In this issue of Blood, the articles by Shaham et al1 and Wang et al2 are the first to identify microRNA 486 (miR-486) as a requisite oncomiR and credible therapeutic target in myeloid leukemia of Down syndrome (ML-DS) and chronic myeloid leukemia (CML) by showing that these 2 leukemias co-opt miR-486 functions in normal erythroid progenitor progrowth and survival activity.
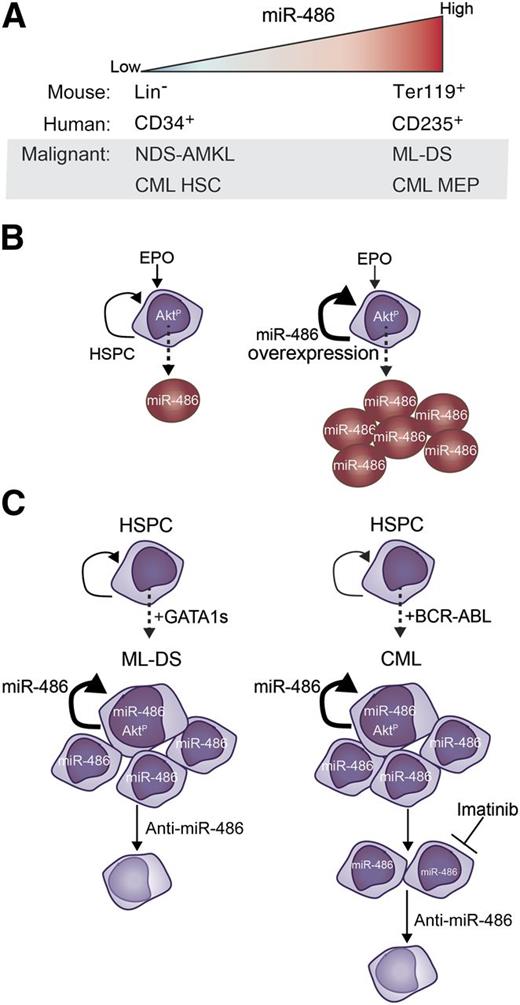
miR-486 is a regulator of normal erythropoiesis and myeloid leukemogenesis. (A) Expression pattern of miR-486 in normal and malignant hematopoiesis. (B) miR-486 expression is upregulated during erythroid differentiation (left), and forced overexpression of miR-486 in hematopoietic stem and progenitor cells pushes the expansion of erythrocyte differentiation (right). miR-486 expression directly controls PTEN and FoxO1 to permit activation of AKT signaling during normal erythroid differentiation. (C) Overexpression of miR-486 cooperates with Gata1s to increase proliferation and self-renewal, and knockdown of miR-486 in ML-DS induces cell death (left). Expression of miR-486 synergizes with BCR-ABL to promote cell proliferation (right). Imatinib treatment of CML cells partly reduces miR-486 expression and induces cell death, which is amplified by sponge-mediated knockdown of miR-486. EPO, erythropoietin; HSC, hematopoietic stem cell; HSPC, hematopoietic stem and progenitor cell; NDS, non-DS.
miR-486 is a regulator of normal erythropoiesis and myeloid leukemogenesis. (A) Expression pattern of miR-486 in normal and malignant hematopoiesis. (B) miR-486 expression is upregulated during erythroid differentiation (left), and forced overexpression of miR-486 in hematopoietic stem and progenitor cells pushes the expansion of erythrocyte differentiation (right). miR-486 expression directly controls PTEN and FoxO1 to permit activation of AKT signaling during normal erythroid differentiation. (C) Overexpression of miR-486 cooperates with Gata1s to increase proliferation and self-renewal, and knockdown of miR-486 in ML-DS induces cell death (left). Expression of miR-486 synergizes with BCR-ABL to promote cell proliferation (right). Imatinib treatment of CML cells partly reduces miR-486 expression and induces cell death, which is amplified by sponge-mediated knockdown of miR-486. EPO, erythropoietin; HSC, hematopoietic stem cell; HSPC, hematopoietic stem and progenitor cell; NDS, non-DS.
The figure summarizes these 2 independent reports, which delineate the mechanisms leading to the aberrant overexpression of miR-486 in ML-DS and CML (panel A). Their highlights are described in greater detail below. In sum, the articles clearly demonstrate that miR-486 directs erythroid differentiation of normal hematopoietic cells involving activation of the AKT pathway (panel B), which is mirrored by a similar erythroid phenotype signaled through miR-486/AKT in leukemia cells that also acts to promote cell survival (panel C). The extensive and congruent results using human and mouse in vivo and in vitro models combined with primary human leukemia and normal hematopoietic cells underscore the importance of miR-486 as a conserved mediator of erythropoiesis and leukemogenesis. Moreover, these studies provide the initial proof of principle for miR-486 as a therapeutic target in CML and ML-DS, laying the groundwork for follow-up in vivo preclinical testing.
Infants and children with Down syndrome (DS) have significantly increased risk of developing transient myeloproliferative disorder (TMD), which sometimes transforms to myeloid leukemia (ML-DS), the most common subtype being acute megakaryoblastic leukemia (AMKL).3 Acquired somatic mutations in the megakaryocyte/erythroid-lineage specifying transcription factor GATA1 generate a short isoform (GATA1s) that cooperates with trisomy 21 early on in the evolution of TMD and ML-DS.4 Reported herein, microRNA (miRNA) expression analyses on bone marrow from patients with ML-DS, non-DS AMKL, or remission samples led to the discovery by Shaham et al that miR-486 is uniquely overexpressed in ML-DS patients (panel A). On the other hand, Wang et al independently discovered that miR-486 is the most highly expressed miRNA in their cohort of patients with CML, a molecularly, pathologically, and phenotypically distinct myeloid neoplasm from ML-DS. The Philadelphia chromosome t(9;22) rearrangement generating the BCR-ABL tyrosine kinase fusion protein is the most common and the earliest initiating event in CML pathogenesis.
What is driving miR-486 expression? Because GATA1 mutations are exclusively found in ML-DS, the expression pattern of miR-486 hinted that GATA1s might be its upstream regulator in normal and malignant hematopoiesis. Indeed, Shaham et al uncover that (1) miR-486 is encoded within the GATA1 target gene ANK15 ; (2) miR-486 positively correlates with GATA1s in primary ML-DS; and (3) miR-486 expression changes concordantly with manipulation of GATA1 or GATA1s in human ML-DS cell lines. Conversely, in CML, Wang et al find that expression of BCR-ABL leads to significant elevation of miR-486 expression in CD34+ cells. Unlike in ML-DS, wherein GATA1s can directly activate miR-486, modulation of BCR-ABL activity with either expression of a kinase-inactive BCR-ABL mutant or treatment with a tyrosine kinase inhibitor only partially restored miR-486 expression, suggesting that miR-486 may be regulated by BCR-ABL kinase-dependent and kinase-independent mechanisms in CML. Because malignant hematopoietic stem cells in chronic-phase CML maintain self-renewal and multilineage potential with clonally expanded granulocytic, megakaryocytic, and erythroid progenitor compartments,6 the authors dissected miR-486 expression in the hematopoietic stem/progenitor populations of CML patient samples and found it most highly expressed in the megakaryocyte-erythroid progenitor (MEP) fraction.
Is there a role for miR-486 in hematopoiesis? Because GATA1s regulates megakaryocytic-erythroid differentiation, Shaham et al measured miR-486 steady-state levels in primary murine hematopoietic cells but found detectable expression only in the erythrocytes from both wild-type and Gata1s knockin mice. Underscoring this finding, miR-486 expression was robustly induced when human GATA1- and GATA1s-overexpressing MEP-like cell lines were differentiated to the erythroid lineage with cytokines. In agreement with this, Wang et al found that miR-486 expression is significantly induced upon erythroid, but not myeloid, cytokine-stimulated differentiation of human CD34+ cells in vitro. Exogenous overexpression of miR-486 in human CD34+ cells significantly enhances cytokine-stimulated erythroid differentiation in vitro. Conversely, knockdown of miR-486 in human CD34+ cells significantly reduced cytokine-induced erythroid differentiation in vitro and significantly diminished the frequency of CD235+ erythroid populations in mouse xenografts in vivo. Collectively, the results of these 2 independent studies solidify a role for miR-486 in erythroid differentiation, a phenomenon they show is conserved from mice to humans.
In building upon this new link between miR-486 and erythroid differentiation, both groups reveal implications that extend beyond normal hematopoiesis. Expression of miR-486 is tightly associated with expression of the erythroid marker glycophorin A (GYPA or CD235) in ML-DS patient blasts and BCR-ABL–expressing human CD34+ cells. In ML-DS, Shaham et al find that knockdown of miR-486 significantly reduces the expression of GYPA, shifting ML-DS cells from a CD235+CD61+ erythromegakaryocytic immunophenotype to a CD235−CD61+ megakaryocytic phenotype. Although ML-DS cells exhibit slowed growth kinetics and undergo a significant increase in apoptosis upon miR-486 knockdown, the amount of cell death is not likely to account for the dramatic shift in CD235 expression. Wang et al knocked down miR-486 in an erythroleukemia cell line and in human CD34+ cells expressing BCR-ABL, which caused a significant induction in cell death that was further enhanced with imatinib treatment. This was concomitant with decreased GYPA expression. Importantly, the authors show that knockdown of miR-486 does not cause cell death of normal human CD34+ cells. The overall survival (OS) of patients with newly diagnosed chronic-phase CML is significantly increased by treatment with tyrosine kinase inhibitors such as imatinib. However, ∼20% of patients are resistant or become treatment refractory. CML patients acquire additional mutations or epigenetic alterations causing the transition into the blast-crisis leukemia phase.7 Children with ML-DS treated with standard low-dose chemotherapy do somewhat better, with a 3-year OS of 80%.8 Data from these 2 reports strongly suggest that antagonism of miR-486 may work best as a combination therapy with the current standard of care.
Conflict-of-interest disclosure: The authors declare no competing financial interests.