In this issue of Blood, Begonja et al describe a role for the membrane sculpting protein PACSIN2 (protein kinase C and casein kinase II substrate 2 in neurons) in membrane organization in megakaryocytes and platelets.1
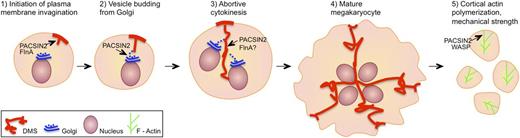
Proposed roles of PACSIN2 in megakaryocyte and platelet biology. (1) DMS formation is initiated by the activation of PACSIN2 tubulating activity by FlnA, leading to focal plasma membrane invagination. (2) PACSIN2 promotes vesicle budding from Golgi, thus facilitating the expansion of the DMS network. PACSIN normally localizes to the cleavage furrow of mitotic cells, where it couples the actomyosin contractile ring to the plasma membrane. (3-4) During megakaryocyte endomitosis, PACSIN2 could recruit FlnA that may decrease actomyosin activity, leading to abortive cytokinesis and multinucleation. (5) In platelets, PACSIN could support plasma membrane stability by activating WASP in the vicinity of the plasma membrane, thus promoting the polymerization of cortical actin.
Proposed roles of PACSIN2 in megakaryocyte and platelet biology. (1) DMS formation is initiated by the activation of PACSIN2 tubulating activity by FlnA, leading to focal plasma membrane invagination. (2) PACSIN2 promotes vesicle budding from Golgi, thus facilitating the expansion of the DMS network. PACSIN normally localizes to the cleavage furrow of mitotic cells, where it couples the actomyosin contractile ring to the plasma membrane. (3-4) During megakaryocyte endomitosis, PACSIN2 could recruit FlnA that may decrease actomyosin activity, leading to abortive cytokinesis and multinucleation. (5) In platelets, PACSIN could support plasma membrane stability by activating WASP in the vicinity of the plasma membrane, thus promoting the polymerization of cortical actin.
F-BAR (Fes-CIP4 homology [FCH] Bin-amphiphysin-Rvs) proteins are structurally well-characterized proteins that mediate the formation of complex membrane structures by binding membranes through their conserved amino-terminal F-BAR domains.2 Many of these proteins have adaptor functions mediated by their carboxy-terminal muniscin homology (μHD) or Src homology 3 (SH3) domains. FCH-only proteins, FCHo1 and FCHo2, initiate the formation of clathrin-coated pits and recruit components of the endocytic machinery through their μHD domains, thus acting both as membrane-sculpting proteins and endocytic hubs.3 Through their SH3 domains, other F-BAR family members, such as the PACSINs and CIP4 (Cdc42-interacting protein 4), recruit and activate members of the Wiskott-Aldrich syndrome (WASP) family of proteins, thus coordinating membrane reshaping with cytoskeletal remodeling, whereas the proline serine threonine phosphatase interacting protein (PSTPIP) subfamily member, PSTPIP1, finely tunes WASP activity by mediating its interaction with and dephosphorylation by the nonreceptor protein tyrosine phosphatase, PTPN12.2 Extensive research has focused on the role of F-BAR proteins in the development and homeostasis of the nervous system. However, although well represented in the hematopoietic system,2 their functions in blood cells are less understood.
PACSINs (also known as syndapins) are F-BAR proteins that control multiple processes, including the generation of membrane curvature, the assembly and fission of caveolae and actin, and microtubule polymerization, which are important for morphogenesis, endocytosis, synaptic vesicle recycling, cell migration, and cytokinesis.4 PACSINs have 3 structurally different isoforms that differ in their biochemical properties and patterns of expression, reflecting different tissue-specific functions.2,4
The demarcation membrane system (DMS) of megakaryocytes forms the plasma membrane of future platelets. During megakaryocyte maturation, development of the DMS is initiated by focal tubular membrane invagination at the cell periphery followed by expansion of the tubular membranes through membrane delivery from the Golgi complex and endoplasmic reticulum-mediated lipid transfer. This gives rise to an extensive intracellular membrane network that remains continuous with the cell surface.5 Begonja et al show that the ubiquitously expressed PACSIN2 is one of the most abundant F-BAR proteins in platelets and propose that it contributes to the formation of the DMS in megakaryocytes. In platelets, PACSIN2 associates with filamin A (FlnA), a cytoskeletal protein that stabilizes platelet membranes subjected to shear stress and promotes platelet adhesion to the von Willebrand factor by bridging the adhesion glycoprotein GPIbα to the actin cytoskeleton.6,7 Interestingly, although ∼50% of FlnA is associated with the cytoskeleton, PACSIN2 is selectively associated with soluble FlnA. This interaction involves a putative FlnA binding motif located between PACSIN2 amino acids 174 and 182, near a loop at the tip of the F-BAR domain necessary for membrane tubulation and FlnA repeat 20. Although the localization of the FlnA binding motif suggests that the interaction between PACSIN2 and FlnA might disrupt PACSIN2 membrane- binding and tubulating activities, FlnA binding enhances the membrane-sculpting activity of PACSIN2 in vitro and dissociates from membrane-bound PACSIN2 during tubulation. These data uncover a novel mechanism for activation of PACSIN2.
To explore the biological relevance of this finding, the authors used super-resolution structured illumination microscopy to examine the localization of PACSIN2, FlnA, and GPIbα in mouse and human platelets and megakaryocytes. PACSIN2 and GPIbα colocalize in clusters resembling the focal membrane invaginations associated with the open canalicular system of platelets or the DMS of megakaryocytes,5 suggesting that PACSIN2 is involved in the initiation of membrane invagination during the formation of these structures. Although in most wild-type platelets PACSIN2 was concentrated in a single focus per cell, in FlnA-null platelets, PACSIN2 formed multiple foci. The cause of the formation of multiple foci is unclear. Because the amount of PACSIN2 was increased in FlnA-null platelets, this may reflect a propensity of PACSIN2 to aggregate at sites of high concentration (eg, sites of membrane tubulation). In contrast, in FlnA-null megakaryocytes, PACSIN2 levels were normal, PACSIN2 was dispersed throughout the cell, and PACSIN2-coated membrane tubules were substantially decreased. Similar results were obtained by expressing a PACSIN2 mutant unable to interact with FlnA, indicating that FlnA binding promotes PACSIN2-mediated membrane tubulation in megakaryocytes. Based on these data and on the fact that FlnA-null megakaryocytes exhibit defects in DMS formation, the authors suggest that PACSIN2 contributes to the formation of the DMS.
The findings of Begonja et al raise important new questions concerning the role of PACSIN2 in platelets that can only be answered by examining platelet and megakaryocyte membrane organization and mechanical strength following targeted inactivation of Pacsin2 or following short hairpin RNA-mediated depletion in megakaryocytic cell lines.8 Indeed, through its roles in membrane tubulation and stimulation of actin polymerization, PACSIN2 may contribute to the mechanical stability of platelets and to the formation of the DMS (see figure).
Eckly et al5 suggested that formation of the DMS is coupled to the process of abortive cytokinesis and that the tubular membrane invaginations of the DMS represent a megakaryocyte-specific pseudo-cleavage furrow. As Drosophila PACSIN regulates cleavage furrow assembly9 and FlnA inhibits actomyosin activity in platelets,10 determining how the interaction of PACSIN2 with FlnA regulates megakaryocyte ploidy is of particular interest.
Conflict-of-interest disclosure: The authors declare no competing financial interests.