Abstract
Therapeutic blockade of immune checkpoint pathways, in particular cytotoxic T-lymphocyte associated protein 4 and programmed-death 1 (PD-1), has become a paradigm-shifting treatment in solid tumor oncology. Hematologic malignancies (HMs), many of which are known to have clinically exploitable immune sensitivity, are a natural target for this type of treatment. Several clinical trials of checkpoint blockade have been conducted in HM, with preliminary results suggesting the therapeutic usefulness of this approach across several tumor types. In particular, the results of PD-1 blockade in Hodgkin lymphoma (HL) are remarkable, and raise hope that it may alter the treatment landscape in this disease. However, numerous questions remain about the optimal role of checkpoint blockade both in HL and beyond. Those questions are the focus of this review, in the hope that, if we are at the dawn of a new day in HM immunotherapy, we may begin to envision its morning.
Introduction
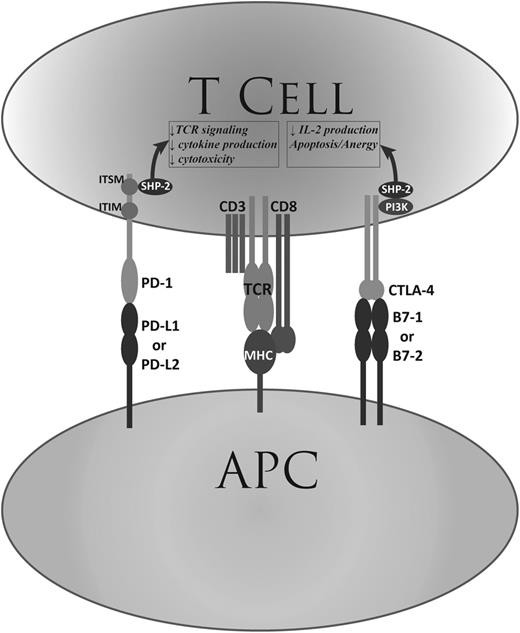
Harnessing the power of the human immune system to combat cancer has been a long-standing dream in oncology. In recent years, an improved understanding of the interaction between the immune system and tumors has spawned new and powerful forms of immunotherapy. One remarkable such advance in cancer immunotherapy has been immune checkpoint blockade. This therapeutic strategy was born from the recognition that tumors can evade the host immune system by usurping immune checkpoint pathways, such as the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed-death 1 (PD-1) pathways. The biology of those pathways has been extensively reviewed elsewhere.1-3 Briefly, the engagement of checkpoint receptors on the surface of T cells by their cognate ligands (B7-1 and B7-2 for CTLA-4, PD ligand 1 [PD-L1] and PD-L2 for PD-1) leads to temporary downregulation of T-cell function (Figure 1). CTLA-4 is upregulated on naïve T cells upon strong antigenic stimulus, and through pleiotropic mechanisms controls the function of regulatory T cells and the establishment of peripheral T-cell tolerance. Signaling through the PD-1 pathway is important in the context of chronic antigenic stimulation of T cells; in that setting, the engagement of PD-1 by its ligands leads to decreased T-cell proliferation, cytotoxicity, and cytokine production, and increased susceptibility to apoptosis. This plays an important role in the generation and maintenance of peripheral tolerance. PD-1 also increases the generation of regulatory T cells, which further helps to attenuate immune responses. The inhibition of T-cell activity by PD-1 engagement appears stronger than by CTLA-4 engagement,4 even though the phenotype of PD-1 knockout mice is less severe than that of CTLA-4 knockout.5,6
PD-1 and CTLA-4. Shown is a simplified representation of the function of the PD-1 and CTLA-4 immune checkpoint pathways. APC, antigen-presenting cell; CD, cluster of differentiation; IL-2, interleukin-2; ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif; TCR, T-cell receptor.
PD-1 and CTLA-4. Shown is a simplified representation of the function of the PD-1 and CTLA-4 immune checkpoint pathways. APC, antigen-presenting cell; CD, cluster of differentiation; IL-2, interleukin-2; ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif; TCR, T-cell receptor.
By expressing the ligands of checkpoint receptors, tumors can selectively block antitumor immune responses. Using monoclonal antibodies (mAbs) directed against the receptors or ligands involved in those pathways, it is now possible to reverse this tumor-induced downregulation of T-cell function and augment antitumor immune activity at the priming (CTLA-4) or tissue effector (PD-1) phase. The first promising clinical results with checkpoint blockade therapy (CBT) were in the treatment of solid tumors, especially melanoma. In this disease, checkpoint blocking antibodies have shown practice-changing activity,7-10 with the potential to induce durable responses.11 Several other solid tumors such as lung cancer, renal cell cancer, head and neck cancer, and urothelial cancer can also be targeted with CBT,9,12-15 based on the results of a large number of clinical trials.
Hematologic malignancies (HMs) offer a particularly fertile ground for immunotherapy. This is most convincingly evidenced by the results of adoptive immunotherapy through allogeneic stem cell transplantation (SCT), which has curative potential for nearly all HMs. Those diseases therefore represent a natural target for CBT, and several trials have now been reported in this field, which we will use as the basis for our discussion. The field of CBT in HM is still in its infancy, an age with great promise but with many more questions than answers. Those questions, and the ways in which we may begin to address them, are the intended focus of this review.
PD-1 blockade in Hodgkin lymphoma (HL)
Based on the success of PD-1 blocking mAbs in the treatment of solid tumors, two phase 1 studies were initiated testing those antibodies in a broad array of HMs. The first study (NCT01592370) tested the safety and single-agent activity of the anti-PD1 mAb nivolumab in patients with relapsed or refractory (R/R) multiple myeloma (MM), non-HL (NHL), and classical HL. The second study (NCT01953692, KEYNOTE-013) tested another PD-1 mAb, pembrolizumab, in R/R myelodysplastic syndromes (MDS), MM, NHL, and HL. Preliminary results from both studies have now been reported.16,17
The inclusion of HL in these two phase 1 studies is noteworthy, and a tribute to the power of translational research. It has long been recognized that HL differs from other lymphomas in being characterized pathologically by rare tumor cells, the Hodgkin Reed-Sternberg (RS) cells, which are surrounded by an extensive but ineffective immune infiltrate. Recent analyses integrating high-resolution copy-number data and transcriptional profiles identified PD-L1 and PD-L2 as key targets of 9p24.1 amplification, which is a recurrent genetic abnormality in HL.18 The 9p24.1 amplicon also includes Janus kinase 2, and gene dosage-dependent Janus kinase 2/signal transducer and activator of transcription activity further induces PD-1 ligand transcription.18 These copy-number dependent mechanisms, as well as other less frequent rearrangements,19 lead to genetically determined overexpression of the PD-1 ligands on the RS cell surface. Epstein-Barr virus infection, also common in HL, is another mechanism of PD-L1 overexpression,20 consistent with the known ability of the virus to usurp the PD-1 pathway to allow viral persistence in the host.1 As a result of these two mechanisms (9p24.1 alterations and Epstein-Barr virus infection), a large proportion of classical HL tumors have increased surface expression of PD-L1.21 This strongly suggested that HL may have a genetic dependence on the PD-1 pathway for survival, and that targeting this pathway could effectively cripple the tumor’s ability to escape immune surveillance. HL was therefore included as an independent expansion cohort in both phase 1 studies, and the clinical results resoundingly validated the preclinical hypothesis. Both studies enrolled patients with multiply R/R HL, with a median of 4 to 5 lines of prior systemic therapy; most had had prior brentuximab and prior autologous stem cell transplantation (ASCT). Despite this, single-agent PD-1 blockade yielded overall response rates of 87% (with a complete response [CR] rate of 17%) and 65% (CR rate = 21%) with nivolumab and pembrolizumab, respectively.16,17 Although the median follow-up is still short, many of the responses appear durable, with some patients now in continued remission for over a year.
Another important finding in both studies was the favorable safety profile in patients with HM, as was also demonstrated by prior studies of another anti–PD-1 mAb, pidlizumab.22-24 This is a salient result, as many patients on those studies had previously received other agents with potential lung toxicity, including radiation, high-dose carmustine, and brentuximab vedotin, raising the concern that PD-1 blockade could lead to a high incidence of pneumonitis in this patient population. In fact, the incidence of pneumonitis in those trials, although not negligible, did not appear excessive, with 13 cases (including 3 severe and 1 fatal case) among 134 patients. Overall, the safety profile of PD-1 blockade in HM appeared similar to that in patients with solid tumors; the rate of severe (grade 3) drug-related adverse events was around 20% in both trials, and only 2 life-threatening (grade 4) and 1 fatal treatment-related toxicities were reported among 134 patients.16,17 PD-1 blockade therefore appears to be a tolerable treatment in HM.
The clinical results in HL must be confirmed and extended in larger cohorts of patients with R/R disease; those studies are planned or already underway. Naturally, there is also interest in using PD-1 blockade earlier in the treatment course of patients, in an attempt to increase cure rates in high-risk patients, or to diminish the toxicity of treatment in lower-risk patients. In theory, PD-1 blockade, alone or in combination, could be used in frontline therapy or in early salvage, and those studies are highly anticipated.
PD-1 blockade beyond HL
The data on PD-1 blockade in HL encompass the largest published experience to date on single-agent checkpoint blockade in a homogeneous tumor type. The success of this strategy in HL raises a broad question: may we expect similar successes with PD-1 blockade, or more generally with CBT, across the spectrum of HM?
NHL
CBT has already been tested in various subtypes of NHL. The first reported study was a phase 1 study of the anti–CTLA-4 antibody ipilimumab in patients with B-cell NHL.25 Among 18 patients, 2 responded, 1 with diffuse large B-cell lymphoma (DLBCL) and 1 with follicular lymphoma (FL). Such a response rate of 11% is generally of limited clinical interest, but the results are important; first, this represented the first demonstration that CBT could in fact achieve a favorable therapeutic result. Second, the 2 responses were durable, lasting over 31 months in the patient with DLBCL. The ability to control an aggressive HM for so long with single-agent checkpoint blockade, even in a single patient, was justification enough for further studies. A similarly interesting result was reported in a phase 1 study of the anti-PD1 antibody pidilizumab23 in a broad array of HMs, which yielded a CR in a patient with FL. The activity of this agent in FL was pursued in a subsequent phase 2 trial.24 The response rate (66%) and CR rate (52%) were notable; however, in this study, pidilizumab was administered in combination with rituximab, and only to patients with rituximab-sensitive disease. The CR rate of 52% is higher than one would expect from rituximab alone in this population, but the use of combination therapy in patients who had demonstrated sensitivity to the CBT partner makes the interpretation of the results challenging. Despite this caveat, correlative studies performed in this trial suggested that endogenous antitumor activity was enhanced by PD-1 blockade and associated with the quality of response; this finding, combined with other studies demonstrating the importance and complexity of PD-1 in the FL microenvironment,26-28 strengthens the scientific foundation for using CBT in this disease. Further support for the activity of CBT in DLBCL and FL came from the aforementioned phase 1 study of nivolumab. Among patients with DLBCL, the response rate was 36%, and among patients with FL it was 40%.29 CBT therefore seems to have activity in at least FL and DLBCL, although the biology that underlies those results is likely to be quite different for the 2 diseases and needs to be better defined. Finally, nivolumab also demonstrated clinical activity in a few patients with systemic and cutaneous T-cell NHL (T-NHL).29 This is a tantalizing result given the general resistance of T-NHL to therapy, but further studies will be required to understand its clinical and scientific implications, given the small sample size and the biological heterogeneity of T-NHL.
MM
MM was also included as an independent expansion arm in the phase 1 studies of nivolumab and pembrolizumab. This inclusion was based on promising preclinical data that demonstrated expression of PD-1 and PD-L1 on MM cells and in the MM microenvironment.30,31 Furthermore, animal models suggested that PD-1 blockade could enhance the effect of immunotherapy.32 In the phase 1 study of pidilizumab mentioned above, 1 patient with MM had stable disease,23 supporting the possible therapeutic effect of PD-1 blockade. Despite this, the results of nivolumab in this disease were disappointing, with no objective response seen among 27 patients treated.29 However, 18 patients (67%) had stable disease, which may be a relevant finding as discussed below.
Myeloid malignancies
Similar to lymphoid malignancies, there is an accumulating body of scientific evidence supporting a role for the PD-1 pathway in myeloid malignancies, especially MDS. PD-L1 is expressed on MDS blasts, possibly at a higher level in high-risk disease and in more refractory disease33 ; furthermore, this expression is enhanced by treatment with hypomethylating agents.34 This suggests that PD-L1 expression may be associated with more aggressive disease behavior and treatment resistance, and that PD-1 blockade could be therapeutically useful in this disease. The activity of pembrolizumab in MDS after hypomethylating agent failure was tested in the KEYNOTE-013 trial, while another ongoing trial (NCT02117219) is testing PD-L1 blockade in a similar patient population. At this time, results are not yet available for either trial. There is also data for a role of the PD-1 pathway in acute myeloid leukemia and chronic myelogenous leukemia,35-39 but at present there are no clinical results of PD-1 blockade in those diseases.
Taken together, the above considerations suggest that the PD-1 pathway is engaged by many different HMs, and that PD-1 ligand expression by tumor cells or in the microenvironment often correlates with more aggressive or refractory disease. Despite this, the clinical results reported to date using anti–PD-1 antibodies are very different between HL and other HMs, and so far no other HM has shown the same sensitivity to PD-1 blockade as HL. This raises two fundamental related questions: why do other HMs seem to respond so differently than HL, and what if anything can be done to augment the therapeutic activity of PD-1 blockade outside of HL?
The simplest explanation for the observed vulnerability of HL to PD-1 blockade is the frequent genetically or virally driven overexpression of PD-L1 on RS cells. Under this assumption, the overexpression of PD-L1 on the cell surface would be enough to confer sensitivity to PD-1 blockade; and the lower responses in other HMs would be attributable to a lower rate of PD-L1 overexpression in those tumors. This explanation is certainly plausible since at least in NHL, the documented prevalence of PD-L1 overexpression, as assessed by immunohistochemistry, is lower than in HL.21,40 If this hypothesis were true, then the most obvious development of PD-1 blockade in HM would require selecting PD-L1 expressing malignancies as targets and would forego treatment of non–PD-L1 expressing ones.
Yet, there are several reasons to doubt that selecting tumors for PD-L1 expression will be the best way to optimize PD-1 blockade in HMs. First, it may be relevant to consider the role of PD-L2. Less is known about how PD-L2 expression is determined and how it may impact response to PD-1 blockade. In solid tumors, results with anti–PD-1 and anti–PD-L1 antibodies seemed roughly comparable,9,12 suggesting that disrupting the PD-1/PD-L2 interaction may be clinically less relevant than disrupting the PD-1/PD-L1 interaction. However, it is noteworthy that the genetic amplification event in HL involves both PD-L1 and PD-L2, raising the possibility that PD-L2 may contribute to sensitivity to PD-1 blockade. PD-L2 is also frequently overexpressed in primary mediastinal lymphoma,41 and may therefore represent an important therapeutic target at least in some HMs; this argues first for the necessity to develop widely usable diagnostic antibodies to detect and quantify PD-L2 expression and also for the potential benefit, at least in lymphoma, of targeting the receptor PD-1 (in order to disrupt both the PD-1/PD-L1 and PD-1/PD-L2 interactions) rather than the ligand PD-L1 (which would not affect the PD-1/PD-L2 interaction).
Even for PD-L1 itself, expression of the molecule on the tumor cell surface may not be the sole determinant of sensitivity to PD-1 blockade. The very definition of PD-L1 “positivity” is not clear, and depends on the sensitivity of the diagnostic mAb and the threshold chosen for positivity. At present, different companies and academic centers are using different antibodies, and we have little data to help choose the best diagnostic antibody or threshold. Moreover, although initial data obtained in solid tumors suggested that PD-L1 expression on tumor cells was the strongest determinant of response to PD-1 blockade, there is a meaningful response rate even in apparently PD-L1–negative tumors15,42,43 ; in fact, at least in some cases, the tumor microenvironment may determine response more strongly than PD-L1 tumor expression.15 It must be remembered that PD-L1 expression on tumor cells is a dynamic process, induced by interferon signaling and other factors. Therefore, the immune environment around the tumor likely has a direct impact on PD-L1 expression, and hence, likely also on sensitivity to PD-1 blockade; and the determination of PD-L1 expression on an archival tumor sample may not accurately reflect the degree of PD-1 engagement by tumor cells at the time that therapy is started. Finally, it is interesting to note that HL tumor cells frequently have abnormal major histocompatibility class I (MHC-I) expression, possibly driven by frequent β2-microglobulin mutations,44,45 and may also have downregulated MHC class II expression.19 This raises the question of how an active antitumor immune response can be generated by PD-1 blockade without effective MHC expression. In this light, one could consider that what is most distinctive in HL is that PD-L1 expression is fixed by genetic or viral factors, and hence, less subject to dynamic changes or microenvironmental influences; HL may therefore have a form of signal addiction to PD-1 that explains its high vulnerability to PD-1 blockade.
If fixed upregulation of PD-L1 is the mechanism that explains the sensitivity of HL to PD-1 blockade, then other tumor types with similar patterns of PD-L1/PD-L2 expression may be similarly promising targets for anti–PD-1 antibodies. Primary mediastinal large cell lymphoma may be such a candidate, as it often harbors 9p24.1 amplification18 or rearrangement,46 and concomitant overexpression of PD-L1 and PD-L2. Moreover, certain subtypes of DLBCL, which can be identified by their gene expression signature or by the presence of viral infection of the tumor, also seem to have a high degree of PD-L1 expression,21 which may be similarly fixed as in HL. Those tumors, though not common among DLBCLs, may represent good targets for PD-1 blockade. The studies of nivolumab and pembrolizumab included a few patients with primary mediastinal large cell lymphoma, but the clinical outcome data are not mature enough to confirm this hypothesis.
Other settings for PD-1 and CTLA-4 blockade
Another way to deploy CBT is to target settings where, rather than having a particularly susceptible HM type, it is the immune system itself that is optimally poised for such targeting. This distinction may be especially relevant for immunotherapy, since the state of the immune system and its ability to mount an effective antitumor response are likely to vary significantly over time for a given patient. Front-line treatment may be one such preferred setting, since presumably the immune system in untreated patients is less degraded than in the same patients after they have received extensive chemotherapy or SCT; if so, the magnitude of antitumor responses with CBT could be greater early on in the treatment course than in multiply relapsed disease. This hypothesis is likely to be tested soon in HL, as the promising data in R/R patients are fueling interest in front-line studies; yet, given the current paradigms of drug development, it seems less likely that front-line PD-1 blockade will be attempted in other tumor types in the absence of more robust evidence of activity in R/R patients.
Checkpoint blockade after SCT
Another potentially fertile testing ground for checkpoint blockade is the post-ASCT setting. This is a state characterized both by minimal residual disease and by a remodeling immune system with a relative preponderance in the first few months after transplantation of the lymphocyte subsets that are the likely targets of PD-1 blockade.47,48 This has already been tested in a phase 2 trial of the anti–PD-1 antibody pidilizumab, administered to patients with DLBCL after ASCT.22 In this trial, 72 patients received 3 doses of post-ASCT pidilizumab. The 18-month progression-free survival (PFS) in the 66 eligible patients was 72%, which met the study’s predefined primary end point. Of note, the 18-month PFS was 70% among the 24 patients who had a positive positron emission tomography scan after pre-ASCT salvage therapy. We now recognize this to be a high-risk feature in patients undergoing ASCT.49,50 These results compared favorably to the 52% PFS in an otherwise similarly high-risk historical control population. Moreover, among the patients who had measurable disease after ASCT, the response rate after pidilizumab treatment was 51% (with a 34% CR rate). PD-1 blockade post-ASCT may therefore have important therapeutic efficacy. This is especially important since this is a setting where cure is achievable and is often the last such setting for patients with R/R DLBCL or HL. If post-ASCT CBT can increase the cure rate of ASCT, this would be a very important therapeutic achievement. Further testing in this setting is underway (NCT02362997, NCT02331368).
Finally, CBT may also be uniquely useful after allogeneic hematopoietic cell transplantation. In this setting, one is already relying on a grafted immune system to cure R/R HMs, and immune checkpoint engagement may represent an important mechanism of tumor survival.51,52 Clinical trials have already been performed with CTLA-4 blockade. In a phase 1 study, ipilimumab was administered in a single dose to patients with relapsed HM after allogeneic SCT. Treatment appeared safe, which is a major milestone in a post-allogeneic SCT CBT trial.53 Specifically, with 29 patients treated up to a dose of 3.0 mg/kg, there was no severe graft-versus-host disease (GVHD), no dose-limiting toxicity, and only 3 drug-related grade 3 or 4 events. Furthermore, there was evidence of antitumor activity: 2 patients with HL achieved CR and 1 patient with mantle cell lymphoma achieved a partial remission. A trial is currently underway (NCT01822509) testing repeated dosing of ipilimumab in this setting (4 doses every 3 weeks, followed by maintenance treatment every 12 weeks). Preliminary results have been reported.54 So far, 13 patients have been treated at doses of 3 and 10 mg/kg. The only dose-limiting toxicity was a single case of chronic GVHD, with no case of acute GVHD. Immune-related events were noted but readily manageable. One patient with HL achieved a partial remission, and 3 additional patients (1 with HL, 1 with AML, and 1 with T-NHL) had stable disease. In general, CTLA-4 blockade has been less studied than PD-1 blockade in HM, and the field has followed the results in patients with solid tumors, where PD-1 blockade has to date been associated with a better ratio of efficacy to toxicity. However, the post-allogeneic SCT setting could be different, as preclinical studies in murine models have raised the possibility that blocking PD-L1 could result in significant GVHD.55
The possible association of PD-L1/PD-1 with GVHD control has another important corollary: patients with HM who are treated with PD-1 blockade may eventually proceed on to allogeneic SCT, and the anti-PD1 antibodies administered before SCT could continue to affect immune activity after SCT. It is therefore possible that they could affect the post-allogeneic SCT course, which could in principle translate not only to better efficacy but also to increased toxicity. It will be critical to describe the outcome of those patients as the data matures, in order for us to learn whether allogeneic SCT can be safely performed after CBT, and what the best transplantation type and timing may be in this setting.
Beyond PD-1 and CTLA-4 blockade
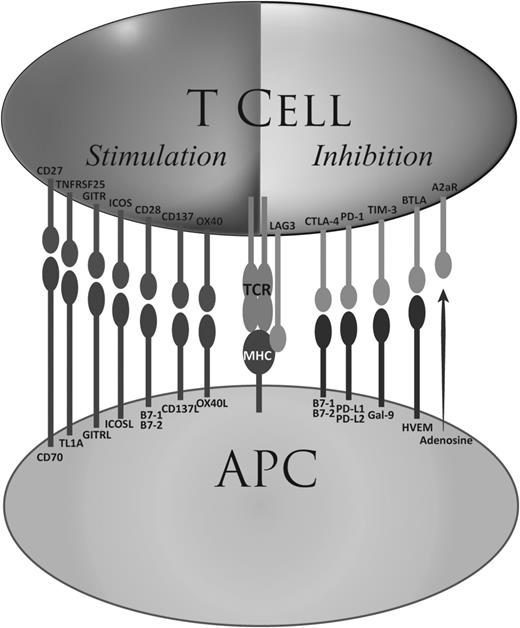
The interaction between T cells and APCs is extremely intricate, and there are now a large number of co-receptors identified, which may serve as up or downregulators of the T-cell response (Figure 2). With the therapeutic successes of CTLA-4 and PD-1 blockade, it is a natural step to target other co-receptors or their cognate ligands.56 Already, mAbs against some of those are in clinical trials. LAG-3, another immune checkpoint, is being targeted in an ongoing phase 1 study in HM (NCT02061761); CD137, a positive regulator of T-cell function, is also targetable using the agonist mAb urelumab, which is in trial for NHL (NCT01775631 and NCT01471210); and CD27 can be targeted using the mAb varlilumab, which is in testing for HM (NCT01460134) with already a documented clinical response.57 Other co-receptors, such as OX-40 and TIM-3 on T cells or killer-cell immunoglobulin-like receptors on natural killer cells are also now targetable and will likely be tested as therapeutic targets in HM in the near future. As our knowledge of immune checkpoints expands, it is likely that even more drugs will reach the clinic over the next few years.
Stimulatory and inhibitory co-receptors. A partial list of currently known stimulatory and inhibitory T-cell co-receptors are shown together with their cognate ligands. HVEM, herpesvirus-entry mediator.
Stimulatory and inhibitory co-receptors. A partial list of currently known stimulatory and inhibitory T-cell co-receptors are shown together with their cognate ligands. HVEM, herpesvirus-entry mediator.
From single-agent to combination treatment
As discussed above, much remains to be learned about PD-1 and CTLA-4 blockade, including which tumors to target and in which setting to most effectively target them. In addition, other checkpoint antibodies are now entering clinical trials as mentioned above. Already, there is a third active avenue of clinical research in CBT, which is the testing of combination therapy. There are at least 3 broad possible ways to approach combination CBT. One is to combine CBT with conventional cytotoxic agents. The hypothesis to support this is that some cytotoxic therapies can provide “immunogenic apoptosis” by releasing tumor antigens at the site of the tumor and allowing better presentation of tumor antigens by APCs58 ; this could not only provide cytoreduction but also immunologically increase the activity of checkpoint blockade.59-61 To some extent, the potential benefit of concomitant cytotoxic therapy may need to be balanced against the inhibition of immune function that attends chemotherapy, as many drugs will affect lymphocytes as well as tumor cells. In theory, it may be best to partner CBT with agents that are relatively sparing of T-cell function, but at present there is no published clinical data to support this assumption. CBT/chemotherapy combination trials will therefore demand special attention to the chosen partners and the sequencing schedules. There is little mature clinical data to date, although many clinical trials are in progress in solid tumors using chemotherapy and CBT. Several trials have also been launched in HM combining CBT with other therapies, such as anti-CD20, anti-CD19 mAbs, or lenalidomide (NCT01775631, NCT02036502, NCT02271945, and NCT02077959).
The second option is to combine different checkpoint agents in an attempt to achieve more complete disinhibition or enhancement of immune function. There is emerging preclinical evidence of the possible benefit of combined CBT in solid tumors62 and in HM,63 and this approach has already met with clinical success in melanoma treatment.64 Several trials are underway testing this approach in HM: for example, a phase 1 study is testing the combination of nivolumab + ipilimumab (NCT01592370), whereas another trial testing nivolumab + urelumab includes an NHL cohort (NCT02253992). It is too early to know whether this strategy will be as fruitful in HM as it appears to be in melanoma, but the results of those trials will likely inform the general direction of CBT combination therapy in the near future.
The last option for combination therapy is to combine CBT with other types of immunotherapy, including cellular immunotherapies such as chimeric antigen receptor (CAR) T cells, tumor vaccines, or oncolytic viral therapy. Those trials are more demanding in terms of infrastructure, and therefore will likely be slower to launch and complete. Nonetheless, this strategy has already been tested in MM, combining a tumor vaccine with pidilizumab, with interesting preliminary results.65 Some HMs with a prior record of successful vaccine therapy, such as FL where CBT seems to also have some efficacy,24,29 may be particularly fruitful targets for this approach. Here, it is important to remember that in principle, for CBT to be effective, there must be tumor-specific antigens able to elicit a response from the disinhibited immune system. Much work is being done currently to identify and target those tumor-specific antigens, fueling the hope that the fields of neo-antigen discovery/targeting and checkpoint blockade will soon combine synergistically. Recent progress with the use of oncolytic viruses may also lend itself to combination anti-cancer therapy with checkpoint blockade.66 Finally, given the ground-shifting therapeutic results obtained in some HMs with CAR–T cells and bi-specific engaging antibodies, 2 strategies that force an interaction between T cells and tumor cells, it is tempting to test whether CBT could enhance the efficacy of this form of immunotherapy.
Checkpoint blockade desiderata
We stand on the verge of an explosion in the field of CBT in hematologic oncology, as has already occurred in solid tumor oncology. Some of the early results with PD-1 blockade hint at a potential paradigm shift in HL, and there are today a very large number of ongoing, planned, and proposed trials. In fact, clinical testing is already outpacing our understanding of the underlying checkpoint biology. All of these trials will doubtlessly provide useful information, likely provide evidence of activity in new tumors and new settings, and possibly change the course of treatment of some HMs. Yet, in order to maximize the gain from those trials, we must take care to extract from them all of the information that we can, and take this knowledge back to the laboratory in order to judiciously design future trials. To this end, there are several issues that may deserve special mention.
The first issue is that of end point choice and response assessment. Current response assessment in HM, revised as recently as the Lugano classification for lymphoma,67 is optimized for conventional cytotoxic therapy. However, the experience with CBT in solid tumors has shown that early progression and delayed responses are not uncommon and are associated with true therapeutic benefit68 ; this has led to the proposal of immune-related response criteria as a more appropriate method of response assessment in solid tumors.69,70 It will be critical to adapt this experience to the world of HM. There will soon be enough clinical data from the various CBT trials, at least in lymphoma, to establish patterns of response by computed tomography and positron emission tomography imaging, to examine the implication of early progression, and to assess the possible therapeutic benefit of stable disease. All of this information can then be used to revise current response criteria. The implications for the field of developing appropriate response criteria for immunotherapy are enormous, since without such modifications, we may not recognize therapeutically valuable results. For example, one may consider the lack of objective responses in MM with nivolumab a disappointing result.29 However, the finding of a 67% stable disease rate, with a previous report of stable disease in a patient with MM treated with pidilizumab,23 could instead suggest a dependence of MM cells on PD-1 for growth and the presence of escape survival pathways that could be simultaneously targeted, as predicted by animal studies.63
A second imperative in CBT may be to focus scientifically on the determinants of response and of resistance to CBT. As discussed above, the demonstration of ligand overexpression on the tumor cell surface may not ultimately be the best predictor of response to CBT; more valuable information may lie in the composition and architecture of the tumor microenvironment, or in the composition and status of peripheral blood and bone marrow immune cells. Furthermore, much may be learned from comparison of tumor, tumor microenvironment, or marrow/peripheral blood composition between pretreatment and posttreatment tumor samples both in responders and in nonresponders. It is possible that, upon treatment with a particular checkpoint inhibitor, the tumor could downregulate expression of the relevant checkpoint ligand, or upregulate that of alternative checkpoint ligands; knowledge of those escape pathways would directly impact on the design of CBT combination therapy. A recent study in melanoma-bearing mice demonstrated that treatment with radiation and anti-CTLA4 mAb led to increased expression of PD-L1,71 suggesting that parallel checkpoint pathways may indeed serve as escape mechanisms for checkpoint blockade. Our ability to further decipher those resistance mechanisms and correlate them with clinical outcome should facilitate the choice of rational combination therapies from the explosively growing number of possible clinical trials. The potential rewards of this type of work will ideally motivate the scientific sharing of specimens, assays, and data among institutions and pharmaceutical companies through HM-specific immuno-oncology consortia.
Conclusions
Immunotherapy is revolutionizing oncology, and HM should be among the most fertile grounds to employ this strategy. CBT has had a dramatic impact on the treatment of some solid tumors, and seems poised to do the same in at least some types of hematologic cancers. There are many new drugs and targets, many tumors and settings to test them in, and myriad possible combinations. Therefore, the perspectives in CBT for HM are vast and enticing. However, CBT is very unlikely to be a panacea across HM, and much remains to be done in a coordinated and judicious fashion, to maximize its therapeutic potential. Perhaps this phenomenal scientific and clinical opportunity will allow us to redefine not only trial end points, but the very way in which collaborative clinical research is conducted across industry and academia.
Acknowledgments
The author thanks Drs Margaret Shipp and Gordon Freeman for their assistance with this manuscript.
Authorship
Contribution: P.A. wrote the paper.
Conflict-of-interest disclosure: P.A. has received research funding from Merck, Bristol-Myers Squibb, and consultancy fees from Merck.
Correspondence: Philippe Armand, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: parmand@partners.org.