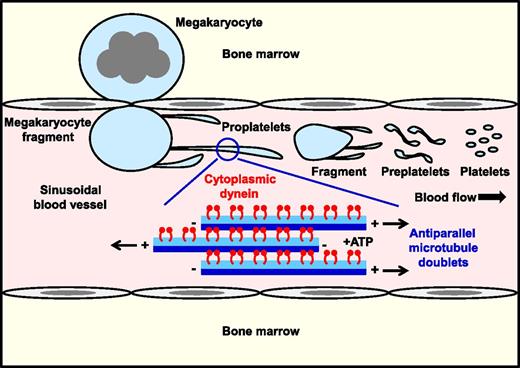
In this issue of Blood, Bender et al provide compelling evidence that the motor protein cytoplasmic dynein provides the necessary force for microtubule sliding and proplatelet elongation from megakaryocytes (see figure).1
Cytoplasmic dynein drives microtubule sliding and proplatelet elongation. Dynein-mediated sliding of antiparallel microtubule doublets within proplatelet shafts drives elongation under static and physiological shear force in vitro. Presumably, the same occurs in vivo, where shear force applied to megakaryocyte fragments extruded into sinusoidal blood vessels triggers the highly dynamic process of proplatelet formation and platelet biogenesis.
Cytoplasmic dynein drives microtubule sliding and proplatelet elongation. Dynein-mediated sliding of antiparallel microtubule doublets within proplatelet shafts drives elongation under static and physiological shear force in vitro. Presumably, the same occurs in vivo, where shear force applied to megakaryocyte fragments extruded into sinusoidal blood vessels triggers the highly dynamic process of proplatelet formation and platelet biogenesis.
Microtubule sliding has been proposed to drive proplatelet elongation, but direct proof of this mechanism has been lacking. Bender et al report a highly dynamic process involving repetitive phases of extension, pause, and retraction that is independent of de novo microtubule growth. They also show that physiological shear forces generated in a microfluidic platelet bioreactor accelerate proplatelet extension by reducing the pause phase.1,2 Better understanding the molecular basis of platelet biogenesis will yield improved strategies for treating thrombocytopenia and thrombocythemia, and optimize conditions for culturing platelets in vitro for experimental and therapeutic purposes.
Previous findings by Hartwig and colleagues led to the hypothesis that dynein-driven microtubule sliding underlies proplatelet elongation.3 They showed that microtubules continuously polymerize in megakaryocytes in vitro at a rate that is considerably faster than the average rate of proplatelet formation in vitro.3 Because this was not impaired by nocodazole, which blocks microtubule assembly, it was concluded that tubulin polymerization was unlikely to be the primary driver of this process. Evidence supporting the role of dynein was that this was an adenosine triphosphate (ATP)-dependent process, and disruption of dynein function by overexpression of the dynamitin subunit in megakaryocytes severely impaired proplatelet formation.3 To address this hypothesis, Bender et al used fluorescence loss after photoconversion (FLAC) time lapse and fluorescence recovery after photobleaching (FRAP) to directly visualize and quantify the rates of proplatelet elongation in fetal liver-derived mouse megakaryocytes expressing β1-tubulin tagged with the photoconvertible fluorophore Dendra2 (β1-tubulin-Dendra2).1 Experiments were performed in the presence and absence of structurally distinct inhibitors of dynein, under static and physiological shear stress. Bender et al establish that proplatelet elongation is not a continuous process once initiated, but rather undergoes repetitive phases of extension, pause, and retraction back to the megakaryocyte cell body, the rate of which is considerably increased when megakaryocytes are exposed to shear. Shear shortened the pause phase, but microtubule sliding remained essential for proplatelets to form. The same applies to released proplatelets, which subfragment in the circulation. The physiological significance of the pause and retraction phases remains unclear and warrants further investigation. Resolving the mechanisms underlying all 3 phases could lead to pharmacologically or genetically based means of manipulating the rate of platelet production.
Microtubule mobility during the process of proplatelet elongation was tracked in β1-tubulin-Dendra2–expressing primary mouse megakaryocytes using FLAC microscopy, which enables spots to be marked along the proplatelet shaft by irreversibly photoconverting Dendra2 from green to red with a 405-nm laser. FRAP microscopy was used to further analyze the kinetics of elongation of released proplatelets expressing β1-tubulin-Dendra2. A 488-nm laser was used to bleach a 1.5-μm spot and fluorescence recovery rates determined as an indicator of microtubule sliding. Incomplete recovery in the bleached region was most likely due to immobile microtubules. Similar findings were obtained using human umbilical cord blood–derived megakaryocytes,1 demonstrating that this is a common mechanism, and adding credence to mouse megakaryocytes as a model for resolving the mechanism of platelet production in humans.
Three structurally distinct compounds were used to inhibit dynein in this study, namely, sodium orthovanadate (Na3VO4), erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA), and ciliobrevin D, the last being the newest and most selective dynein inhibitor of the 3.4 Na3VO4 and EHNA have known off-target effects, the former inhibiting protein-tyrosine phosphatases5 and the latter inhibiting phosphodiesterases,6 in addition to dynein ATPase activity. However, the fact that all 3 compounds had similar inhibitory effects on proplatelet elongation suggests they act through the same mechanism. In contrast, inhibition of tubulin polymerization by nocodazole had no effect on proplatelet elongation, as previously reported, and taxol, which stabilizes microtubules, inhibited microtubule sliding by an as yet unknown mechanism.1
Findings from this study fill an important gap in our knowledge of the mechanism driving platelet formation. It unifies previous in vitro findings by Hartwig and Italiano,7 and in vivo findings by Junt et al8 and Pertuy et al,9 demonstrating that large fragments of megakaryocytes enter the sinusoidal lumen where they further subfragment into platelets in the circulation. Thus, the terminal stages of platelet production are actively taking place within the blood, where platelets adopt their final shape and size,8,10 rather than immediately at the point of entry, as is sometimes depicted in schematic representations. All the while, the dynein is driving microtubule sliding and proplatelet elongation.
The application of quantitative fluorescence microscopy techniques to address a fundamental question of hematology makes this study a must-read. It also highlights key unanswered questions, including: how shear force gets converted into biochemical signals that increase dynein function and proplatelet formation, how this mechanism is suppressed in the bone marrow, how microtubule cross-linking and twisting influence the direction of net microtubule movement, and how platelets are ultimately released from the tips of proplatelet shafts in the circulation. Watch this space.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal