In this issue of Blood, Ivanciu and Camire describe engineered factor (F)Xa variants with a spectrum of properties that broaden the utility of FXa as a treatment for bleeding.1
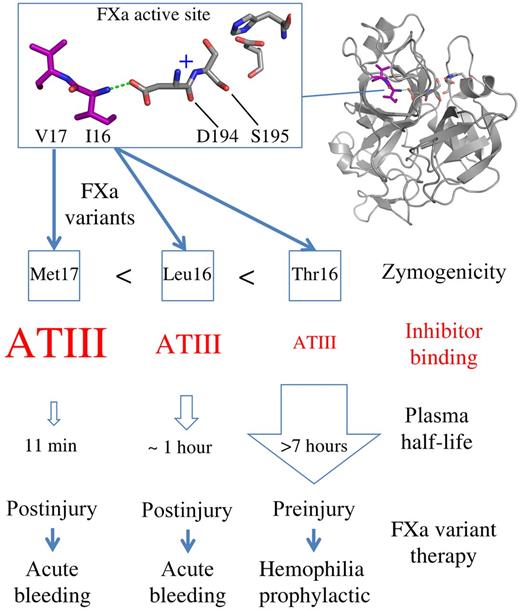
Representation of the FXa residues Val17 and Ile16 (purple) buried in the FXa active site adjacent to Ser195, His57, and Asp102. Ile16 interacts with Asp194 (dashed green line) forming the oxyanion hole (indicated as +). FXa variants induce zymogenicity to different degrees (Ile16Thr > Ile16Leu > Val17Met). The greater the zymogenicity, the weaker the inhibitor binding (red) and the longer the plasma half-life. This provides a toolkit of prohemostatic FXa variants that potentially broadens the applicability of FXa to different clinical settings involving bleeding.
Representation of the FXa residues Val17 and Ile16 (purple) buried in the FXa active site adjacent to Ser195, His57, and Asp102. Ile16 interacts with Asp194 (dashed green line) forming the oxyanion hole (indicated as +). FXa variants induce zymogenicity to different degrees (Ile16Thr > Ile16Leu > Val17Met). The greater the zymogenicity, the weaker the inhibitor binding (red) and the longer the plasma half-life. This provides a toolkit of prohemostatic FXa variants that potentially broadens the applicability of FXa to different clinical settings involving bleeding.
Treatment of hemophilia A and B requires replacement of the deficient coagulation factor (FVIII or FIX, respectively) to restore thrombin generation at the site of tissue injury. This is successfully used for treatment of hemophilia but has the common problem of developing an inhibitory antibody against the infused protein. As an alternative strategy, FXa has been investigated as a means of bypassing the intrinsic pathway because FXa bound to damaged or activated endothelium in the presence of the cofactor FVa converts prothrombin to thrombin.2 One problem with this approach is that administering wild-type FXa (wt-FXa) is ineffective because it has a very short half-life in plasma due to the high concentration of circulating inhibitors (particularly antithrombin III) that prevent passage to the activated surface. Strategies to improve these pharmacokinetic properties with protein engineering could involve mutation of surface residues or providing additional N-linked glycan residues. Ivanciu and colleagues have developed an innovative approach to tackle this problem by engineering alternative amino acids at the N-terminal tip of the highly conserved FXa activation loop.1 The figure shows that two key amino acids (Val17 and Ile16 shown in purple) are buried in the hydrophobic core of the FXa enzyme. Ile16 does not contact the substrate prothrombin but is indirectly critical for formation of the oxyanion hole and FXa selectivity pocket by interacting with Asp194. This interaction alone is not sufficient to produce a mature FXa active site competent to cleave prothrombin, which requires the additional presence of the cofactor FVa.
Biochemical studies mutating FXa Val17 and Ile16 revealed that substitutions here are less efficient at forming the active site resulting in an enzyme that is zymogen-like.3 How do the mutations at the tip of the activation loop work to improve FXa utility in the treatment of bleeding? Subsequent studies revealed that zymogen-like FXa tip variants do not bind circulating inhibitors, which effectively broadens the functional half-life and provides safe passage to the site of injury.4 This observation together with the ability of FVa to combine with the FXa variants to produce an active prothrombinase complex was the recipe for effectively bypassing the intrinsic pathway. These studies were then extended into a mouse model of hemophilia to demonstrate that the FXa Ile16Leu variant was more effective than FVIIa as a replacement therapy.5
Ivanciu and Camire1 present data that further advance these studies to characterize a total of 11 variants mutating FXa residues Ile16 and Val17. Variants Val17Met, Ile16Leu, Ile16Met, Val17Thr, Val17Ser, and Ile16Thr are characterized in detail in terms of enzyme activity, plasma half-life, clotting times, and thrombin generation in hemophilic plasma. The results reveal that these variants were effective in mouse injury models and achieved a broadening of the spectrum of FXa zymogen-like activities. At one end of the spectrum, variants Val17Met and Val17Leu (termed group 1)1 are characterized as having high activity. This type of variant can be achieved only by modifying Val17, which is more remote to the oxyanion hole than Ile16 and only partially buried in the active FXa structure (see figure). In terms of circulating plasma half-life, the group 1 variants were characterized as having a 10-fold advantage compared with the wt-FXa. In the middle of the spectrum, variants Ile16Leu and Ile16Met (group 2) have an intermediate zymogenicity with a plasma half-life up to 100-fold greater than wt-FXa. Finally, at the other end of the spectrum, the Ile16Thr and Val17Ser variants (group 3) are the most zymogen-like and have a plasma half-life up to 350-fold greater than wt-FXa (6-7 hours). It is notable that this latter type of variant is achieved only by removing the hydrophobic nature of the amino acid whereby introduction of the small polar-uncharged amino acid (Thr or Ser) into the activation loop tip achieves the correct balance of inducing FXa zymogenicity without compromising the ability of FVa to rescue activity in the prothrombinase complex.
The time of administration was also investigated, and postinjury administration of high-activity FXa variants such as Val17Met were shown to be the most efficacious in the mouse model. Interestingly, variants involving the polar-uncharged amino acids (Ile16Thr, Val17Ser) with long functional half-lives are effective when administered before injury and may be suitable for prophylactic treatment. This provides a toolkit of FXa variants which can be tailor-made for different clinical contexts involving bleeding (see figure).1 The authors draw attention to potential issues with immunogenicity of the FXa variants because of altering a conserved amino acid; however, this may not be a serious issue because there are a large number of human serine proteases that also use variations of the Ile-Val-Gly-Gly activation loop sequence to which the immune system has already been exposed. Overall, this is a fascinating story of the FXa zymogen-like tip variants advancing from a basic science biochemical investigation into clinical trials as a promising new therapy for bleeding.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal