Key Points
KTd is an effective induction and consolidation regimen for transplant-eligible MM patients.
The KTd regimen is safe and well tolerated with a notable lack of peripheral neuropathy.
Abstract
This multicenter phase 2 study of the European Myeloma Network investigated the combination of carfilzomib, thalidomide, and dexamethasone (KTd) as induction/consolidation therapy for transplant-eligible patients with previously untreated multiple myeloma (N = 91). During KTd induction therapy, patients received 4 cycles of carfilzomib 20/27 mg/m2 (n = 50), 20/36 mg/m2 (n = 20), 20/45 mg/m2 (n = 21), or 20/56 mg/m2 (n = 20) on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle; thalidomide 200 mg on days 1 to 28; and dexamethasone 20 mg on days 1, 2, 8, 9, 15, and 16. After autologous stem cell transplantation, patients proceeded to KTd consolidation therapy, where the target doses of carfilzomib were 27 mg/m2, 36 mg/m2, 45 mg/m2, or 56 mg/m2, respectively, and thalidomide 50 mg. Common grade 3/4 adverse events included respiratory (15%), gastrointestinal (12%), and skin disorders (10%); polyneuropathy was infrequent (1%). Complete response rates after induction and consolidation treatment were 25% and 63%, respectively; rates of very good partial response or better after induction and consolidation were 68% and 89%, respectively. At a median follow-up of 23 months, the 36-month progression-free survival rate was 72%. The KTd induction and consolidation regimens were active, safe, and well tolerated. This study was registered at http://www.trialregister.nl as #NTR2422.
Introduction
In transplant-eligible patients with newly diagnosed multiple myeloma (MM), the quality of response following autologous stem cell transplantation (ASCT) has been linked with improved progression-free survival (PFS) and overall survival.1-7 Consequently, appropriate selection of induction and consolidation regimens is essential for achieving the maximum response and improving patient outcomes following ASCT. The guidelines of both the National Comprehensive Care Network and European Society of Medical Oncology recommend induction treatment prior to ASCT, with triplet combinations that incorporate bortezomib and dexamethasone, along with an additional agent (eg, thalidomide, lenalidomide, cyclophosphamide, or doxorubicin).8-10
In particular, the combination of bortezomib, thalidomide, and high-dose (40 mg) dexamethasone (VTD) has been extensively investigated as induction or consolidation therapy for transplant-eligible patients in prospective phase 3 studies,5,11,12 and it is one of the most commonly employed treatments in patients with newly diagnosed MM.13 VTD has shown promising extension of PFS as induction therapy (relative to TD12 ) and when used as consolidation therapy following ASCT (compared with TD or no consolidation therapy13,14 ).
The clinical use of bortezomib within the VTD setting is hampered by the concurrent neurotoxicity profile with thalidomide and the consequent high incidence of grade 2 to 4 peripheral neuropathy (PN). In patients treated with bortezomib-based regimens, PN is a significant cause of treatment discontinuations and dose reductions that can potentially lead to suboptimal outcomes. In a phase 3 study of VTD induction therapy, 60% of patients experienced grade ≥2 PN, 14% experienced grade 3/4 PN, 2% discontinued VTD due to PN, and 25% required bortezomib dose reductions due to a PN adverse event (AE).12 Lower doses of bortezomib5 and thalidomide and/or reduced treatment cycles can reduce the incidence of PN but may also reduce efficacy.12 Subcutaneous administration of bortezomib to patients with relapsed MM is associated with a lower rate of PN without compromising efficacy,15 but this administration route has not been validated when bortezomib is used within VTD combination therapy in patients with newly diagnosed MM.
Treatment regimens with a more favorable safety profile, particularly an improved PN profile, could potentially improve outcomes following induction and consolidation therapy. The combination of bortezomib, lenalidomide, and dexamethasone may have lower rates of PN than VTD,16 but this regimen is not a standard of care for frontline treatment outside of the United States because of a lack of registration. Therefore, combination treatments based on alternative agents are needed in frontline MM therapy. Carfilzomib is a selective proteasome inhibitor that was approved in 2012 in the United States as a single-agent treatment of patients with relapsed and refractory MM. Carfilzomib is currently being investigated in combination with other agents (eg, immunomodulatory agents, histone deacetylase inhibitors, corticosteroids, and/or alkylating agents) for treatment of patients with newly diagnosed MM17-22 as well as patients with relapsed and/or refractory MM.23-27 Specifically, the combination of carfilzomib with lenalidomide and dexamethasone (KRd) has been shown to be safe and tolerable with encouraging activity in patients with newly diagnosed MM18,22 and also in patients with relapsed MM.28 In particular, low rates of PN have been reported using KRd as a frontline regimen (23% all grades, 5.7% grade ≥2).18 This highlights the potential effectiveness of carfilzomib within the framework of frontline combination therapy that uses a proteasome inhibitor, an immunomodulatory agent, and a corticosteroid, such as dexamethasone.
We report herein the results of a phase 2 trial examining the safety and efficacy of carfilzomib plus thalidomide and low-dose (20 mg) dexamethasone (KTd) as induction and consolidation therapy in previously untreated patients with MM. This is the first study to evaluate KTd in transplant-eligible patients with newly diagnosed MM and the first study in this setting to use a carfilzomib-based regimen as both an induction and consolidation treatment strategy.
Patients and methods
Patients
Transplant-eligible patients aged 18 to 65 years with previously untreated MM (International Staging System [ISS] stage 1-3) and World Health Organization (WHO) performance status 0 to 3 could be enrolled. A WHO performance of 3 was allowed only if it was caused by MM rather than a comorbid condition.
Exclusion criteria included grade 3/4 neuropathy or grade 2 painful PN, known intolerance of thalidomide, New York Heart Association class II to IV heart failure, systematic amyloid light-chain amyloidosis, nonsecretory MM, Waldenström macroglobulinemia or immunoglobulin M (IgM) MM, and history of active malignancy during the past 5 years with the exception of basal cell carcinoma or stage 0 cervical cancer. Laboratory exclusion criteria included creatinine clearance <15 mL/min, absolute neutrophil count <1.0 × 109/L, hemoglobin <4.9 mmol/L, and platelet count <75 × 109/L.
The study protocol was approved by the appropriate institutional review boards and ethics committees, and the study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and the European Clinical Trial Directive as implemented in Dutch law. All patients provided written informed consent.
Study design and treatment
This was a multicenter, single-arm, open-label, phase 2 trial. The treatment schema is illustrated in Figure 1. Patients received KTd induction therapy in 28-day cycles for up to 4 cycles. During induction therapy, carfilzomib was administered IV over 2 to 10 minutes on days 1, 2, 8, 9, 15, and 16. Patients were originally enrolled in the first dosing cohort where the target starting dose of carfilzomib was 20 mg/m2 for days 1 and 2 of cycle 1 and was escalated to a target dose of 27 mg/m2 for days 8, 9, 15, and 16 of cycle 1 and for days 1, 2, 8, 9, 15, and 16 of cycles 2 to 4 (20/27 mg/m2). In addition, thalidomide 200 mg was given orally on days 1 to 28 and dexamethasone 20 mg was given orally on days 1, 2, 8, 9, 15, and 16. Following KTd induction therapy, all eligible patients underwent stem cell harvest (SCH) after priming with 4-g/m2 IV cyclophosphamide and daily 10-µg/kg granulocyte colony-stimulating factor. After treatment with high-dose melphalan (HDM; 200 mg/m2) and ASCT, patients received 4 cycles of KTd consolidation therapy. The schedule for the consolidation regimen was the same as the schedule for the induction regimen, except that the target dose of carfilzomib was 27 mg/m2, 36 mg/m2, or 45 mg/m2 on days 1 and 2 of cycle 1 and the target dose of thalidomide was 50 mg.
Dosing schedule and treatment schema. Patients received up to 4 cycles (28 days each) of KTd induction therapy before initiating SCH, HDM, and ASCT. Carfilzomib was administered IV over 2 to 10 minutes on days 1, 2, 8, 9, 15, and 16 at a starting dose of 20 mg/m2 for days 1 and 2 of cycle 1 and escalated to a target dose of 27 mg/m2 (cohort 1), 36 mg/m2 (cohort 2), 45 mg/m2 (cohort 3), or 56 mg/m2 (cohort 4, not shown) for days 8, 9, 15, and 16 of cycle 1 and days 1, 2, 8, 9, 15, and 16 of cycles 2 to 4. Thalidomide 200 mg was administered on days 1 to 28, and dexamethasone 20 mg was administered on days 1, 2, 8, 9, 15, and 16. SCH was performed using cyclophosphamide 2 to 4 g/m2 and granulocyte colony-stimulating factor according to institutional protocols. After ASCT, up to 4 cycles (28 days each) of consolidation therapy were initiated. The KTd consolidation regimen was similar to the induction regimen, except that the target doses of carfilzomib and thalidomide were 27 mg/m2 and 50 mg, respectively.
Dosing schedule and treatment schema. Patients received up to 4 cycles (28 days each) of KTd induction therapy before initiating SCH, HDM, and ASCT. Carfilzomib was administered IV over 2 to 10 minutes on days 1, 2, 8, 9, 15, and 16 at a starting dose of 20 mg/m2 for days 1 and 2 of cycle 1 and escalated to a target dose of 27 mg/m2 (cohort 1), 36 mg/m2 (cohort 2), 45 mg/m2 (cohort 3), or 56 mg/m2 (cohort 4, not shown) for days 8, 9, 15, and 16 of cycle 1 and days 1, 2, 8, 9, 15, and 16 of cycles 2 to 4. Thalidomide 200 mg was administered on days 1 to 28, and dexamethasone 20 mg was administered on days 1, 2, 8, 9, 15, and 16. SCH was performed using cyclophosphamide 2 to 4 g/m2 and granulocyte colony-stimulating factor according to institutional protocols. After ASCT, up to 4 cycles (28 days each) of consolidation therapy were initiated. The KTd consolidation regimen was similar to the induction regimen, except that the target doses of carfilzomib and thalidomide were 27 mg/m2 and 50 mg, respectively.
The dose and schedule of carfilzomib in the first dosing cohort (20/27 mg/m2) were selected based on results from large single-arm studies of single-agent carfilzomib in relapsed and/or refractory MM, demonstrating that this regimen is effective and well tolerated without cumulative toxicity.29,30 Thalidomide and dexamethasone dosing was based on the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’Adulto) trial experience.11 Because the results of the lowest dosing cohort (carfilzomib 20/27 mg/m2) were promising, 3 additional dose levels (carfilzomib 20/36 mg/m2, 20/45 mg/m2, and 20/56 mg/m2) were added via amendments. The fourth cohort (n = 20), in which carfilzomib was escalated to a target dose of 56 mg/m2 in the induction and consolidation schedule, was recently completed and will be reported later.
Patients were required to maintain adequate hydration during cycle 1 to reduce the risk of tumor lysis syndrome. Oral hydration approximately equal to 30 mL/kg per day was initiated 48 hours before the first dose of carfilzomib. In addition, 250 to 500 mL of IV fluids was given before and after each carfilzomib dose in cycle 1. If lactate dehydrogenase or uric acid was elevated at day 1 of cycle 2, then IV hydration was repeated for cycle 2. The goal of the hydration program was to maintain robust urinary output (ie, ≥2 L/day). Patients at high risk for tumor lysis syndrome were also permitted to receive allopurinol or rasburicase prophylaxis. Patients were recommended to receive antibiotic prophylaxis with ciprofloxacin or other fluoroquinolone (or trimethoprim/sulfamethoxazole if fluoroquinolones were contraindicated). In addition, patients were recommended to receive acyclovir or similar anti-varicella agent prophylaxis. Bisphosphonates and erythropoietic agents were permitted during the study. If clinically indicated, patients were allowed to receive red blood cell or platelet infusions and palliative radiation therapy. Antithrombotic prophylaxis consisted of aspirin or low-molecular-weight heparin.
The primary objective of the study was to establish the response to carfilzomib in combination with thalidomide and dexamethasone in patients with MM at first presentation. To support this primary objective, the primary end point of the study was to determine the proportion of patients who obtained a complete response (CR) or very good partial response (VGPR) after induction therapy. Secondary objectives of the study included investigation of the clinical efficacy and toxicity of KTd in remission induction and consolidation treatment of MM at first presentation, effect of KTd induction therapy on SCH, and PFS. The secondary end points to support these objectives included the improvement of response after ASCT and consolidation therapy, PFS, evaluation of SCH success, and the safety of the induction and consolidation treatments.
This study was registered at http://www.trialregister.nl as #NTR2422.
Assessments
Response assessments were conducted after each induction cycle and at 2-month intervals during consolidation treatment. Responses were based on assessments by study investigators and were classified according to International Myeloma Working Group Uniform Response Criteria,31 with categories for CR, VGPR, and partial response (PR). Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria of Adverse Events, version 4.0.
Clinical and molecular assessment consisted of the analysis of bone marrow aspirate which was conducted at screening to quantify myeloma cell involvement, and the conduction of cytogenetics and fluorescence in situ hybridization studies. The following cytogenetic abnormalities in CD138+ purified MM cells were evaluated as prognostic variables: 1p/q, t(4;14)(p16;q32), t(14;16)(q32;q23), del(13q), del(17p), numerical abnormalities 9 or 11 (ie, hyperdiploidy), and complex cytogenetic abnormalities.
Statistical analysis
All analyses were performed according to the intention-to-treat principle that was restricted to eligible patients. A true CR + VGPR rate of ≥45% after induction treatment was considered necessary to show sufficient therapeutic activity, whereas a true CR + VGPR rate of ≤25% was considered too low to warrant further investigation in future clinical trials. To detect this clinically relevant CR + VGPR rate with power 1 − β = 0.80 (2-sided significance level α = 0.05), it was determined that ≥41 patients should be included in the study. To test the null hypothesis proportion of 25% vs the alternative hypothesis proportion of 45%, a 2-sided 95% confidence interval (CI) was constructed around the observed CR + VGPR rate after induction treatment. The study was deemed successful if the lower boundary of the 95% CI was >25%.
Exploratory subgroup analyses evaluated the potential effect of risk status, defined by cytogenetic/ fluorescence in situ hybridization criteria and ISS stage, on the response to KTd therapy. Patients were considered to be at high risk if they had t(4;14) and/or del(17p) and/or add1q and/or ISS stage 3.32
Time-to-event end points were analyzed using the Kaplan-Meier method,33 and descriptive statistics were used to summarize continuous and categorical data. PFS was defined as the time from registration to progression or death from any cause.31 Patients known to be alive and free of progression at the last day of contact were censored. Statistical analysis was performed using Stata v13.1 software (StataCorp, College Station, TX). All authors had access to the primary clinical trial data reported herein.
Results
Patients and treatment
This multicenter study was performed at a total of 8 Dutch centers. A total of 91 patients (27 mg/m2: n = 50; 36 mg/m2: n = 20; 45 mg/m2: n = 21) were enrolled between September 16, 2010, and May 30, 2013, and the database was closed for analysis as of September 9, 2014. Two patients did not fully comply with the eligibility criteria (1 was 66 years of age, and the other had nonsecretory MM). Their inclusion was discussed with and approved by the principal investigator. Baseline demographic and disease characteristics are summarized in Table 1. Patients ranged in age from 29 to 66 years, with a median age of 58 years. The majority of patients were male (67%). A total of 45% of patients had a WHO performance status of 1 or 2; the status of 7% was unknown. Thirty-eight percent of patients were considered to be at high risk based on ISS stage and cytogenetics, whereas another 40% of patients were considered to be at standard risk (Table 2 and supplemental Table 1 available at the Blood Web site). The remaining 22% of patients had an unknown risk status, mainly due to missing cytogenetics. A total of 7 of 81 patients (9%) had a history of grade 1/2 PN at study entry, whereas 10 patients did not have a baseline assessment of PN recorded. Baseline median creatinine clearance was 60 mL/min (range, 26-118 mL/min), and the baseline median hemoglobin level was 7.0 mmol/L (range, 4.3-10.4 mmol/L).
Baseline patient characteristics
| Characteristic . | N = 91 . |
|---|---|
| Male, n (%) | 61 (67) |
| Age | |
| Median (range), years | 58 (29-66) |
| ISS stage, n (%) | |
| 1 | 38 (42) |
| 2 | 31 (34) |
| 3 | 22 (24) |
| WHO performance status, n (%) | |
| 0 | 42 (46) |
| 1 | 37 (41) |
| 2 | 4 (4) |
| 3 | 2 (2) |
| Unknown | 6 (7) |
| M-protein isotype, n (%) | |
| IgA | 20 (22) |
| IgG | 48 (53) |
| IgD | 3 (3) |
| Light-chain disease | 17 (19) |
| Unknown | 3 (3) |
| M-protein light chain, n (%) | |
| κ | 59 (65) |
| Λ | 29 (32) |
| Unknown | 3 (3) |
| Lytic bone lesions, n (%) | |
| 0 | 22 (24) |
| 1 | 8 (9) |
| 2 | 6 (7) |
| ≥3 | 53 (58) |
| Unknown | 2 (2) |
| Genetic abnormalities, n (%)* | |
| add1q | 11 (15) |
| t(4;14)(p16;q32) | 4 (5) |
| del(17p13) | 8 (11) |
| Risk status, n (%)† | |
| Standard | 36 (40) |
| High | 35 (38) |
| Unknown | 20 (22) |
| Grade 1/2 PNP‡ | 7 (9) |
| Median β2-microglobulin (range), mg/L | 3.6 (1.4-23.6) |
| Median hemoglobin (range), mmol/L | 7.0 (4.3-10.4) |
| Median calcium (range), mmol/L | 2.3 (2.0-4.1) |
| Median creatinine (range), µmol/L | 79 (40-345) |
| Median creatinine clearance (range), mL/min§ | 60 (26-118) |
| Characteristic . | N = 91 . |
|---|---|
| Male, n (%) | 61 (67) |
| Age | |
| Median (range), years | 58 (29-66) |
| ISS stage, n (%) | |
| 1 | 38 (42) |
| 2 | 31 (34) |
| 3 | 22 (24) |
| WHO performance status, n (%) | |
| 0 | 42 (46) |
| 1 | 37 (41) |
| 2 | 4 (4) |
| 3 | 2 (2) |
| Unknown | 6 (7) |
| M-protein isotype, n (%) | |
| IgA | 20 (22) |
| IgG | 48 (53) |
| IgD | 3 (3) |
| Light-chain disease | 17 (19) |
| Unknown | 3 (3) |
| M-protein light chain, n (%) | |
| κ | 59 (65) |
| Λ | 29 (32) |
| Unknown | 3 (3) |
| Lytic bone lesions, n (%) | |
| 0 | 22 (24) |
| 1 | 8 (9) |
| 2 | 6 (7) |
| ≥3 | 53 (58) |
| Unknown | 2 (2) |
| Genetic abnormalities, n (%)* | |
| add1q | 11 (15) |
| t(4;14)(p16;q32) | 4 (5) |
| del(17p13) | 8 (11) |
| Risk status, n (%)† | |
| Standard | 36 (40) |
| High | 35 (38) |
| Unknown | 20 (22) |
| Grade 1/2 PNP‡ | 7 (9) |
| Median β2-microglobulin (range), mg/L | 3.6 (1.4-23.6) |
| Median hemoglobin (range), mmol/L | 7.0 (4.3-10.4) |
| Median calcium (range), mmol/L | 2.3 (2.0-4.1) |
| Median creatinine (range), µmol/L | 79 (40-345) |
| Median creatinine clearance (range), mL/min§ | 60 (26-118) |
Ig, immunoglobulin; PNP, polyneuropathy.
A total of 74 patients were evaluable for cytogenetics.
High-risk patients had ISS stage 3 disease and/or del17p and/or t(4;14) and/or add1q cytogenetic abnormalities. The remaining patients with available ISS status and cytogenetics were considered to have a standard risk.
Not recorded in 10 patients.
Evaluated from 36 patients.
Response after induction, after HDM, and after consolidation by carfilzomib dosing level and risk status
| . | Dosing level of carfilzomib . | Risk status by cytogenetics and ISS stage . | ||||
|---|---|---|---|---|---|---|
| 20/27 mg/m2 . | 20/36 mg/m2 . | 20/45 mg/m2 . | All patients . | Standard risk* . | High risk* . | |
| Patients, n | 50 | 20 | 21 | 91 | 36 | 35 |
| Response after induction, n (%) | ||||||
| CR | 10 (20) | 6 (30) | 7 (33) | 23 (25) | 3 (8) | 9 (26) |
| ≥VGPR | 28 (56) | 17 (85) | 17 (81) | 62 (68) | 25 (69) | 22 (63) |
| ≥PR | 46 (92) | 18 (90) | 18 (86) | 82 (90) | 34 (94) | 32 (91) |
| Response after HDM, n (%) | ||||||
| CR | 14 (28) | 8 (40) | 8 (38) | 30 (33) | 9 (25) | 14 (40) |
| ≥VGPR | 32 (64) | 18 (90) | 19 (90) | 69 (76) | 29 (81) | 25 (71) |
| ≥PR | 47 (94) | 20 (100) | 20 (95) | 87 (96) | 34 (94) | 33 (94) |
| Response after consolidation, n (%) | ||||||
| CR | 29 (58) | 14 (70) | 14 (67) | 57 (63) | 21 (58) | 23 (66) |
| ≥VGPR | 43 (86) | 18 (90) | 20 (95) | 81 (89) | 31 (86) | 30 (86) |
| ≥PR | 47 (94) | 20 (100) | 20 (95) | 87 (96) | 34 (94) | 33 (94) |
| . | Dosing level of carfilzomib . | Risk status by cytogenetics and ISS stage . | ||||
|---|---|---|---|---|---|---|
| 20/27 mg/m2 . | 20/36 mg/m2 . | 20/45 mg/m2 . | All patients . | Standard risk* . | High risk* . | |
| Patients, n | 50 | 20 | 21 | 91 | 36 | 35 |
| Response after induction, n (%) | ||||||
| CR | 10 (20) | 6 (30) | 7 (33) | 23 (25) | 3 (8) | 9 (26) |
| ≥VGPR | 28 (56) | 17 (85) | 17 (81) | 62 (68) | 25 (69) | 22 (63) |
| ≥PR | 46 (92) | 18 (90) | 18 (86) | 82 (90) | 34 (94) | 32 (91) |
| Response after HDM, n (%) | ||||||
| CR | 14 (28) | 8 (40) | 8 (38) | 30 (33) | 9 (25) | 14 (40) |
| ≥VGPR | 32 (64) | 18 (90) | 19 (90) | 69 (76) | 29 (81) | 25 (71) |
| ≥PR | 47 (94) | 20 (100) | 20 (95) | 87 (96) | 34 (94) | 33 (94) |
| Response after consolidation, n (%) | ||||||
| CR | 29 (58) | 14 (70) | 14 (67) | 57 (63) | 21 (58) | 23 (66) |
| ≥VGPR | 43 (86) | 18 (90) | 20 (95) | 81 (89) | 31 (86) | 30 (86) |
| ≥PR | 47 (94) | 20 (100) | 20 (95) | 87 (96) | 34 (94) | 33 (94) |
High-risk patients are those with t(4;14) and/or del(17p) and/or add1q and/or ISS stage 3 disease. Standard-risk patients are all remaining patients, excluding 20 patients whose risk status was unknown.
The flow of patients through the protocol and adherence to each stage of treatment is shown in Figure 2. All 91 registered patients started KTd induction therapy, and 83 patients continued on to receive cyclophosphamide priming treatment. Five patients discontinued induction therapy because of the following AEs: grade 3 rash attributable to thalidomide, grade 2 fever with sepsis, grade 1 hyponatremia, and grade 2 exanthema. Two patients discontinued treatment because of progressive disease, and 1 patient was not eligible for further treatment. SCH was successful in 81 of 83 mobilized patients (98%) with >3 × 106 CD34+ yield. Two patients were not eligible for HDM treatment: 1 had insufficient CD34+ yield, and the other had progressive disease after SCH. Two additional patients discontinued treatment after SCH: 1 declined treatment and the other had a nonrelated disease. A total of 79 patients received HDM (200 mg/m2) and ASCT. HDM/ASCT was performed with complete hematologic recovery in all patients. A total of 75 patients initiated the consolidation regimen. Four patients were not eligible for consolidation treatment owing to a long post-ASCT recovery (n = 1), progression (n = 1), refusal (n = 1), or nonrelated disease (n = 1). Four patients completed 2 or 3 cycles of KTd consolidation therapy and discontinued treatment due to disease progression (n = 2), thrombotic thrombocytopenic purpura (n = 1), and overall worsening of constitution (n = 1), respectively. The remaining 71 patients completed all 4 consolidation cycles. Median follow-up from registration was 23.2 months (range, 5.3-44.1 months).
Patient disposition and flow through the study protocol. Cyclo, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; HD, high-dose; SC, stem cell.
Patient disposition and flow through the study protocol. Cyclo, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; HD, high-dose; SC, stem cell.
Efficacy
The response to induction, HDM/ASCT, and consolidation therapy is shown in Table 2. In the overall population (N = 91), 25% of patients achieved a CR, 68% achieved at least a VGPR, and 90% at least a PR after induction therapy. The 95% CI for the ≥VGPR rate after induction therapy was 51% to 72%, leading to a rejection of the null hypothesis of a ≤25% ≥VGPR rate in favor of the alternative hypothesis of a ≥45% rate of ≥VGPR after induction treatment. Furthermore, responses were rapid and increased with additional treatment. The majority of patients (74%) achieved ≥PR within the first induction cycle, and 93% achieved ≥PR after 2 induction cycles. The ≥VGPR rate increased from 68% after induction therapy to 76% after HDM/ASCT, and finally to 89% after 4 cycles of consolidation therapy. After consolidation therapy, standard-risk and high-risk patients (defined by cytogenetics and ISS stage) had similar CR rates (58% vs 66%, respectively). There was no difference in response among the dosing levels (Table 2).
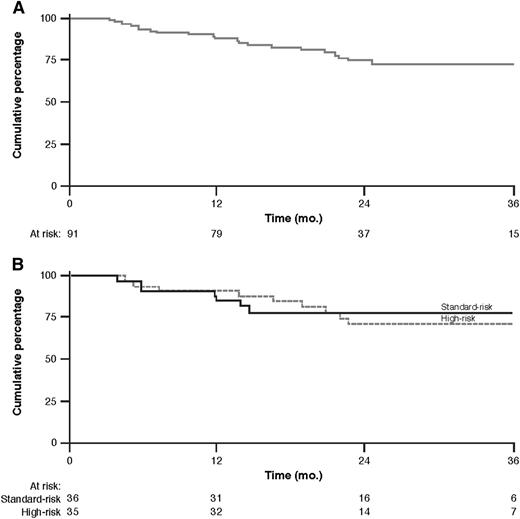
Median PFS was not reached. The PFS rate at 36 months was 72% (95% CI, 60% to 81%) as shown by the Kaplan-Meier curve in Figure 3. Six patients died due to progressive disease.
