Key Points
Dinaciclib is a novel cdk inhibitor that demonstrates single agent activity in myeloma.
Dinaciclib has a safety profile that is easily manageable.
Abstract
Dysregulation of cyclin-dependent kinases is a hallmark of myeloma, and specifically, cdk5 inhibition can enhance the activity of proteasome inhibitors in vitro. Dinaciclib is a novel potent small molecule inhibitor of cyclin-dependent kinases (CDK)1, CDK2, CDK5, and CDK9. Patients with relapsed multiple myeloma and ≤5 prior lines of therapy, with measurable disease, were enrolled. Dinaciclib was administered on day 1 of a 21-day cycle at doses of 30 to 50 mg/m2. Overall, 27 evaluable patients were accrued; the median number of prior therapies was 4. The dose level of 50 mg/m2 was determined to be the maximally tolerated dose. The overall confirmed partial response rate (PR) was 3 of 27 (11%), including 1 patient at the 30 mg/m2 dose (1 very good PR [VGPR]) and 2 patients at the 40 mg/m2 dose (1 VGPR and 1 PR). In addition, 2 patients at the 50 mg/mg2 dose achieved a minimal response (clinical benefit rate, 19%). Leukopenia, thrombocytopenia, gastrointestinal symptoms, alopecia, and fatigue were the most common adverse events. The current study demonstrates single agent activity of dinaciclib in relapsed myeloma, with 2 patients achieving a deep response (VGPR) and 10 patients obtaining some degree of M protein stabilization or decrease. This trial was registered at www.clinicaltrials.gov as #NCT01096342.
Introduction
Treatment paradigms have shifted for myeloma in the last decade with the introduction of 2 classes of effective agents: proteasome inhibitors and immunomodulatory drugs (IMiDs).1 As a result, patients with myeloma are living longer, with median survival that is two- to threefold that of a decade earlier. However, these new therapies have not resulted in eradication of the malignant clone, with the vast majority of patients eventually relapsing and requiring additional therapy.2,3 It is clear that some of the malignant clones in this heterogeneous disease undergo significant evolution in clonal tides and also with acquisition of new genetic abnormalities, especially those that allow evasion the current therapies.4 Given this, it is of the utmost importance that we develop new therapies that work through mechanisms that are unique compared with the current drugs. This is increasingly becoming a reality with a better understanding of the changes that underlie disease evolution, so that new therapeutic targets can be identified and targeted.
Cyclin-dependent kinases (CDKs) are serine/threonine kinases that regulate progression through the cell cycle, complexing with specific cell cycle regulatory cyclins.5 In addition, there are specific CDK inhibitors that are negative regulators of the cell division process.6-8 Multiple myeloma (MM) is characterized by translocations involving the immunoglobulin heavy chain locus or trisomies of odd numbered chromosomes (hyperdiploidy) in the vast majority of patients.9 The recurrent immunoglobulin (Ig)H translocations either directly dysregulate CCND1 (11q13: cyclin D1) or CCND3 (6p21: cyclin D3), or dysregulate transcription factors (16q23: MAF, 20q11: MAFB) or oncogenes (4p16: FGFR3/MMSET) that ultimately transactivate CCND2 (cyclin D2).10 Similar to the translocated MM, hyperdiploid MM also exhibits universal dysregulation of 1 or more cyclin D genes, commonly involving transactivation of CCND1 and/or CCND2. Cell cycle dysregulation in MM is further complemented by loss of endogenous CDK inhibitors (such as CDKN2A/p16 or CDKN2C/p18) and by recurrent dysregulation of MYC (8q24), following translocation of MYC to the IgH locus, gene amplification, or transactivation. Importantly, we have shown through RNA interference-based screens that inhibition of CDK5 results in sensitization of myeloma cells to proteasome inhibitors, a phenomenon mediated through modulation of the proteasome subunit PSMB5.11 These findings led us to examine the therapeutic benefit of targeting CDKs in patients with MM, with a particular focus on CDK5 inhibition.
Dinaciclib (SCH727965) is a novel, potent, small molecule inhibitor of CDKs, selectively inhibiting CDK1, CDK2, CDK5, and CDK9 with 50% inhibitory concentrations (IC50) in the low nanomolar concentration (4, 1, 1, and 4 nM, respectively). Cyclin D/CDK4 complexes were inhibited with an IC50 of 100 nM, whereas extracellular signal-regulated kinase 2 and GSK3B (2 serine threonine kinases closely related to CDK2 and CDK1) were inhibited at an IC50 of 4100 and 800 nM, respectively. Dinaciclib has been well tolerated in initial trials, and clinical efficacy has been observed in patients with chronic lymphocytic leukemia and solid tumors.12,13
Patients and methods
Study design
This was a phase 1/2 study that was initially designed as an open-label, single-arm phase 2 study to evaluate the safety, tolerability, and efficacy of dinaciclib given once every 3 weeks in patients with relapsed and/or refractory MM. The study was subsequently modified into a phase 1/2 study by adding a dose escalation phase to determine the maximally tolerated dose (MTD) of dinaciclib, based on toxicities observed among the first 2 patients. It was open to enrollment at 6 sites in the United States and 1 site in Singapore through a National Institutes of Health–funded Phase 2 Consortium, enrolling patients between August 2009 and September 2011. The study was performed in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice, and with approval of Institutional Review Boards at individual enrolling institutions.
Study objectives
The primary study objective was to determine the efficacy (assessed in terms of the overall response rate) of dinaciclib administered intravenously once every 3 weeks. The secondary objectives included assessment of toxicity and assessment of the rate of minimal response (MR) or better in patients with relapsed and refractory MM in patients with relapsed or refractory MM treated with dinaciclib given intravenously every 3 weeks. The primary objective of the dose escalation phase that was added with the modification was to determine the maximum tolerated dose (MTD) of dinaciclib given intravenously once every 3 weeks in patients with relapsed myeloma.
Patient selection
Patients were eligible to participate in the dose escalation part of the study if they had relapsed MM following ≥1 prior therapy and ≤5 prior therapies. Patients were required to have measurable disease (serum M protein ≥1 g/dL or urine M protein ≥200 mg/24 hours or serum immunoglobulin free light chain ≥ 10 mg/dL along with an abnormal serum immunoglobulin κ to λ free light chain ratio), an Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate hematologic (absolute neutrophil count ≥1000/mm3, platelets ≥ 75 000/mm3), hepatic (total bilirubin within normal limits, alanine/aspartate aminotransferase ≤2.5 × upper limit of normal), and renal (creatinine <2.5 mg/dL) function. Patients with major surgery, serious infection, or radiotherapy or who had any investigational products or myelosuppressive therapy within 21 days of the first dose of dinaciclib were excluded from participation. Concurrent corticosteroid therapy for coexisting conditions in excess of 20 mg daily of prednisone or equivalent was prohibited on study. Patients with an active malignancy, with the exception of nonmelanoma skin cancer or in situ cervical or breast cancer, or any uncontrolled intercurrent illness that would limit compliance with study requirements were excluded from participation. Concurrent use of strong inhibitors or inducers of CYP3A4 were prohibited during the clinical trial.
Drug administration
Dinaciclib was initially administered intravenously on day 1 of a 21-day cycle until disease progression or unacceptable toxicity, for a maximum of 12 cycles. During the dose escalation phase, dose escalation proceeded via a standard 3 + 3 design, based on the occurrence of dose-limiting toxicities (DLTs) in cycle 1. After the first 2 patients were enrolled and treated at 50 mg/m2, based on the toxicities observed, we added a limited dose escalation phase, with 3 planned cohorts at 30, 40, and 50 mg/m2. In addition to the dose escalation, routine growth factor support was added as detailed below, and patients received hydration before and after dinaciclib. DLTs were defined as ≥1 of the following toxicities considered related to dinaciclib: (1) grade 4 neutropenia lasting >7 days or grade 3 neutropenia with fever and/or infection, (2) grade 4 thrombocytopenia lasting >7 days, (3) any grade ≥3 nonhematologic toxicity except grade 3 nausea, vomiting, or diarrhea adequately managed by maximal supportive care, or (4) delay of >2 weeks in starting cycle 2 due to lack of recovery from drug-related toxicities in cycle 1. The MTD was the highest dose level with ≤1 patient experiencing DLTs during cycle 1. Standard supportive care measures were allowed for management of nausea and diarrhea, when observed. After the trial modification, routine growth factor was added to the regimen including the dose escalation phase patients, and a dose of pegfilgrastim was administered 24 hours after administration of dinaciclib. Following the initial modification, patients also received 500 mL of normal saline before and after the dinaciclib infusion, and they received 20 mg dexamethasone prior to dinaciclib infusion.
Assessments
Adverse events (AEs) were graded using the National Cancer Institute’s Common Terminology Criteria for AEs, version 3.0 (NCI-CTCAE v3.0). Myeloma disease response was done in accordance with the International Myeloma Working Group uniform criteria, incorporating the additional category of MR.14 The individual investigators performed response assessments.
Statistical analyses
Toxicity and response data were summarized using descriptive statistics. Progression-free survival was defined as the time from registration until disease progression or death due to any cause. Overall survival was defined as the time from registration to death due to any cause. Duration of response was defined as the time from first evidence of response (MR or better) to time of progression. The distributions of time to event end points were estimated using the Kaplan-Meier method.
Results
Twenty-nine patients were accrued to this study overall (19 for phase 1 and 10 for phase 2) from July 2009 to November 2012. Two patients were considered not evaluable and were replaced and therefore not included in any analysis. One patient failed to complete the 3 weeks of therapy on cycle 1 and was not evaluable for DLT or response. The other patient did not have a 24-hour urine available from baseline and hence was excluded from analysis. The phase 2 analysis included the 6 patients treated at the phase 1 dose level of 50 mg/m2 and the first 9 patients accrued to the phase 2 portion (15 evaluable patients). Overall, evaluable patients had a median age of 66 years (range, 49-81 years). Median time from diagnosis to registration was 3.5 years. The median number of prior therapies was 4 (range, 1-5); all patients had been exposed to IMiDs and corticosteroids, and 89% and 82% had prior bortezomib and alkylators, respectively. The patient characteristics are summarized in Table 1.
Baseline patient characteristics
| . | 30 mg/m2 (N = 4) . | 40 mg/m2 (N = 6) . | 50 mg/m2 (N = 6) . | Original 50 mg/m2 (N = 2) . | Phase 2 (50 mg/m2) (N = 9) . | Total (N = 27) . |
|---|---|---|---|---|---|---|
| Age | ||||||
| Median (range) | 70.0 (57.0-76.0) | 62.5 (49.0-69.0) | 61.5 (59.0-75.0) | 72.5 (70.0-75.0) | 70.0 (57.0-76.0) | 66.0 (49.0-81.0) |
| Gender | ||||||
| Female | 1 (25.0%) | 4 (66.7%) | 3 (50.0%) | 0 (0.0%) | 1 (25.0%) | 13 (48.1%) |
| Male | 3 (75.0%) | 2 (33.3%) | 3 (50.0%) | 2 (100.0%) | 3 (75.0%) | 14 (51.9%) |
| Months from diagnosis to registration | ||||||
| Median (range) | 31.9 (20.9-47.1) | 43.8 (10.1-67.9) | 66.7 (6.7-134.3) | 113.6 (20.1-207.1) | 31.9 (20.9-47.1) | 41.6 (6.7-207.1) |
| International staging | ||||||
| Stage 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 1 (3.7%) |
| Stage 2 | 3 (75.0%) | 2 (33.3%) | 3 (50.0%) | 1 (50.0%) | 2 (22.2%) | 11 (40.7%) |
| Stage 3 | 1 (25.0%) | 4 (66.7%) | 3 (50.0%) | 1 (50.0%) | 6 (66.7%) | 15 (55.6%) |
| Prior exposure | ||||||
| Lenalidomide | 2 (50.0%) | 6 (100.0%) | 4 (66.7%) | 1 (50.0%) | 6 (66.7%) | 19 (70.4%) |
| Bortezomib | 3 (75.0%) | 5 (83.3%) | 6 (100.0%) | 2 (100.0%) | 8 (88.9%) | 24 (88.9%) |
| Metaphase cytogenetics | ||||||
| Normal | 2 (50.0%) | 2 (33.3%) | 2 (33.3%) | 1 (50.0%) | 3 (33.3%) | 10 (37.0%) |
| Abnormal | 2 (50.0%) | 3 (50.0%) | 4 (66.7%) | 1 (50.0%) | 5 (55.6%) | 15 (55.6%) |
| Not done | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 2 (7.4%) |
| FISH result | ||||||
| 13q- | 2 | 2 | 2 | 0 | 3 | 9 |
| 17p- | 1 | 1 | 1 | 0 | 2 | 5 |
| t(11;14) | 2 | 2 | 1 | 0 | 1 | 6 |
| t(4;14) | 1 | 0 | 1 | 0 | 0 | 2 |
| t(14;16) | 0 | 0 | 0 | 1 | 1 | 2 |
| . | 30 mg/m2 (N = 4) . | 40 mg/m2 (N = 6) . | 50 mg/m2 (N = 6) . | Original 50 mg/m2 (N = 2) . | Phase 2 (50 mg/m2) (N = 9) . | Total (N = 27) . |
|---|---|---|---|---|---|---|
| Age | ||||||
| Median (range) | 70.0 (57.0-76.0) | 62.5 (49.0-69.0) | 61.5 (59.0-75.0) | 72.5 (70.0-75.0) | 70.0 (57.0-76.0) | 66.0 (49.0-81.0) |
| Gender | ||||||
| Female | 1 (25.0%) | 4 (66.7%) | 3 (50.0%) | 0 (0.0%) | 1 (25.0%) | 13 (48.1%) |
| Male | 3 (75.0%) | 2 (33.3%) | 3 (50.0%) | 2 (100.0%) | 3 (75.0%) | 14 (51.9%) |
| Months from diagnosis to registration | ||||||
| Median (range) | 31.9 (20.9-47.1) | 43.8 (10.1-67.9) | 66.7 (6.7-134.3) | 113.6 (20.1-207.1) | 31.9 (20.9-47.1) | 41.6 (6.7-207.1) |
| International staging | ||||||
| Stage 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 1 (3.7%) |
| Stage 2 | 3 (75.0%) | 2 (33.3%) | 3 (50.0%) | 1 (50.0%) | 2 (22.2%) | 11 (40.7%) |
| Stage 3 | 1 (25.0%) | 4 (66.7%) | 3 (50.0%) | 1 (50.0%) | 6 (66.7%) | 15 (55.6%) |
| Prior exposure | ||||||
| Lenalidomide | 2 (50.0%) | 6 (100.0%) | 4 (66.7%) | 1 (50.0%) | 6 (66.7%) | 19 (70.4%) |
| Bortezomib | 3 (75.0%) | 5 (83.3%) | 6 (100.0%) | 2 (100.0%) | 8 (88.9%) | 24 (88.9%) |
| Metaphase cytogenetics | ||||||
| Normal | 2 (50.0%) | 2 (33.3%) | 2 (33.3%) | 1 (50.0%) | 3 (33.3%) | 10 (37.0%) |
| Abnormal | 2 (50.0%) | 3 (50.0%) | 4 (66.7%) | 1 (50.0%) | 5 (55.6%) | 15 (55.6%) |
| Not done | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 2 (7.4%) |
| FISH result | ||||||
| 13q- | 2 | 2 | 2 | 0 | 3 | 9 |
| 17p- | 1 | 1 | 1 | 0 | 2 | 5 |
| t(11;14) | 2 | 2 | 1 | 0 | 1 | 6 |
| t(4;14) | 1 | 0 | 1 | 0 | 0 | 2 |
| t(14;16) | 0 | 0 | 0 | 1 | 1 | 2 |
Patient disposition and treatment exposure
The 27 patients analyzed received a median of 2 cycles (range, 1-12) of treatment. All patients discontinued treatment; discontinuations were due to disease progression (18 patients), alternative treatment (2 patients), adverse event (4 patients), completed study per protocol (1 patient), other medical problems (1 patient), and refused further treatment (1 patient). Among the 27 enrolled patients, 21 patients had disease progression, and 15 patients died. Median follow-up for patients still alive is 14.5 months (range, 8.2-21.7 months).
MTD determination
Overall, 16 patients were enrolled at the 3 dose levels in the dose escalation phase, including 6 patients at 50 mg/m2, the highest dose tested, and the same dose used for the initial 2 patients prior to modification. One dose-limiting toxicity was seen over all dose levels, which was a patient at 40 mg/m2 who experienced grade 3 constipation possibly related to treatment; 50 mg/m2 was determined to be the MTD for the phase 2 portion.
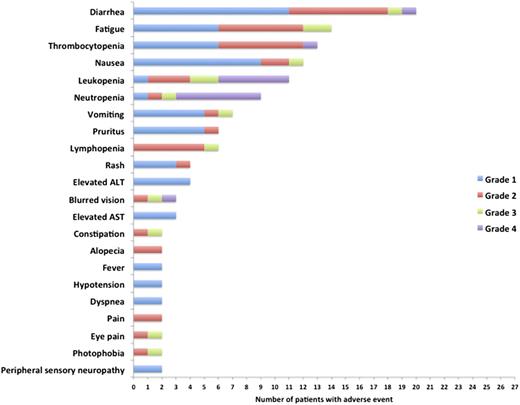
Adverse events and dose delays
Two of the first 3 patients accrued experienced grade 4 adverse events in cycle 1: one patient developed leukopenia and neutropenia, and the other patient developed leukopenia, neutropenia, and stomatitis. These events prompted the safety analysis dose escalation portion of the trial to be added. The most common toxicities observed across the study included hematologic toxicity (leukopenia and thrombocytopenia), gastrointestinal toxicity (nausea and diarrhea), and fatigue. The frequencies of grade 3 and 4 toxicities are as shown in Figure 1. Two patients reported a constellation of blurry vision, photophobia, and eye pain. Detailed evaluation in one of the patients was suggestive of keratitis, and the symptoms resolved in both patients without any sequelae. AEs considered to be at least possibly related to treatment, seen among the 15 patients treated at the phase 2 dose level (50 mg/m2), are as shown in Table 2. Overall, 4 patients had dose delays on 4 cycles due to gastrointestinal intolerance (1 cycle), hematologic toxicity (1 cycle), patient scheduling (1 cycle), and fatigue (1 cycle). Five patients had dosing adjustments on 5 cycles due to nonhematologic adverse events (3 patients, 1 cycle each), gastrointestinal intolerance (1 cycle), and fatigue (1 cycle). Overall, 4 patients discontinued therapy due to toxicities and included 2 patients with ocular toxicities and 1 for nausea and vomiting, and the fourth patient went off the study due to multiple grade 3 toxicities.
The distribution of all adverse events seen in the trial, which were considered at least possibly related to the study drug administration and seen in ≥2 patients across the study.
The distribution of all adverse events seen in the trial, which were considered at least possibly related to the study drug administration and seen in ≥2 patients across the study.
