrombinase resulted in only a modest reduction in prothrombin activation. As a result, the variant was effective at alleviating the hemophilic phenotype in mice when given up to an hour before injury.
An important finding from this study is that certain variants performed much better than others depending on when the proteins were administered. By overcoming the endogenous inhibitors and imparting a prolonged half-life, the lower activity zymogen-like variants were particularly effective when infused before injury. Variants with moderate activity apparently have the right mix of activity and half-life so that they are most effective when used in the acute setting. Together, these findings are important because they highlight that it is unlikely that one type of hemostatic agent will be effective in treating or preventing all types of clinical bleeding. Thus, in situations where re-bleeding occurs, the therapeutic intervention would need a suitable half-life to retain hemostatic potency long enough to address the injury. However, if there is an active bleed, a procoagulant hemostatic that is more potent and faster acting, but quickly eliminated, may be more effective and safer.
We also show that the variants are safe as measured by changes in markers of coagulation.7 After the variants were administered, platelets, D-dimer, and fibrinogen levels were unchanged. TAT levels transiently increased only at the highest dose (450 μg/kg) of FXa-I16T, but they quickly returned to baseline. These data likely reflect the low activity of the variants toward physiologic substrates in the absence of circulating FVa. Although these studies are performed in mice, they are important because the possibility of systemic activation of coagulation is a major concern with any procoagulant therapy. Another area of concern not investigated here is the potential immunogenicity of the variants. Although the complexity of the immune system makes predictions very difficult, an antibody response to a particular zymogen-like FXa variant could be mounted. Clearly these studies are difficult to do in animals with any predictive value, even if species-specific proteins are used. Nevertheless, a recent phase III clinical trial with an FVIIa variant suggest that the immune response to a mutant protein must be taken very seriously.35
In summary, we exploited the FX zymogen-to-protease transition and identified a series of variants with a range of activities and half-lives that are effective for the treatment of hemophilia. The in vivo data show that the physiologic circulating inhibitors provide a steep barrier to overcome because they rapidly neutralize the most active of the zymogen-like variants. However, through mutagenesis, the right balance of activity and half-life can be attained and, depending on the timing of administration and potentially the type of injury, a highly efficacious bypass agent can be identified. Still, the data show that the most zymogen-like variant can evade interactions with inhibitors best and attain a prolonged half-life and thus would be most effective in situations in which the molecule is administered before injury. The shorter half-life but more potent FXa variants were most effective in stopping acute bleeding. In fact, in this situation, a short half-life could be beneficial from a safety standpoint. We conclude that zymogen-like FXa variants are robust procoagulant hemostatic agents that, because of their mechanism of action, could be used for a range of bleeding situations not just restricted to hemophilia.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Drs Sriram Krishnaswamy and Valder R. Arruda (Children’s Hospital of Philadelphia/The University of Pennsylvania) for critical review of the manuscript.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute grant P01 HL-74124, Project 2 and by research funding from Pfizer (R.M.C).
Authorship
Contribution: L.I. conducted research, analyzed data, and wrote the paper; and R.M.C analyzed data and wrote the paper.
Conflict-of-interest disclosure: R.M.C. receives licensing fees and research funding from Pfizer. The remaining author declares no competing financial interests.
Correspondence: Rodney M. Camire, Division of Hematology, The Children’s Hospital of Philadelphia, 5018 Colket Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: rcamire@mail.med.upenn.edu.

![Figure 2. In vitro half-life studies in HB plasma. Factor Xa (20 nM wt-FXa [●]; zymogen-like 1: FXa-V17M [□]; zymogen-like 2: FXa-I16L [▪], FXa-I16M [▼], FXa-V17T [▿]; zymogen-like 3: FXa-V17S [△] and 60 nM FXa-I16T [▲]) were incubated in (A) human or (B) mouse HB plasma at the indicated time points, and residual activity was assessed by clotting assay. The solid lines were drawn after analysis of data sets to an exponential decay function with the following fitted parameters for half-life. The fitted parameters for the (A) human proteins can be found in Table 2 and those for (B) mouse proteins are as follows: FXa-I16T ∼60 minutes, FXa-V17S ∼17 minutes, FXa-I16M ∼9 minutes, FXa-I16L ∼5 minutes7, FXa-V17M ∼1.2 minutes, and wt-FXa 0.15 minutes. The data are representative of 3 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/1/10.1182_blood-2015-03-634329/4/m_94f2.jpeg?Expires=1769204333&Signature=z~7Pfltnqe~Tw780Jmc6w7ozERUcgBhlgqVyXF0RjJINxAiPr1I3kMDzm4o83VVaM2L-cv19EZewXfvcE38Kjq98P8aGSDbi-SGvliio-1mCzaD4JdS1TxrLZTtqmU4damK3Cb~qgMp2WX9Mag4ulAe1Pujj8L~UBsPMzWmas4mkwsQrNx~hXTGg5JbaJQ9SIpFZqEedDrVzfhXuaT2b1~Qn3CXIFWVedIsyEug4uXWMBDrkB91o4wtr8~LsxJsiJoHs7VoZ4YwbEngVxH6AIqEESjGmODh7tOIZ7~-mgtHxJPnO86RhWJnM2VhWAeJ2PRvrjVrV-D2~h70st1h17Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal