Key Points
Syk is required for increased B-cell activation and cGVHD generation and maintenance.
The Syk inhibitor fostamatinib can treat murine cGVHD and increase human cGVHD B-cell death.
Abstract
Novel therapies for chronic graft-versus-host disease (cGVHD) are needed. Aberrant B-cell activation has been demonstrated in mice and humans with cGVHD. Having previously found that human cGVHD B cells are activated and primed for survival, we sought to further evaluate the role of the spleen tyrosine kinase (Syk) in cGVHD in multiple murine models and human peripheral blood cells. In a murine model of multiorgan system, nonsclerodermatous disease with bronchiolitis obliterans where cGVHD is dependent on antibody and germinal center (GC) B cells, we found that activation of Syk was necessary in donor B cells, but not T cells, for disease progression. Bone marrow–specific Syk deletion in vivo was effective in treating established cGVHD, as was a small-molecule inhibitor of Syk, fostamatinib, which normalized GC formation and decreased activated CD80/86+ dendritic cells. In multiple distinct models of sclerodermatous cGVHD, clinical and pathological disease manifestations were not eliminated when mice were therapeutically treated with fostamatinib, though both clinical and immunologic effects could be observed in one of these scleroderma models. We further demonstrated that Syk inhibition was effective at inducing apoptosis of human cGVHD B cells. Together, these data demonstrate a therapeutic potential of targeting B-cell Syk signaling in cGVHD.
Introduction
The development of chronic graft-versus-host disease (cGVHD) is a major complication following allogeneic hematopoietic stem cell transplantation. Discovery of new therapies has been limited by the absence of murine models that closely represent the clinical human disease and pathogenesis.1,2 Herein, we use multiple mouse models to assess the effectiveness of therapies during cGVHD. We use a mouse model of antibody-dependent multiorgan system cGVHD, previously demonstrated to mimic several aspects of human cGVHD pathology, with the exception of sclerodermatous cGVHD,3,4 and 3 models of sclerodermatous cGVHD with skin manifestations.5,6
Although the exact mechanisms of cGVHD remain unknown, recent studies have elucidated a role for antibody production by B cells. The increase of B cells in the germinal center (GC) has been shown to be necessary for the development of an antibody-facilitated multiorgan system cGVHD model that includes bronchiolitis obliterans (BO).3 Spleen tyrosine kinase (Syk) is activated by B-cell receptor (BCR) engagement. After antigen-BCR engagement, Syk is phosphorylated at Y348, allowing for B-cell survival and proliferation. Increased proximal BCR signaling was recently described in cGVHD-patient B cells.7
Syk also affects myeloid cell (macrophages; neutrophils and dendritic cells [DCs]) including phagocytosis, signal transduction via activating Fc receptors, and antigen-uptake, internalization, and upregulation of costimulatory molecules in DCs.8 Syk also has been demonstrated to have an impact on cell migration of monocytes.9 Given the function of Syk in myeloid cells and the critical role of macrophages and DCs in cGVHD,5,10 Syk inhibition may have additional advantages of treating cGVHD via macrophage and DC effects.
We sought to determine whether B-cell Syk activation is a critical component of cGVHD pathophysiology. First, we demonstrated that Syk is hyperresponsive in B cells during cGVHD and that Syk is necessary in B cells, but not T cells, for murine cGVHD progression. Next, we demonstrated that the deletion of Syk in donor BM cells in vivo in mice with established cGVHD with BO was able to reverse disease, a finding that was phenocopied by the in vivo molecular inhibition of Syk with fostamatinib. Inhibition of Syk decreased the frequency of GCs and expression of the activation costimulatory molecules CD80 and CD86 in CD11c+ cells in vivo. In multiple distinct models of sclerodermatous cGVHD, clinical and pathological disease manifestations were not eliminated when mice were therapeutically treated with fostamatinib, though both clinical and immunologic effects could be observed in one of the sclerodermatous cGVHD models. Human cGVHD B cells had increased death when treated with fostamatinib, demonstrating BCR-activated B cells can be preferentially targeted. These data together suggest that Syk could be a novel therapeutic for cGVHD patients.
Materials and methods
Mice
C57Bl/6 (B6; H2b) mice were purchased from the National Cancer Institute. B10.BR (H2k) mice were purchased from The Jackson Laboratories. Female C57Bl/6 (B6) (H-2b, CD45.2) and B6D2F1 (H-2b/d, CD45.2) mice were purchased from the Animal Resources Center. BALB/c, B10.D2-Thy1.2, and B10-.D2-Thy1.1 mice were propagated in the animal facility at the Johns Hopkins University Cancer Research Building I. The Syk fl/fl × ERT2-cre mice were provided by R.A.C. from Columbia University. Deletion of Syk (Syk knockout [KO]) occurred with administration of tamoxifen (Sigma) by mouth for 5 days and confirmed by western blot. Mice were housed in a specific-pathogen–free facility and used with the approval of each institution’s animal care committee.
BM transplantation
For model 1 (systemic cGVHD with BO, major histocompatibility complex [MHC] disparate), B10.BR recipients were conditioned with cyclophosphamide and total body irradiation (8.3 Gy). Recipients received 10 × 106 T-cell–depleted (TCD) BM cells from B6 wild-type (WT) or BM cells from donors that were induced to delete Syk by tamoxifen administration. Cohorts received no supplemental T cells or purified splenic T cells (0.1 × 106) from WT vs KO donors.3 In one experiment, as indicated, mice were given donor BM from Syk fl/fl × ERT2-cre or WT mice with or without purified T cells; on day 28 post–BM transplantation, mice were given tamoxifen to delete Syk from donor BM cells. For model 2 (sclerodermatous cGVHD, semiallogeneic recipients), on day −1, B6D2F1 recipient mice received total body irradiation (11 Gy; 137Cs source), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity.5 On day 0, recipients underwent transplantation with 5 × 106 BM with or without 1 × 106 purified T cells from B6 mice. Non-GVHD control groups were injected with TCD BM grafts. For model 3 (sclerodermatous cGVHD with granulocyte colony-stimulating factor [G-CSF]–mobilized splenocytes, MHC disparate), on day −1, mice received total-body irradiation (1 Gy; 137Cs source), delivered as in model 2. On day 0, B6 mice each received 25 × 106 donor splenocytes from G-GSF–mobilized BALB/c donors. Spleens were depleted of T cells as previously described for non-GVHD control groups.11 Transplanted mice were monitored daily, and those with GVHD clinical scores of ≥610,11 were sacrificed and the date of death registered as the next day in accordance with institutional animal ethics committee guidelines. For model 4 (sclerodermatous cGVHD, minor histocompatibility antigen disparate), a single lethal irradiation dose of 7.75 Gy was administered using a 137Cs irradiator. Animals were reconstituted with 107 B10.D2-Thy1.2 TCD BM cells (107) alone or supplemented 1.8 × 106 CD4+ and 0.9 × 106 CD8+ Thy1.1+ T cells, reflecting T cells found in 1.2 × 107 B10.D2 donor splenocytes, a dose that reproducibly induces GVHD.6 Purified populations of donor T cells were obtained using T-cell isolation kits (Dynabeads; Invitrogen, Carlsbad, CA).
Where indicated, cGVHD recipients were given R788 (30 mg/kg per animal twice daily by mouth) or 0.1% carboxymethylcellulose vehicle from days 28 to 56 (BO model 1), days 14 to 28 (B6 into B6D2F1, scleroderma, model 2), days 7 to 21 (G-CSF–mobilized BALB/c splenocytes into B6 recipients, scleroderma model 3), or B10.D2 into BALB/c (experiment 1, days 21-35; experiment 2, days 14-28). Both prodrug, R788, and its active metabolite, R406 (both known as fostamatinib), were kindly provided by Rigel Pharmaceuticals. Lung function was assessed as previously described.12
Patient samples
Samples were obtained from patients following written informed consent in accordance with the Declaration of Helsinki. Viably frozen peripheral blood samples from patient with either active clinical manifestations of cGVHD or no active cGVHD were randomly selected from cell banks for study (patient characteristic details are shown in supplemental Table 1, available on the Blood Web site). The institutional review boards at the University of North Carolina Chapel Hill, Duke University Medical Center, and the Dana-Farber Cancer Institute approved all studies.
Analysis of Syk activation and in vitro effects of Syk inhibition
For analysis of Syk phosphorylation in murine cGVHD, B cells were purified from murine cGVHD spleens or BM-only controls on day 60 in the cGVHD/BO model and activated by 5 μg/mL anti-immunoglobulin M (anti-IgM) antigen-binding fragment (Jackson Research), fixed with BD Cytofix Fixation Buffer and permeabilized with BD Perm Buffer III. Cells were subsequently stained with anti-Syk Y348 PE (eBioscience) as previously described.13
For analysis of the effects of in vitro Syk inhibition, cryopreserved peripheral blood mononuclear cells (PBMCs) from cGVHD or non-cGVHD control patients were thawed and allowed to rest overnight. Patient PBMCs were incubated with the Syk inhibitor, R406 (0.01 or 0.1 μM), or DMSA control (0.1%) for 48 hours at 37°C. Apoptosis was measured by gating on CD19+ or CD19− cells and costaining with Annexin V and 7-AAD.
Flow cytometry
GC B cells and CD11c+ cells were labeled with anti-CD19 (clone eBio1D3), anti-CD11c (clone N418), anti-CD80 (clone 16-10A1), anti-CD86 (clone GL1), anti-MHCII (clone M5/114.15.2), anti-GL7 (clone GL-7) (eBioscience), anti-CD4 (clone RM4-5), anti-CD95 (clone Jo2) (BD Biosciences), anti-F4/80 (clone BM8), anti-CXCR4 (clone L276F12), anti-Ly6G (clone 1A8), anti-CD3 (clone 145-2C11), anti-Ly6C (clone HK1.4), anti-CD8 (clone 53-6.7), and anti-CD11b (clone M1/70) (BioLegend) and analyzed on BD LSRFortessa. Ficolled human PBMCs were stimulated and stained for Annexin V as previously described.14,15
Frozen tissue preparation, pathology, and immunofluorescence.
Organs harvested were embedded in optimal cutting temperature compound, snap frozen, and stored at −80°. GC detection and pathologic scoring were accomplished as previously described.4 At various times posttransplantation, GVHD target tissues were harvested, fixed in 10% formalin for 24 hours, embedded in paraffin, and processed to generate 5-µm-thick sections. Hematoxylin and eosin–stained sections of skin were examined in a blinded fashion using a semiquantitative scoring system for GVHD as previously published.4,16 Samples were scored from 0 to 4 for epidermal and dermal inflammation, dermal fibrosis and subcutaneous fibrosis, epidermal apoptosis, and loss of subcutaneous fat.
Statistics
Group comparisons of pathology, pulmonary function tests, cell counts, and flow cytometry data were analyzed by Student t test or 1-way analysis of variance.
Results
Syk phosphorylation is increased during murine cGVHD and is necessary in donor-BM–derived cells for the development and maintenance of cGVHD
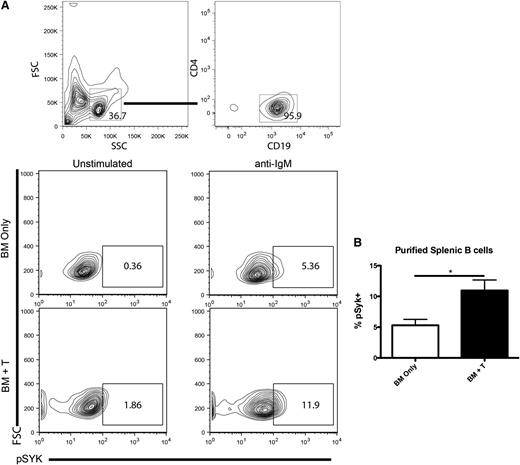
To determine if Syk activation is important in murine cGVHD, purified B cells (Figure 1A) from day-60 cGVHD spleens were assayed for Syk phosphorylation. Purified B cells from cGVHD mice were activated with anti-IgM and the amount of phosphorylated Syk (pSyk) at Y348 was measured. There was an increase in the percentage pSyk in both BCR-stimulated B cells and unstimulated B cells in cGVHD (Figure 1B). These data extend a recent report that human B cells have increased pSyk during cGVHD.7
Syk activation during cGVHD. (A) B cells were purified from the spleens of healthy control (BM only) or cGVHD (BM + T) mice and stimulated with anti-IgM at 5 μg/mL. Splenocytes from B10.BR mice transplanted with B6 BM and low numbers of T cells were analyzed for pSyk at Y348 after ex vivo stimulation by 5 μg/mL of anti-IgM. (B) Cumulative expression of phosphorylated Syk. *P < .5. Error bars represent standard error of the mean (SEM); n = 8 representative data from 2 experiments.
Syk activation during cGVHD. (A) B cells were purified from the spleens of healthy control (BM only) or cGVHD (BM + T) mice and stimulated with anti-IgM at 5 μg/mL. Splenocytes from B10.BR mice transplanted with B6 BM and low numbers of T cells were analyzed for pSyk at Y348 after ex vivo stimulation by 5 μg/mL of anti-IgM. (B) Cumulative expression of phosphorylated Syk. *P < .5. Error bars represent standard error of the mean (SEM); n = 8 representative data from 2 experiments.
To test whether Syk is necessary for cGVHD progression, mice were transplanted with WT or induced Syk KO BM with WT T cells and analyzed for disease on day 60. Mice receiving Syk KO BM and WT T cells did not develop cGVHD pulmonary dysfunction when compared with those receiving WT BM and T cells (Figure 2A). Because Syk is necessary for the proliferation of B cells following antigen stimulation,17 we analyzed the spleens of transplanted mice to determine if there was a defect in the maintenance of Syk KO BM–derived B cells. We found that total B-cell frequency in mice receiving Syk KO BM was decreased eightfold (Figure 2B). These data are consistent with the dependency of activated B cells on Syk for proliferation and survival and a requirement for activated donor-derived BM B cells in cGVHD pathogenesis.3,4
Presence of Syk in BM-derived B cells, but not donor T cells, is necessary for development of cGVHD pathology in a BO model. (A) Day-60 pulmonary function tests of mice transplanted with tamoxifen-induced Syk KO BM and WT T cells. (B) Frequency of B cells in transplanted mice on day 60 after transplant. (C) Pulmonary function tests from mice transplanted with WT BM and either WT T cells or Syk KO T cells. (D) Pathology scores from lung and liver of mice transplanted with Syk KO T cells. *P < .5; **P < .01. Error bars represent SEM; representative data from 3 independent experiments with n = 8 per group.
Presence of Syk in BM-derived B cells, but not donor T cells, is necessary for development of cGVHD pathology in a BO model. (A) Day-60 pulmonary function tests of mice transplanted with tamoxifen-induced Syk KO BM and WT T cells. (B) Frequency of B cells in transplanted mice on day 60 after transplant. (C) Pulmonary function tests from mice transplanted with WT BM and either WT T cells or Syk KO T cells. (D) Pathology scores from lung and liver of mice transplanted with Syk KO T cells. *P < .5; **P < .01. Error bars represent SEM; representative data from 3 independent experiments with n = 8 per group.
In contrast to Syk dependence in BM-derived cells, the addition of Syk KO donor T cells did not attenuate the functional manifestations of BO (with the exception of resistance) compared with Syk WT T-cell–transplanted mice (Figure 2C). In addition, cGVHD pathology in the lungs and liver was similar in recipients of Syk WT vs KO T cells (Figure 2D). Whereas T-cell effects after Syk inhibition were previously described in acute18 and sclerodermatous cGVHD,19 in this systemic cGVHD model with BO and without scleroderma, donor T-cell Syk was not fully required for cGVHD development.
To determine whether Syk function in BM-derived B cells was essential for sustaining cGVHD in mice with established disease, recipients were given donor BM from Syk fl/fl × ERT2-cre or WT mice with or without purified T cells. On day 28 post–BM transplantation, mice were given tamoxifen. Induced deletion of Syk in donor BM cells significantly improved all pulmonary function parameters to levels of non-cGVHD controls (Figure 3).
Genetic deletion of Syk during active disease prevents pathogenic pulmonary function. Mice were transplanted with either WT or Syk fl/fl × ERT2-Cre BM. On day 8, mice were treated with tamoxifen for 5 days to delete Syk in donor-BM–derived cells. Pulmonary function tests of mice on day 60. *P < .5; ** P < .01. Error bars represent SEM; n = 10 per group.
Genetic deletion of Syk during active disease prevents pathogenic pulmonary function. Mice were transplanted with either WT or Syk fl/fl × ERT2-Cre BM. On day 8, mice were treated with tamoxifen for 5 days to delete Syk in donor-BM–derived cells. Pulmonary function tests of mice on day 60. *P < .5; ** P < .01. Error bars represent SEM; n = 10 per group.
Therapeutic pharmacologic inhibition of Syk decreases pathology in a murine BO model of cGVHD but had little clinical therapeutic effect in scleroderma models of cGVHD
Fostamatinib is a potent small-molecule inhibitor of Syk. Studies in rheumatoid arthritis have demonstrated efficacy with fostamatinib in randomized phase 2 clinical trials.20,21 Fostamatinib was notably safe in patients treated for non-Hodgkin lymphoma, including some who had received prior autologous hematopoietic cell transplantation.22 Additionally, Leonhardt et al18 demonstrated that Syk inhibition decreased costimulatory molecules on antigen-presenting cells and increased survival of mice during acute GVHD while preserving antitumor and antiviral immunity. In addition, other studies have demonstrated the importance of BCR signaling in the development of murine cGVHD.23
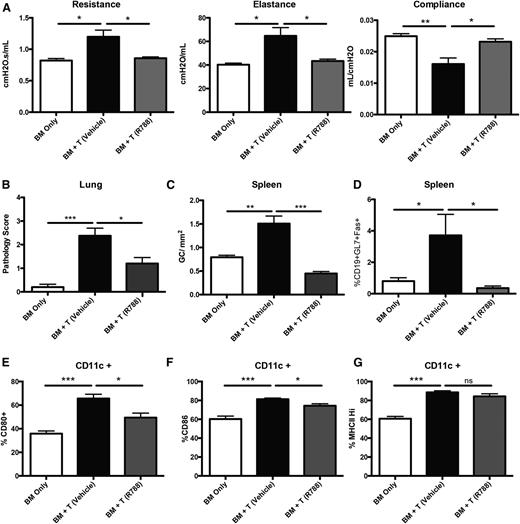
Having demonstrated the requirement for Syk in donor BM cells in mice with established cGVHD and BO, we next sought to determine whether drug treatment with fostamatinib in mice with established cGVHD would be similarly efficacious as Syk deletion in donor BM cells.3,4 Mice receiving fostamatinib beginning on day 28 had restoration of pulmonary function, similar to the healthy transplanted BM-only controls (Figure 4A). Improvement in pulmonary function correlated with a reduction in cGVHD pathology in the lung (Figure 4B). The number of GC reactions in the spleen was decreased in fostamatinib vs vehicle-treated cGVHD mice (Figure 4C). This was highlighted by a decrease in frequency of splenic GC B cells in fostamatinib-treated cGVHD mice, matching BM-only controls (Figure 4D). These data demonstrate the importance of Syk in B-cell signaling during active disease.
Inhibition of Syk by R788 decreases cGVHD in a murine BO model. (A) Day-56 pulmonary function tests on mice treated with 30 mg/kg R788 twice daily from days 28 to 56. (B) GVHD pathology scores from the lungs of mice on day 60. (C) Number of GCs present in situ in spleens of mice. (D) Frequency of GC B cells (gated on CD19+GL7+CD95hi) present in the spleens of transplanted mice on day 60. (E-G) Frequency of CD11c cells expressing CD80, CD86, and MHC class II in the spleens of transplanted mice on day 60. *P < .5; **P < .01; ***P < .001. Error bars represent SEM; representative data from 3 independent experiments with n = 8 per group.
Inhibition of Syk by R788 decreases cGVHD in a murine BO model. (A) Day-56 pulmonary function tests on mice treated with 30 mg/kg R788 twice daily from days 28 to 56. (B) GVHD pathology scores from the lungs of mice on day 60. (C) Number of GCs present in situ in spleens of mice. (D) Frequency of GC B cells (gated on CD19+GL7+CD95hi) present in the spleens of transplanted mice on day 60. (E-G) Frequency of CD11c cells expressing CD80, CD86, and MHC class II in the spleens of transplanted mice on day 60. *P < .5; **P < .01; ***P < .001. Error bars represent SEM; representative data from 3 independent experiments with n = 8 per group.
Previous reports demonstrated a decrease in the activation of CD11c+ cells18 in mice with acute GVHD treated with fostamatinib. In the BO cGVHD model, we found increased expression of costimulatory molecules CD80 and CD86 on CD11c+ spleen cells compared with BM-only transplanted mice. When these cGVHD mice are treated with fostamatinib beginning on day 28, we found a significant decrease in the frequency of CD11c+ cells expressing CD80 and CD86, though without affecting MHC class II expression (Figure 4E-G). These data highlight a potentially beneficial alternative effect of Syk inhibition in CD11c+ myeloid cells during cGVHD.
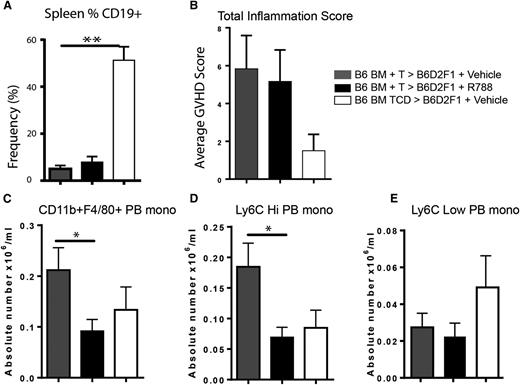
In order to determine whether the effects of Syk inhibition attenuate murine sclerodermatous cGVHD in our hands, we used three different cGVHD scleroderma models involving lethally irradiated recipients: a B6 into B6D2F1 model (model 2), G-CSF–mobilized BALB/c splenocytes into B6 (model 3), and a minor-mismatch model of B10.D2 into BALB/c (model 4). In the B6 into F1 model (fostamatinib, days 14-28) and in the G-CSF-mobilized BALB/c into B6 models (fostamatinib, days 7-21), the frequency of splenic CD19+ B cells in vehicle- and fostamatinib-treated cGVHD were more than tenfold and fivefold fold lower than non-cGVHD, BM-only controls in both the B6 into B6D2F1 and G-CSF BALB/c into B6 models, respectively (Figure 5A and supplemental Figure 1A). Neither a significant beneficial clinical therapeutic effect nor a pathological effect as reflected by total skin inflammation score was seen in either model (Figure 5B and supplemental Figure 1B). However, we found a significant decrease in peripheral blood CD11b+F4/80+ and Ly6Chi monocytes numbers in the B6 into B6D2F1 model after treatment with fostamatinib in the B6 into B6D2F1 model (Figure 5C-E), which was also seen in the G-CSF–mobilized BALB/c into B6 model but did not reach statistical significance (supplemental Figure 1C-E).
Fostamatinib does not alter skin inflammation in a B6 into B6D2F1 model of sclerodermatous GVHD but decreases peripheral blood Ly6Chi monocytes. (A) Frequency of CD19+ B cells in spleens of mice on day 21 in vehicle-treated (gray bars), R788-treated (black bars), and non-GVHD controls (white bars). (B) Total inflammation score. (C) Absolute number of CD11b+ F4/80+ peripheral blood monocytes. Peripheral blood monocytes were examines for (D) Ly6C high or (E) Ly6C low expression. *P < .5; **P < .01. Error bars represent SEM; n = 6 per group. PB mono, peripheral blood monocyte.
Fostamatinib does not alter skin inflammation in a B6 into B6D2F1 model of sclerodermatous GVHD but decreases peripheral blood Ly6Chi monocytes. (A) Frequency of CD19+ B cells in spleens of mice on day 21 in vehicle-treated (gray bars), R788-treated (black bars), and non-GVHD controls (white bars). (B) Total inflammation score. (C) Absolute number of CD11b+ F4/80+ peripheral blood monocytes. Peripheral blood monocytes were examines for (D) Ly6C high or (E) Ly6C low expression. *P < .5; **P < .01. Error bars represent SEM; n = 6 per group. PB mono, peripheral blood monocyte.
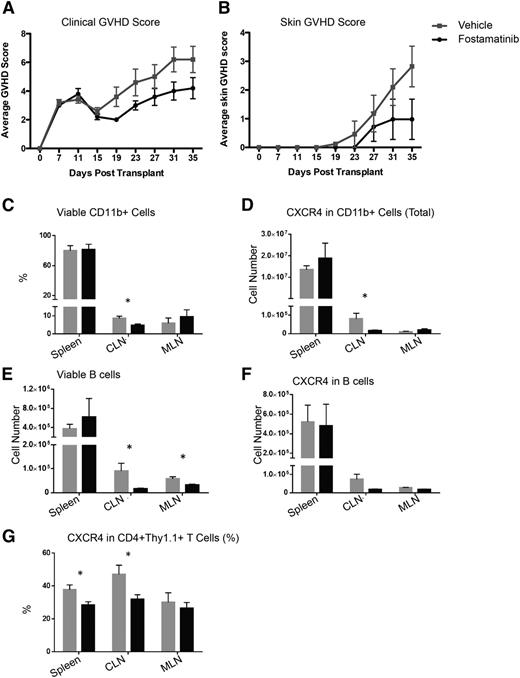
In the B10.D2 into BALB/c model of scleroderma, fostamatinib treatment from days 21 to 35 did not impact skin disease or clinical score (not shown). However, beginning fostamatinib treatment 1 week earlier, on day 14, as used by Huu et al,19 did significantly improve these parameters on day 19, and there was a reduction in the both mean skin score from 3 to 1 and the GVHD clinical score from 6 to 4 in the fostamatinib- vs vehicle-treated controls on day 35, the end of the study (Figure 6A-B). In the latter experiment, there also was a decrease in total numbers of viable cervical and mesenteric lymph node (but not splenic) B cells and frequency of CD11b+ cervical lymph node cells. CXCR4+ T cells in the spleen and cervical lymph node were also decreased in fostamatinib- vs vehicle-treated cGVHD mice (Figure 6C-G), suggesting an impairment to the overall immune response and migration of T cells and myeloid cells to tissues. Taken together, these data suggest that early posttransplant treatment of sclerodermatous cGVHD may attenuate disease progression.
Fostamatinib has little clinical therapeutic benefit in a B10.D2 into BALB/c sclerodermatous cGVHD model but decreases expression of CXCR4 on CD11b+ and CD4+ cells. (A) Clinical GVHD scores or (B) skin GVHD scores in vehicle-treated (gray squares) or R788-treated (black circles) mice in the B10.D2 into BALB/c model. (C) Frequency of viable CD11b+ cells. (D) Expression of CXCR4 on CD11b+ cells. (E) Absolute number CD19+ B cells. (F) Expression of CXCR4 on B cells. (G) Expression of CXCR4 on CD4+ cells. *P < .5. Error bars represent SEM; n = 5 per group. CLN, cervical lymph node; MLN, mesenteric lymph node.
Fostamatinib has little clinical therapeutic benefit in a B10.D2 into BALB/c sclerodermatous cGVHD model but decreases expression of CXCR4 on CD11b+ and CD4+ cells. (A) Clinical GVHD scores or (B) skin GVHD scores in vehicle-treated (gray squares) or R788-treated (black circles) mice in the B10.D2 into BALB/c model. (C) Frequency of viable CD11b+ cells. (D) Expression of CXCR4 on CD11b+ cells. (E) Absolute number CD19+ B cells. (F) Expression of CXCR4 on B cells. (G) Expression of CXCR4 on CD4+ cells. *P < .5. Error bars represent SEM; n = 5 per group. CLN, cervical lymph node; MLN, mesenteric lymph node.
Fostamatinib increases apoptosis in B cells purified from cGVHD patients
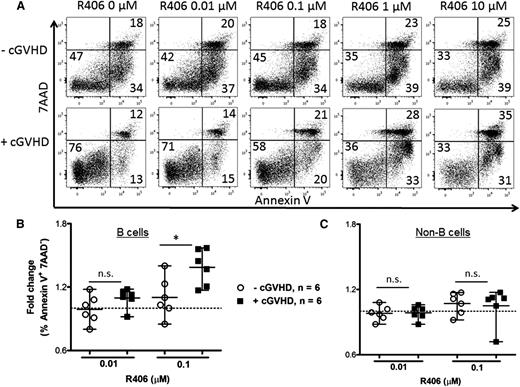
To determine if human cGVHD B cells are more susceptible to Syk blockade, human peripheral blood (supplemental Table 1) was treated in vitro with R406, the active form of R788. B cells from patients with active cGVHD had increased apoptotic and total cell death compared with patients with inactive or no cGVHD after R406 (Figure 7). These data, consistent with work by Allen et al,7 reveal that R406 preferentially kills cGVHD B cells via apoptosis. Global targeting of B cells with rituximab has been met with mixed success, possibly due to altered B-cell homeostasis perpetuated in some patients.24,25 We now demonstrate that constitutively activated B cells can be selectively targeted in cGVHD by Syk inhibition (Figure 7B). Consistent with the lack of a requirement for Syk in donor T cells in the cGVHD BO model, fostamatinib did not induce apoptosis in the non–B-cell population (Figure 7C). In 3 samples from each patient group, T-cell–specific anti-CD3 antibody was included to stimulate T-cell proliferation in the presence of R406; no increase in T-cell death was observed under these conditions. Together, these data point to the therapeutic potential of fostamatinib and pSyk targeting in cGVHD patients.
Increased apoptosis in human B cells when treated with R788. (A) Representative flow plots of human PBMCs with or without cGVHD. Consistent with previous work,36 unmanipulated, untreated cGVHD B cells had superior survival compared with B cells from patients without disease. PBMCs from patients without cGVHD (n = 6, open circles) and with cGVHD (n = 6, filled squares) treated with R406 (0, 0.01, and 0.1 μM) as indicated for 48 hours. Apoptotic B cells were defined as CD19+ Annexin V+ 7AAD− cells (B) or as CD19− non–B cells (C). Fold increase in apoptosis by R406 divided by phosphate-buffered saline is depicted. Data are median ± range pooled from 2 independent experiments. *P < .5; **P < .01; ***P < .001. n.s., not significant.
Increased apoptosis in human B cells when treated with R788. (A) Representative flow plots of human PBMCs with or without cGVHD. Consistent with previous work,36 unmanipulated, untreated cGVHD B cells had superior survival compared with B cells from patients without disease. PBMCs from patients without cGVHD (n = 6, open circles) and with cGVHD (n = 6, filled squares) treated with R406 (0, 0.01, and 0.1 μM) as indicated for 48 hours. Apoptotic B cells were defined as CD19+ Annexin V+ 7AAD− cells (B) or as CD19− non–B cells (C). Fold increase in apoptosis by R406 divided by phosphate-buffered saline is depicted. Data are median ± range pooled from 2 independent experiments. *P < .5; **P < .01; ***P < .001. n.s., not significant.
Discussion
Here, we demonstrated that pSyk was upregulated in murine cGVHD B cells in a multiorgan system model of cGVHD with BO that is dependent upon donor T-cell support of antibody isotype switching and immunoglobulin deposition. Such cGVHD B cells were hyperresponsive to B-cell activation signals in vitro. Syk function in donor-BM–derived B cells, but not T cells, was required for cGVHD generation, and Syk deletion in donor BM cells in mice with established cGVHD on day 28 post–BM transplantation reversed disease. A small-molecule inhibitor of pSyk, fostamatinib, which has been tested in the clinic for autoimmune diseases, was able to treat established cGVHD when therapy was initiated on day 28. In cGVHD patients, previous studies have shown that cGVHD B cells have high levels of pSyk, and in the current study, we now show that B cells from patients with active cGVHD, but not B cells from patients without active cGVHD, were induced into apoptosis in vitro by the active metabolite of fostamatinib. Whereas fostamatinib was highly effective in reversing cGVHD in the BO model, clinical and immunologic effects in sclerodermatous cGVHD appeared to be more modest under the conditions tested.
It is unclear if B cells are intrinsically or extrinsically affected during cGVHD. B cells might increase their activation state based on pathogen-activating molecular patterns released during conditioning treatment and activating Toll-like receptors on B cells. In contrast, there is potential that increased activating factors such as B-cell activating factor (BAFF) are causing B cells to increase response from factors such as Syk, because BAFF receptor may be reliant on Syk for its signaling.26 The requirement of Syk in BM-derived B cells for cGVHD development in the BO model also allowed us to elucidate a potential mechanism of disease. BCR stimulation with antigen leads to phosphorylation of proximally located Syk to initiate downstream signaling for B-cell survival and proliferation. Syk signaling is necessary for survival of B cells during the GC reaction. The decrease in B cells after Syk-deficient BM transplant in the BO model suggests a mechanistic link between BCR-antigen engagement that requires Syk for B-cell promotion and perpetuation. The absence or relatively modest effects of fostamatinib in sclerodermatous cGVHD is consistent with the findings that there were few splenic B cells present in our studies in fostamatinib- or vehicle-treated sclerodermatous cGVHD mice when analyzed 4 to 5 weeks posttransplant. These B-cell data contrast with the significantly increased splenic GC B-cell frequency seen in cGVHD mice with BO.3,4
Studies of cGVHD patient peripheral blood have demonstrated an important role of B cells in the development of disease. The production of auto- and alloantibodies has previously been associated with the development of cGVHD in patients.27-30 The dysregulation of B cells could be due to the significant increase in BAFF present during cGVHD.15,31 B cells in patients with cGVHD were more sensitive to Syk inhibition compared with B cells from patients without active cGVHD, consistent with our previous finding that these cells have increased activation of Syk and the proximal BCR signalosome.7 We demonstrated that these cells had an increase in cell death rates compared with controls. Like BCR-activated B-cell lymphomas,32 only those cGVHD B cells activated through the BCR pathway are presumably susceptible to killing at low drug concentration. Together, these data demonstrate the relative selective ability of fostamatinib to target the activated B cells in cGVHD patients.
The role of Syk in donor T cells was demonstrated to be of little importance in the development of murine cGVHD in the BO model. However, in the minor antigen-disparate sclerodermatous cGVHD model, we did find a decrease in the expression of CXCR4 on T cells when mice with established clinical cGVHD were treated with R788, consistent with Huu et al,19 who also demonstrated increased pSyk in T cells on day 14 posttransplant and a reduction in the proliferation of T cells from cGVHD mice when exposed in vitro to the active fostamatinib metabolite, R406, and mice (4-6 per group) treated with fostamatinib in vivo exhibited an ∼1-week shift in cGVHD onset and progression when treatment was initiated on day 14 posttransplant, resulting in significantly lower average skin scores and body weight between days 21 to 42 posttransplant.19 The decrease in migration of T cells to effector tissues could have a beneficial effect on the development of disease. Thus, although treatment did not fully restore health in our study in the B10.D2 into BALB/c mode, there was a decrease in the progression of disease associated with a lowered frequency of CXCR4+ T cells that may contribute to disease reduction.
Despite the apparent lack of Syk dependency of donor T cells in cGVHD with BO, we demonstrate that the Syk inhibitor fostamatinib is capable of blocking the cGVHD GC response that is dependent upon follicular helper T cells and GC B cells.3 These data point to a T-cell–independent effect of fostamatinib in abrogating the cGVHD GC response, which is essential for cGVHD generation and progression in the BO model.
In addition to targeting B cells during cGVHD, there is evidence that antigen-presenting cells are susceptible to fostamatinib treatment. For example, in an acute GVHD model, Leonhardt et al18 showed that fostamatinib reduced costimulatory molecule expression and disrupted cytoskeletal organization, along with blocking T-cell proliferation and migration, resulting in acute GVHD amelioration with preservation of graft-versus-leukemia and antiviral immunity. Consistent with other reports that inhibition of Syk can decrease costimulatory molecule expression,19,33-35 we demonstrated in vivo a decrease in the frequency of activated (CD80+, CD86+) DCs in mice with cGVHD and BO.
Although we could not discern effects of fostamatinib on the total skin inflammatory scores in either MHC-disparate model of sclerodermatous cGVHD, in the B6 into B6D2F1 model, fostamatinib significantly decreased the absolute number of CD11b+F4/80+ monocytes and the inflammatory Ly6Chi, but not the Ly6Clo, noninflammatory subset in peripheral blood. These data are interesting in light of the role of Syk in macrophages and monocytes and our recent study implicating monocytes and macrophages in cGVHD generation and maintenance in the 2 scleroderma cGVHD models as well as in the BO model.5 Notably, there was a significant decrease in CD11b+ cells in the cervical lymph node of fostamatinib-treated mice in the minor antigen-disparate B10.D2 into BALB/c sclerodermous cGVHD model. Monocyte and macrophages may contribute to cGVHD in these models by antigen-presentation capacity or the elaboration of profibrogenic proteins such as transforming growth factor β.5 Thus, the impact of fostamatinib in cGVHD models may depend in part on the stage of disease progression as well as the dominant underlying mechanisms of disease pathogenesis.
In summary, our data clearly indicate, via biochemical, genetic, and pharmacologic approaches, a role of Syk in donor BM-derived B cells in a multiorgan system model of cGVHD with BO. Further, we have translated these findings into a therapeutic using a pharmacologic small-molecule Syk inhibitor, fostamatinib, that has progressed through phase 2 clinical trials20,21 Fostamatinib ameliorated disease in mice with cGVHD and BO and has biological effects in some models of scleroderma. These preclinical murine and human data point to consideration of clinical trials of fostamatinib or another Syk inhibitor alone or in combination with other similar agents23 for the treatment of cGVHD.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rigel Pharmaceuticals for providing fostamatinib (R788/R406). This work was supported in part by National Institutes of Health National Cancer Institute grants P01 CA142106-06A1 and 5P01-CA047741-20; National Heart, Lung, and Blood Institute grants R01 HL126530 and K08HL107756; National Institute of Allergy and Infectious Diseases grants P01 AI 056299, and T32 AI 007313; and Leukemia and Lymphoma Society translational research grants 6458-15 and 6462-15. R.F. is supported by the Children′s Cancer Research Fund, Minneapolis, MN.
Authorship
Contribution: R.F. designed experiments, performed experiments, and wrote the paper; J.L.A., A.V., K.P.M., K.P., K.A.A., J.D., and J.C.P. designed and performed experiments; A.P.-M. performed histological analyses, discussed experimental design, and edited the paper; P.A.T. performed experiments and edited the paper; J.S.S., W.J.M., G.R.H., K.P.M., L.L., I.M., J.K., C.S.C., R.J.S., J.H.A., and J.R. designed experiments and edited the paper; N.J.C. and R.A.C. provided reagents, discussed experiments, and edited the paper; and S.S. and B.R.B. designed experiments and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Department of Pediatrics, Masonic Cancer Center, University of Minnesota, 420 Delaware St SE, MMC 109, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
Author notes
R.A.C., S.S., and B.R.B. contributed equally to this study.