Frequency of adverse events seen at the Phase 2 dose of 50 mg/m2, considered at least possibly related to treatment and seen in ≥2 patients
| Toxicity . | Grade . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All . | ||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Thrombocytopenia | 4 | 26.7 | 4 | 26.7 | 1 | 6.7 | 9 | 60.0 | ||
| Leukopenia | 2 | 13.3 | 1 | 6.7 | 1 | 6.7 | 4 | 26.7 | ||
| Neutropenia | 1 | 6.7 | 1 | 6.7 | 1 | 6.7 | 1 | 6.7 | 4 | 26.7 |
| Lymphopenia | 4 | 26.7 | 4 | 26.7 | ||||||
| Diarrhea | 9 | 60.0 | 2 | 13.3 | 1 | 6.7 | 1 | 6.7 | 13 | 86.7 |
| Nausea | 6 | 40.0 | 2 | 13.3 | 8 | 53.3 | ||||
| Vomiting | 4 | 26.7 | 1 | 6.7 | 5 | 33.3 | ||||
| Fatigue | 5 | 33.3 | 4 | 26.7 | 1 | 6.7 | 10 | 66.7 | ||
| Aspartate aminotransferase increased | 3 | 20.0 | 3 | 20.0 | ||||||
| Alanine aminotransferase increased | 2 | 13.3 | 2 | 13.3 | ||||||
| Peripheral sensory neuropathy | 2 | 13.3 | 2 | 13.3 | ||||||
| Vision blurred | 1 | 6.7 | 1 | 6.7 | 2 | 13.3 | ||||
| Hypotension | 2 | 13.3 | 2 | 13.3 | ||||||
| Pruritus | 2 | 13.3 | 2 | 13.3 | ||||||
| Dry eye syndrome | 1 | 6.7 | 1 | 6.7 | ||||||
| Toxicity . | Grade . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All . | ||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Thrombocytopenia | 4 | 26.7 | 4 | 26.7 | 1 | 6.7 | 9 | 60.0 | ||
| Leukopenia | 2 | 13.3 | 1 | 6.7 | 1 | 6.7 | 4 | 26.7 | ||
| Neutropenia | 1 | 6.7 | 1 | 6.7 | 1 | 6.7 | 1 | 6.7 | 4 | 26.7 |
| Lymphopenia | 4 | 26.7 | 4 | 26.7 | ||||||
| Diarrhea | 9 | 60.0 | 2 | 13.3 | 1 | 6.7 | 1 | 6.7 | 13 | 86.7 |
| Nausea | 6 | 40.0 | 2 | 13.3 | 8 | 53.3 | ||||
| Vomiting | 4 | 26.7 | 1 | 6.7 | 5 | 33.3 | ||||
| Fatigue | 5 | 33.3 | 4 | 26.7 | 1 | 6.7 | 10 | 66.7 | ||
| Aspartate aminotransferase increased | 3 | 20.0 | 3 | 20.0 | ||||||
| Alanine aminotransferase increased | 2 | 13.3 | 2 | 13.3 | ||||||
| Peripheral sensory neuropathy | 2 | 13.3 | 2 | 13.3 | ||||||
| Vision blurred | 1 | 6.7 | 1 | 6.7 | 2 | 13.3 | ||||
| Hypotension | 2 | 13.3 | 2 | 13.3 | ||||||
| Pruritus | 2 | 13.3 | 2 | 13.3 | ||||||
| Dry eye syndrome | 1 | 6.7 | 1 | 6.7 | ||||||
Disease response
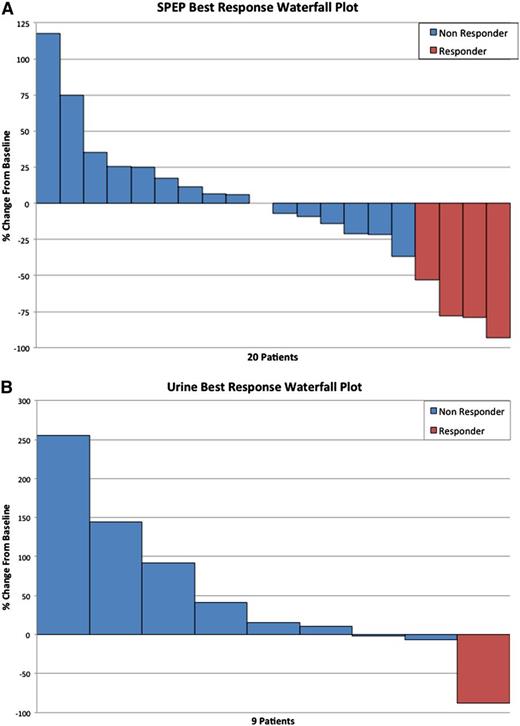
Overall, 5 of 27 (19%) patients had a confirmed minor response or better, including 3 patients (11%) with a PR or better (Table 3). One patient at the 30 mg/m2 dose (1 VGPR), no patients at the original 50 mg/m2 dose, 2 patients at the 40 mg/m2 dose (1 VGPR and 1 PR), 1 patient at the 50 mg/mg2 dose (1 MR), and 1 phase 2 patient (1 MR) responded. The waterfall plot (Figure 2A) demonstrates the spectrum of serum M protein decreases among patients with measurable serum M protein at study entry. Figure 2B demonstrates the M protein kinetics across the disease course in one of the patients with a VGPR as the best response. There were no significant differences between the responding patients and the nonresponding patients with respect to any clinical or laboratory characteristics.
Response to therapy
| Response category . | 30 mg/m2 (N = 4) . | 40 mg/m2 (N = 6) . | All 50 mg/m2 (N = 17) . | All (N = 27) . |
|---|---|---|---|---|
| VGPR | 1 | 1 | 0 | 2 |
| PR | 0 | 1 | 0 | 1 |
| MR | 0 | 0 | 2 | 2 |
| SD | 1 | 4 | 8 | 13 |
| PD | 2 | 0 | 6 | 8 |
| NA | 0 | 0 | 1 | 1 |
| Response category . | 30 mg/m2 (N = 4) . | 40 mg/m2 (N = 6) . | All 50 mg/m2 (N = 17) . | All (N = 27) . |
|---|---|---|---|---|
| VGPR | 1 | 1 | 0 | 2 |
| PR | 0 | 1 | 0 | 1 |
| MR | 0 | 0 | 2 | 2 |
| SD | 1 | 4 | 8 | 13 |
| PD | 2 | 0 | 6 | 8 |
| NA | 0 | 0 | 1 | 1 |
Monoclonal protein response to treatment with dinaciclib. (A) Waterfall plot of the serum M protein responses among patients with a measurable M protein on serum protein electrophoresis at study entry. (B) Waterfall plot limited to patients with 24-hour urine M spike as measurable disease at study entry.
Monoclonal protein response to treatment with dinaciclib. (A) Waterfall plot of the serum M protein responses among patients with a measurable M protein on serum protein electrophoresis at study entry. (B) Waterfall plot limited to patients with 24-hour urine M spike as measurable disease at study entry.
The median progression-free survival across the entire study was 3.5 months (95% confidence interval [CI]: 1.4, 7.3). The median duration of response was 7.7 months (95% CI: 5.3, 8.3), and the median overall survival was 18.8 months (95% CI: 5.9, 23.7).
Discussion
Abnormalities involving regulators of the cell cycle process are common to all cancers and appear to play a prominent role in MM as well. Although the myeloma cells tend to have low proliferative rates compared with many of the other hematologic malignancies, a high proliferative rate when present has been associated with poor outcome in these patients. There exists a strong rationale to target CDKs from a therapeutic perspective, given the universal presence of cyclin dysregulation in the myeloma cell. Several drugs targeting the CDKs have been explored as treatment options in myeloma. The best studied of these drugs is probably flavopiridol, which unfortunately did not demonstrate any clinical activity despite demonstrating potent activity against myeloma cell lines and primary myeloma cells.15,16 Several other CDK inhibitors have been studied recently in the preclinical setting showing promising activity, and some of these molecules are currently undergoing clinical evaluation.17-24
In addition to the critical role of CDKs in the myeloma biology in general, our studies have shown a unique role for CDK5 in the context of therapy with proteasome inhibitors. In an RNA interference-based screen to identify mediators of bortezomib resistance, CDK5 was identified as a top target.11 Subsequent studies in vitro using short interfering RNA-based inhibition of CDK5 or inhibition of CDK5 by dinaciclib resulted in sensitization of myeloma cells to bortezomib- or carfilzomib-induced apoptosis. Detailed mechanistic studies demonstrated this effect to be at least partially mediated by inhibition of one of the proteasome subunits, PSMB5. Interestingly, the highest expression of CDK5 was seen in the myeloma cells and among normal tissues in the neural tissue. The expression of CDK5 was clearly higher in the myeloma cells and myeloma cell lines compared with normal plasma cells or plasma cells from patients with monoclonal gammopathy of undetermined significance (MGUS). Given the CDK inhibition profile of dinaciclib, with CDK5 inhibition occurring at low concentrations, we wanted to explore the activity of the drug in myeloma as an initial step prior to evaluating the drug in combination with bortezomib.
Dinaciclib is one of the first drugs in its class that appear to have single agent activity in myeloma. In the current study, we saw encouraging responses including very good partial responses in patients with relapsed disease. The responses were seen at all dose levels studied, suggesting that MTD doses may not be required for activity. Overall, 1 in 5 patients derived clinical benefit from the single agent dinaciclib, including MRs, which have been shown to be of clinical value in the relapsed population.25 This also increases the options for drug combinations, where overlapping toxicities such as hematologic toxicity may require the use of lower doses. In the current study, we were not able to demonstrate any particular characteristics of these patients that predicted for a response. We examined the relationship between M protein response and the cytogenetic types, the types of previous therapies, type of monoclonal protein (light chain vs intact immunoglobulin), and plasma cell labeling index where available. Other studies examining the relationship between response and expression of cyclins are underway. Dinaciclib also has been shown to have promising clinical activity in the setting of chronic lymphocytic leukemia,13 where phase 3 trials are already underway. In another phase 1 study, PD 0332991, an orally bioavailable, selective inhibitor of CDK4/6, was given in combination with bortezomib and different doses and schedules. The most common treatment-related AEs were thrombocytopenia and neutropenia. One patient achieved a very good partial response, 1 achieved a PR, and 3 had stable disease >3 months.
The toxicity pattern seen here is clearly related to the effect on rapidly cycling cells as highlighted by the hematologic and gastrointestinal toxicities, reminiscent of cytotoxic chemotherapy. The hematologic toxicity seen with the drug prompted us to routinely use growth factor support in these patients, which resulted in reduced hematologic toxicity and allowed timely initiation of treatment cycles. The gastrointestinal side effects were manageable with adequate supportive care. Despite the presence of cdk5 in nerve tissue, we did not notice any specific neurotoxicity.
Given the single agent activity seen with the drug and the promising preclinical data of combining dinaciclib with proteasome inhibitors, we are currently exploring a clinical trial combining dinaciclib with bortezomib. We believe that the combinations may allow patients to derive additional benefit from the proteasome inhibitors by decreasing the resistance to proteasome inhibitors. Continued efforts devoted to the development to rational combinations will be critical in continuing to improve the survival of patients with myeloma.26
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are deeply indebted to the patients who participated in this trial and all investigators and support personnel at all participating trial sites who facilitated patient care and data collection.
Research was supported in part by Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA90628) and the Mayo Phase 2 Consortium (N01-CM-2011-00099).
Authorship
Contribution: S.K.K. was involved in the design of the concept, data collection, analysis, and writing the paper; B.L. and B.F. were involved with the data analysis and writing; and W.J.C., J.Z., N.C., R.F., V.R., C.E., and A.K.S. were involved with enrolling patients and writing the manuscript.
Conflict-of-interest disclosure: S.K.K. has sponsored research from Celgene, Genzyme (Sanofi), Onyx, Millennium, Janssen, and Novartis and also has consulted for Celgene, Genzyme (Sanofi), Onyx, and Millennium. R.F. has received a patent for the prognostication of MM based on genetic categorization of the disease; has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Onyx, Binding Site, Millennium, and AMGEN; and has sponsored research from Celgene and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55906; e-mail: kumar.shaji@mayo.edu