PFS. Kaplan-Meier curve of PFS for all patients (A) and by risk group (B).
Safety
The incidence of AEs occurring during induction therapy and across all treatment cycles is summarized in Table 3. Any grade blood and lymphatic system disorder AE occurring during induction therapy was reported in 8% of patients, whereas grade 3/4 blood and lymphatic system disorder AEs were reported in 5%. These rates increased to 16% and 7%, respectively, at the completion of consolidation therapy. Dermatologic, respiratory, and gastrointestinal disorders were among the most common nonhematologic grade 3/4 AEs experienced during all treatment cycles, affecting 10%, 15%, and 12% of patients, respectively. At the lowest dose level, skin rash was observed in 40% of patients, which was attributed to the use of cotrimoxazole and was not allowed in later cohorts. Whereas 9% of patients had preexisting grade 1/2 PNP at study entry, any grade PNP was experienced by 40% of patients during all treatment cycles. Grade ≥2 PNP events were reported in 18% of patients, 1 patient (1%) experienced grade 3 PNP, and there were no reports of grade 4 PNP over all treatment cycles. Most cases of PNP were deemed to be thalidomide-related; of 59 PNP AEs reported, 47 were at least possibly related to thalidomide. Any cardiac-related AE was reported in 16% of patients after induction therapy. Ten of these cardiac events were grade 1/2, and the remaining 5 were grade 3. After consolidation therapy, the rate of any cardiac-related AE increased to 19%; 5% of patients experienced a grade 3 cardiac event, and no grade 4 events were reported. Grade 3 cardiac events consisted of heart failure (n = 3), dyspnea (n = 1), and chest pain (n = 1). Two cardiac-related AEs (grade 2 atrial fibrillation and grade 3 dyspnea) were possibly caused by carfilzomib, 1 cardiac AE (grade 1 atrial flutter) was attributed to thalidomide, and 20 cardiac AEs were considered unrelated to treatment or could not be attributed to a specific treatment. Grade 3 or 4 respiratory adverse events included infection/pneumonia (n = 6), dyspnea (n = 7), and pulmonary embolism (n = 2), all of which resolved.
Treatment-emergent adverse events during therapy
| Toxicity . | Induction therapy (N = 91) . | All treatment cycles (N = 91) . | |||
|---|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | SAE . | |
| Hematologic | |||||
| Blood and lymphatic system disorders | 7 (8) | 5 (5) | 15 (16) | 6 (7) | 3 (3) |
| Nonhematologic | |||||
| Gastrointestinal disorders | 49 (54) | 2 (2) | 66 (73) | 11 (12) | 8 (9) |
| General disorders and administration site conditions | 53 (58) | 2 (2) | 62 (68) | 5 (5) | 12 (13) |
| Respiratory, thoracic, and mediastinal disorders | 35 (38) | 9 (10) | 50 (54) | 14 (15) | 9 (10) |
| PNP | 33 (36) | 0 (0) | 36 (40) | 1 (1) | 2 (2) |
| Skin and subcutaneous tissue disorders | 36 (40) | 7 (8) | 41 (45) | 9 (10) | 2 (2) |
| Musculature, skeletal, and connective tissue disorders | 21 (23) | 1 (1) | 38 (42) | 4 (4) | 4 (4) |
| Vascular disorders | 24 (26) | 5 (5) | 27 (30) | 6 (7) | 2 (2) |
| Cardiac disorders | 15 (16) | 5 (5) | 17 (19) | 5 (5) | 5 (5) |
| Infections and infestations | 12 (13) | 0 (0) | 33 (36) | 3 (3) | 0 (0) |
| Metabolism and nutrition disorders | 16 (18) | 7 (8) | 19 (21) | 8 (9) | 7 (8) |
| Investigations | 6 (7) | 4 (4) | 12 (13) | 9 (10) | 0 (0) |
| Eye disorders | 9 (10) | 1 (1) | 15 (16) | 1 (1) | 0 (0) |
| Psychiatric disorders | 7 (8) | 0 (0) | 10 (11) | 0 (0) | 0 (0) |
| Renal and urinary disorders | 7 (8) | 3 (3) | 10 (11) | 3 (3) | 4 (4) |
| Endocrine disorders | 2 (2) | 0 (0) | 3 (3) | 1 (1) | 1 (1) |
| Surgical and medical procedures | 1 (1) | 1 (1) | 3 (3) | 1 (1) | 1 (1) |
| Toxicity . | Induction therapy (N = 91) . | All treatment cycles (N = 91) . | |||
|---|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | SAE . | |
| Hematologic | |||||
| Blood and lymphatic system disorders | 7 (8) | 5 (5) | 15 (16) | 6 (7) | 3 (3) |
| Nonhematologic | |||||
| Gastrointestinal disorders | 49 (54) | 2 (2) | 66 (73) | 11 (12) | 8 (9) |
| General disorders and administration site conditions | 53 (58) | 2 (2) | 62 (68) | 5 (5) | 12 (13) |
| Respiratory, thoracic, and mediastinal disorders | 35 (38) | 9 (10) | 50 (54) | 14 (15) | 9 (10) |
| PNP | 33 (36) | 0 (0) | 36 (40) | 1 (1) | 2 (2) |
| Skin and subcutaneous tissue disorders | 36 (40) | 7 (8) | 41 (45) | 9 (10) | 2 (2) |
| Musculature, skeletal, and connective tissue disorders | 21 (23) | 1 (1) | 38 (42) | 4 (4) | 4 (4) |
| Vascular disorders | 24 (26) | 5 (5) | 27 (30) | 6 (7) | 2 (2) |
| Cardiac disorders | 15 (16) | 5 (5) | 17 (19) | 5 (5) | 5 (5) |
| Infections and infestations | 12 (13) | 0 (0) | 33 (36) | 3 (3) | 0 (0) |
| Metabolism and nutrition disorders | 16 (18) | 7 (8) | 19 (21) | 8 (9) | 7 (8) |
| Investigations | 6 (7) | 4 (4) | 12 (13) | 9 (10) | 0 (0) |
| Eye disorders | 9 (10) | 1 (1) | 15 (16) | 1 (1) | 0 (0) |
| Psychiatric disorders | 7 (8) | 0 (0) | 10 (11) | 0 (0) | 0 (0) |
| Renal and urinary disorders | 7 (8) | 3 (3) | 10 (11) | 3 (3) | 4 (4) |
| Endocrine disorders | 2 (2) | 0 (0) | 3 (3) | 1 (1) | 1 (1) |
| Surgical and medical procedures | 1 (1) | 1 (1) | 3 (3) | 1 (1) | 1 (1) |
Data are n (%) of patients.
Serious AEs (SAEs) were reported in 21 patients in cohort 1, 8 patients in cohort 2, and 7 patients in cohort 3. Therefore, 40% of patients reported at least 1 SAE. The most common SAE was fever (11 patients, 12%).
Overall, the KTd regimen was well tolerated. As noted earlier, only 5 patients (5%) discontinued induction therapy because of excessive toxicity. There were no toxicity-related treatment discontinuations during consolidation therapy. An analysis of treatment adherence to each of the study drugs composing the KTd regimen is shown in Table 4. During the induction phase, the normal treatment completion rate was 59% for carfilzomib, 52% for thalidomide, and 78% for dexamethasone. Dose delays, reductions, and/or interruptions of carfilzomib, thalidomide, and dexamethasone were observed in 35%, 38%, and 16% of patients, respectively. Carfilzomib dose reductions were required in 5% of patients because of preexisting renal insufficiency (n = 1), PNP (n = 1), tumor lysis syndrome (n = 1), chest pain (n = 1), or unknown reasons (n = 1). Premature stoppage of carfilzomib, thalidomide, and dexamethasone administration occurred in 5%, 10%, and 5% of patients, respectively. In general, for carfilzomib and thalidomide, normal completion rates for each of the study drugs were higher during consolidation treatment cycles compared with induction treatment cycles, whereas dose delays, reductions, and/or interruptions were lower compared with induction treatment cycles. Dexamethasone treatment adherence was similar for induction and consolidation treatment cycles.
Cumulative adherence to treatment protocol
| . | Induction (N = 91) . | Consolidation (N = 75) . |
|---|---|---|
| Carfilzomib treatment adherence | ||
| Normal completion | 54 (59) | 52 (69) |
| Dose delay, reduction and/or interruption | 32 (35) | 19 (25) |
| Premature stop | 5 (5) | 4 (5) |
| Thalidomide treatment adherence | ||
| Normal completion | 47 (52) | 56 (75) |
| Dose delay, reduction, and/or interruption | 35 (38) | 9 (12) |
| Premature stop | 9 (10) | 10 (13) |
| Dexamethasone treatment adherence | ||
| Normal completion | 71 (78) | 55 (73) |
| Dose delay, reduction, and/or interruption | 15 (16) | 15 (20) |
| Premature stop | 5 (5) | 5 (7) |
| . | Induction (N = 91) . | Consolidation (N = 75) . |
|---|---|---|
| Carfilzomib treatment adherence | ||
| Normal completion | 54 (59) | 52 (69) |
| Dose delay, reduction and/or interruption | 32 (35) | 19 (25) |
| Premature stop | 5 (5) | 4 (5) |
| Thalidomide treatment adherence | ||
| Normal completion | 47 (52) | 56 (75) |
| Dose delay, reduction, and/or interruption | 35 (38) | 9 (12) |
| Premature stop | 9 (10) | 10 (13) |
| Dexamethasone treatment adherence | ||
| Normal completion | 71 (78) | 55 (73) |
| Dose delay, reduction, and/or interruption | 15 (16) | 15 (20) |
| Premature stop | 5 (5) | 5 (7) |
Discussion
Results from this multicenter phase 2 study demonstrated that the KTd regimen was active, safe, and well tolerated as induction and consolidation therapy in newly diagnosed MM patients planning to undergo ASCT. The response after induction therapy was encouraging, with 68% of patients achieving at least a VGPR, thus meeting the study’s primary end point of a ≥45% ≥VGPR rate. Moreover, responses were rapid and improved with HDM/ASCT and consolidation therapy. A high CR rate (63%) was seen after consolidation therapy and was generally similar between high- and standard-risk groups, based on cytogenetics and ISS stage. With a median follow-up of 23 months, the PFS rate at 36 months was 72%. KTd induction therapy did not adversely affect the feasibility of ASCT. Notably, PNP was mostly grade 1/2, and the majority of cases were attributable to thalidomide. Grade 3/4 PNP was reported infrequently (1%). These data are consistent with the PN rates reported from studies using other carfilzomib combinations in the frontline setting for both transplant-eligible17-19,22 and ineligible patients.20,21 Taken together, these data highlight the potential effectiveness of KTd as a frontline regimen for transplant-eligible patients with previously untreated MM.
Although cross-trial comparisons should be interpreted with caution, the postinduction response data (overall response rate [ORR], 90%; ≥VGPR, 68%) compare favorably in relation to the rates reported from studies with frontline VTD and TD. The ORR of TD in 2 randomized trials was 63%,34,35 while the ≥VGPR rate was 44%.35 In 2 phase 3 trials of VTD induction (GIMEMA and PETHEMA/GEM [Programa para el Estudio y la Terapéutica de las Hemopatías Malignas/Grupo Español de Mieloma]), ORRs of 85% and 93% and ≥VGPR rates of 60% and 62% were reported.11,12 A recent retrospective study assessed the efficacy of both VTD induction and consolidation therapy for the treatment of newly diagnosed MM.13 After consolidation, the CR rate was 52% and the ≥VGPR rate was 83%, which are similar to the postconsolidation rates reported here (63% and 89% for CR and ≥VGPR, respectively).
KTd was well tolerated in initial and later treatment across the 3 dosing cohorts. AEs were manageable. Treatment discontinuations of KTd due to AEs were infrequent at 8%, and these discontinuations all occurred during induction treatment. Dose reductions of carfilzomib due to AEs occurred in 8% of patients. The most common grade 3/4 AEs were dermatologic, gastrointestinal, and respiratory disorders. Notably, with the caveat of cross-trial comparisons, the rate of PNP (16% grade ≥2, 1% grade ≥3) seen in our study was lower than PN rates reported in studies treating patients with VTD induction therapy. The GIMEMA phase 3 study reported a 16% frequency of grade ≥2 PN events and a 10% frequency of grade 3/4 PN events.11 The PETHEMA/GEM phase 3 study reported a 60% frequency of grade ≥2 PN events and a 14% frequency of grade 3/4 PN events.12 Only 1 of 91 patients (1%) in the study presented here required a carfilzomib dose modification because of a PNP AE during KTd induction. In comparison, dose reductions of bortezomib in response to PN AEs have been required in up to 25% of patients during VTD induction.12 SCH was successful in 81 of 83 patients who started with cyclophosphamide following KTd induction. The finding that the KTd induction regimen does not hinder SCH is consistent with other reports of carfilzomib and bortezomib frontline combination regimens.16,18,36 Following induction and SCH, 79 of 81 patients (98%) underwent HDM/ASCT, which is similar to the 73% to 90% of patients able to undergo ASCT following VTD induction.5,11,12 The 3 dosing cohorts in this trial had similar outcome in response and AEs. From the present data, it can be concluded that the maximum tolerated dose of carfilzomib in combination with thalidomide and dexamethasone has not been reached. A fourth dosing cohort at carfilzomib 56 mg/m2 (n = 20) is ongoing.
The results of this study are encouraging, but they will need to be confirmed in future studies, preferably in randomized trials with a larger patient population. Longer follow-up from our study and results from planned and ongoing studies examining higher doses of carfilzomib with thalidomide and dexamethasone will further delineate the role of the KTd regimen in the frontline setting. In addition, the safety and efficacy of regimens based on the combination of carfilzomib with other agents are being explored in the frontline setting for both transplant-eligible and transplant-ineligible patients.17,19-22 These include supplementation of the KTd regimen with additional agents. For example, the Mayo Clinic has recently reported promising results from the CYKLONE phase 2 study in which cyclophosphamide is added to the KTd regimen.19
In newly diagnosed patients who are eligible for ASCT, the combination of carfilzomib plus thalidomide and dexamethasone is a safe, rapidly effective, and well-tolerated induction regimen. Consolidation treatment after ASCT with this regimen results in a significant upgrade of response. Importantly, the favorable PN safety profile may allow for greater treatment adherence and more durable response. The data reported here support further clinical trials to validate the benefit of the KTd regimen for induction and consolidation therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Medical writing and editing services were provided by BlueMomentum, a division of KnowledgePoint360 Group, San Bruno, CA, and supported by funding from Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Authorship
Contribution: B.v.d.H., A.P., S.L., H.L., and P.S. designed the research; all authors performed the research; B.v.d.H. performed the statistical analysis; P.S., B.A., S.L., S.Z., H.L., and B.v.d.H. analyzed and interpreted the data; P.S. wrote the initial draft of the manuscript and edited the final draft; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: P.S. has received research support from Onyx, Janssen, and Celgene. A.P. has served as a consultant or advisor for and received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium, and Onyx. M.J.K. has received research support from Celgene and honoraria from Celgene and Janssen. S.Z. has received research support from and served on the advisory boards of Janssen, Celgene, and Millennium. H.L. has served as a consultant for Mundi-Pharma, Genmab, and Johnson & Johnson and has received research support from Genmab and Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Pieter Sonneveld, Department of Hematology, Erasmus MC Cancer Institute, Erasmus University Medical Center, Rotterdam, The Netherlands; e-mail: p.sonneveld@erasmusmc.nl.