Abstract
Neurologic complications are a major cause of morbidity and mortality in sickle cell disease (SCD). In children with sickle cell anemia, routine use of transcranial Doppler screening, coupled with regular blood transfusion therapy, has decreased the prevalence of overt stroke from ∼11% to 1%. Limited evidence is available to guide acute and chronic management of individuals with SCD and strokes. Current management strategies are based primarily on single arm clinical trials and observational studies, coupled with principles of neurology and hematology. Initial management of a focal neurologic deficit includes evaluation by a multidisciplinary team (a hematologist, neurologist, neuroradiologist, and transfusion medicine specialist); prompt neuro-imaging and an initial blood transfusion (simple followed immediately by an exchange transfusion or only exchange transfusion) is recommended if the hemoglobin is >4 gm/dL and <10 gm/dL. Standard therapy for secondary prevention of strokes and silent cerebral infarcts includes regular blood transfusion therapy and in selected cases, hematopoietic stem cell transplantation. A critical component of the medical care following an infarct is cognitive and physical rehabilitation. We will discuss our strategy of acute and long-term management of strokes in SCD.
Introduction
Strokes in children and adults with sickle cell disease (SCD) continue to be a major cause of morbidity. Understanding the epidemiology of overt strokes is critical to the multidisciplinary, and timely acute and long-term management of strokes. The first neurologic complication associated with SCD was described in a child with sickle cell anemia (SCA), seizures, and acute left hemiparesis in 1923.1 In the 1970s, a single center retrospective cohort study by Powars et al,2 described for the first time the high rate of overt ischemic strokes in children and adults with SCD (∼6%), and their high recurrence rate (∼50%) in the first 2 years after the initial event, and 66% in the 9 years following the initial stroke. In the 1990s, investigators from the Cooperative Study of Sickle Cell Disease provided the most definitive and comprehensive study of the natural history of strokes across the lifespan.3 Children and adults with SCA (hemoglobin SS [HbSS]) have a high prevalence (4.01%) and incidence (0.61 per 100 patient years) of cerebrovascular accidents.3 Among patients with SCA, ischemic strokes were observed to have a bimodal distribution, being more common in children and older adults, and lowest in adults aged 20 to 29 years. Risk factors associated with ischemic strokes include prior transient ischemic attack (TIA), low steady-state hemoglobin (Hb) concentration, rate of, and recent episode of acute chest syndrome (ACS), and elevated systolic blood pressure (BP). Among individuals with SCA, hemorrhagic stroke was most frequent in the 20- to 29-year age group. Associated risk factors included low steady-state Hb and high leukocyte count.3 Similar to observations by Powars et al,2 in this cohort, hemorrhagic strokes were associated with a high mortality rate (24% overall and 26% in individuals with SCA). This review describes our multidisciplinary approach to acute and long-term management of strokes. For illustrative purposes, we describe 2 cases, a child and an adult with SCA, presenting with focal neurologic deficit, highlighting inherent challenges in care, while also emphasizing current approaches in the acute, sub-acute, and long-term management of strokes.
Case presentations: acute stroke and its management
Case 1. A 16-year-old African American female with SCA, HbSS, and a history of overt stroke at 4 years of age, was admitted to the hospital for generalized body pains, fever, shortness of breath, and productive cough. She was initially started on regular blood transfusion therapy, but was switched to hydroxyurea therapy because of her family’s decision to stop regular blood transfusion therapy. At presentation, her vital signs were: BP 140/60 mm Hg, pulse rate 125, temperature 102.5°F, and oxygen saturation was 93% on room air. Baseline Hb was 7 gm/dL. Chest radiographs showed left lower lobe opacity suggestive of lobar pneumonia. On day 6 of hospitalization, she was found on the floor, combative and disoriented with seizure-like activity. She was intubated for airway protection. Initial magnetic resonance imaging (MRI) and magnetic resonance venogram (MRV) revealed new acute infarcts in the left parietal, bilateral temporal, and occipital lobes. Initial supportive care measures included management of severe agitation, delirium, and seizure activity. Hb S percentage (HbS%) on admission was 87%, and she received initial simple transfusion followed subsequently by exchange transfusion, with a goal HbS% of 15% or less and target hematocrit of 28%.
Case 2. A 38-year-old African American male with SCA, HbSS, complicated by a history of diabetes mellitus, hypertension, and no prior strokes, presented to the Emergency Department with dysphasia for a week. Evaluation revealed a right lower facial droop and dysarthria consistent with an infarct in the left hemisphere. His MRI showed an acute lacunar stroke in the left corona radiata, as well as old deep lacunae in bilateral basal ganglia. His MRV of the brain did not reveal any central sinus venous thrombosis (CSVT). He was evaluated by a multidisciplinary team (ie, a hematologist, neurologist, neuroradiologist, and laboratory transfusion medicine specialist). Thrombolysis with tissue plasminogen activator was initially considered but was contraindicated based on prolonged duration of symptoms. Non-SCD–related risk factors were thought to be most likely associated with etiology of the stroke. He was admitted to the stroke service for further management.
Differential diagnosis of acute presentation of focal neurologic deficit
Children and adults with SCD who present with acute focal neurologic deficits should be considered for the following differential diagnosis:
Acute arterial stroke (ischemic infarct): is defined as an acute syndrome caused by the restriction of cerebral blood flow with resultant ischemia and neurologic symptoms, typically with an abnormal MRI of the brain.4 A TIA is considered a transient focal neurologic deficit associated with dysfunction caused by focal brain, spinal cord, or retinal ischemia, without neuroimaging evidence of cerebral infarction.5 In children and adults with SCD, we typically obtain an MRI of the brain to distinguish between a stroke and a TIA because in the former case, strong consideration is given for lifelong regular transfusion therapy, whereas in the latter case, we provide regular blood transfusion therapy only in the presence of other risk factors, such as prior TIAs, conditional transcranial Doppler ultrasound measurements,6 cerebral vasculopathy,7 or the presence of silent cerebral infarcts.8 All of these additional factors increase the likelihood of the patient having a future stroke.
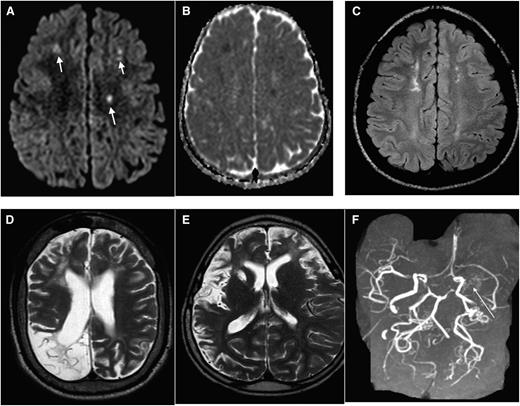
Due primarily to compromised cerebral hemodynamics, many cerebral infarcts are located in the border zone regions of the brain: the area between the cortical territories of the anterior cerebral artery, middle cerebral artery (MCA), and posterior cerebral artery; and in the periventricular white matter between the deep and the superficial arterial systems of the MCA, or between the superficial systems of the MCA and anterior cerebral artery, referred to as the internal border-zone region.9 Figure 1A-F shows a spectrum of MRI abnormalities ranging from cerebral infarcts, encephalomalcia and vasculopathy in individuals with SCD.
Hemorrhagic stroke: is the most common neurologic event in adults with SCD.3 No etiology for hemorrhagic stroke is identified in most cases. Among those with an etiology, subarachnoid hemorrhage is the most common and is often associated with intracranial aneurysm.10,11 The presence of hemorrhagic stroke does not exclude the possibility of ischemic stroke,12 and if a concomitant ischemic stroke is identified, there should be consideration for regular blood transfusion therapy. Other than the presence of aneurysms, risk factors for hemorrhagic stroke include low Hb, high steady-state leukocyte,3 recent blood transfusion (in the last 14 days), treatment with corticosteroids, or ACS.13-17 An evidence-based approach to care is lacking in patients with SCD presenting with acute hemorrhagic stroke. Recognition of associated risk factors for hemorrhagic stroke is important in treatment stratification, including strong consideration for cerebral angiography in order to exclude intracranial aneurysm. An MRI of the brain ruled out cerebral hemorrhage in the pediatric and adult cases described.
Seizures: the prevalence of seizures in children with SCD is 10 times that of the general population.18,19 Prengler et al found in a cohort of individuals with SCD, that those with seizures had increased perfusion and electroencephalographic abnormalities, suggesting that vasculopathy and focal hypoperfusion may be factors in the development of SCD-associated seizures.18 In case 1, an electroencephalogram was done, which revealed generalized nonspecific cerebral dysfunction and no ictal or interictal epileptiform discharges were recorded.
Hemiplegic migraine: migraines, particularly hemiplegic migraine headaches should be part of the differential diagnosis of a patient with SCD who presents with a focal neurologic deficit. Recurrent headaches and migraines are common and undertreated in SCD, with a prevalence of 25% to 30% of children with SCD, higher than seen in the general pediatric population.20,21 Headaches with migraines are seen in half of these patients, whereas tension-type headaches are seen in the remainder.20 Careful consideration must be given to a patient’s history, with particular attention to family history, because hemiplegic migraine headache may be inherited as an autosomal dominant disorder22-24 with incomplete penetrance.25 Repeated atypical presentations of hemiplegia in patients with negative MRI scans and a family history of migraines should prompt clinicians to consider this diagnosis. Although possible, the diagnosis in both cases presented was unlikely based on the very low prevalence, absence of a family history, and no prior episodes. The presence of acute cerebral infarcts on MRI also excluded this differential in the cases presented.
Posterior reversible encephalopathy syndrome (PRES): PRES was initially defined as a reversible clinical–radiologic syndrome, with patients presenting with a constellation of symptoms, including headache, seizure, visual disorders, and altered mental status, and supported by imaging findings that show parietal and occipital involvement of the brain,26 likely resulting from vasogenic edema. PRES can be associated with ACS.27 Evidence exists that not all cases of PRES are reversible, nor do they always involve the posterior brain.27-29 Distinguishing acute cerebral infarcts from PRES can be challenging.30 MRI is the imaging of choice, and although both PRES and cerebral infarcts present with abnormal hyperintensities on fluid sensitive sequences, the distribution of signal abnormality is usually different in PRES vs infarcts. Diffusion weighted image (DWI) typically is often positive in cerebral infarcts; whereas DWIs in PRES may or may not be positive.31,32 The MRI (DWI) of the brain obtained in both case presentations eliminated the diagnosis of PRES. This diagnosis was also less likely in case 1 due to the absence of elevated systemic BP, and the distribution of the lesions on brain MRI (DWI) in case 2 decreased the likelihood of this diagnosis.
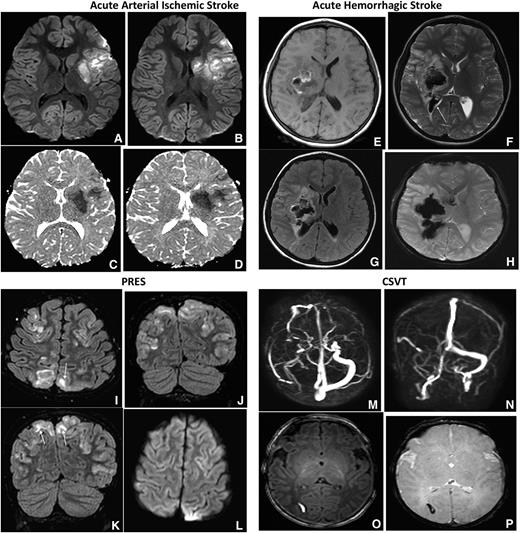
CSVT: is a less common neurologic complication in SCD when compared with ischemic strokes. Despite the low frequency, detecting the presence of CSVT is important because its presence may alter the treatment strategy. Children and adults with SCD and CSVT can present with symptoms that mimic a stroke, such as seizures, coma, cranial nerve palsies, headaches, nausea, and vomiting.33,34 An evaluation with a MRV is the preferred initial imaging study because the imaging sequence only adds a maximum of 7 minutes to the initial MRI to detect an ischemic or hemorrhagic cerebral infarct. In case 1, the MRI and MRV obtained initially excluded the possibility of CSVT, whereas in case 2, CSVT was ruled out with a subsequent MRV. The optimal duration of anticoagulation in patients with SCD presenting with focal neurologic deficit attributable to CSVT is unknown, though recent guidelines recommend 3 to 6 months with periodic reassessment of the thrombus. We recommend following approved guidelines for both acute and chronic management of CSVT, including anticoagulation.35 Figure 2A-P shows MRI findings in the differential diagnosis of patients with SCD presenting with focal neurologic deficit.
MRI in SCD: spectrum of abnormality in SCD as illustrated by different imaging examples. Axial DWI (A) and apparent diffusion coefficient (ADC) (B) map of the brain demonstrating multiple foci of restricted diffusion along bifrontal regions (corresponding to the short arrows in A), along the internal watershed zone most consistent with acute strokes. (C) Axial fluid attenuation inversion recovery (FLAIR) image in a different patient with SCD demonstrate areas of old (silent) infarctions in a similar internal watershed distribution. (D) Axial T2 image shows a large area of right occipital cystic encephalomalacia resulting from prior infarction. (E) Axial T2 image from another patient shows diffuse volume loss, sulcal prominence, and an old right basal ganglia infarct from sickle cell-associated vasculopathy. (F) Maximum intensity projection image from the MRA shows nonvisualization of the left MCA with multiple collateral in the left lenticulostriate distribution (long arrow), consistent with moyamoya collaterals.
MRI in SCD: spectrum of abnormality in SCD as illustrated by different imaging examples. Axial DWI (A) and apparent diffusion coefficient (ADC) (B) map of the brain demonstrating multiple foci of restricted diffusion along bifrontal regions (corresponding to the short arrows in A), along the internal watershed zone most consistent with acute strokes. (C) Axial fluid attenuation inversion recovery (FLAIR) image in a different patient with SCD demonstrate areas of old (silent) infarctions in a similar internal watershed distribution. (D) Axial T2 image shows a large area of right occipital cystic encephalomalacia resulting from prior infarction. (E) Axial T2 image from another patient shows diffuse volume loss, sulcal prominence, and an old right basal ganglia infarct from sickle cell-associated vasculopathy. (F) Maximum intensity projection image from the MRA shows nonvisualization of the left MCA with multiple collateral in the left lenticulostriate distribution (long arrow), consistent with moyamoya collaterals.
Acute arterial ischemic and hemorrhagic stroke, PRES and CSVT. Axial DWI and ADC (shown in A-B and C-D in each of the 4 sets of images, respectively) MRIs of the brain demonstrate increased DWI signal with corresponding decreased ADC signal, consistent with restricted diffusion along the left MCA territory most consistent with acute arterial ischemic stroke. Axial T1-weighted MRI (E), axial T2-weighted MRI (F), axial FLAIR (G), and axial GRE (H) images in patients with SCA demonstrate ill- defined mixed intensity focus within the right basal ganglia region with surrounding vasogenic edema. The lesion demonstrates T1 hyperintensity with hypointensity on axial T2 and GRE consistent with hemorrhage. Also seen is intraventricular extension of blood (F, arrow). GRE MR sequences are most sensitive for the detection of intracranial hemorrhage and may demonstrate more diffuse signal loss than the actual lesion, also known as blooming, as seen in this case. Axial FLAIR (I) and coronal FLAIR images MR (J-K) of the brain demonstrates bilateral near symmetric hyperintense signal involving the parieto-occipital lobes with superior frontal involvement, in a distribution and pattern most consistent with PRES. There is both subcortical (I, K arrows) and cortical involvement. Axial DWI (L) demonstrates no corresponding increased signal to suggest for restricted diffusion. Transverse (M) and coronal (N), maximum intensity projection images from a phase contrast MRV. There is no signal within the right transverse sinus due to thrombosis. Axial T1-weighted MRI (O) demonstrates a small well-defined hyperintense focus within the right occipital white matter with hypointensity on axial GRE (P), consistent with hemorrhagic venous infarction secondary to thrombosis.
Acute arterial ischemic and hemorrhagic stroke, PRES and CSVT. Axial DWI and ADC (shown in A-B and C-D in each of the 4 sets of images, respectively) MRIs of the brain demonstrate increased DWI signal with corresponding decreased ADC signal, consistent with restricted diffusion along the left MCA territory most consistent with acute arterial ischemic stroke. Axial T1-weighted MRI (E), axial T2-weighted MRI (F), axial FLAIR (G), and axial GRE (H) images in patients with SCA demonstrate ill- defined mixed intensity focus within the right basal ganglia region with surrounding vasogenic edema. The lesion demonstrates T1 hyperintensity with hypointensity on axial T2 and GRE consistent with hemorrhage. Also seen is intraventricular extension of blood (F, arrow). GRE MR sequences are most sensitive for the detection of intracranial hemorrhage and may demonstrate more diffuse signal loss than the actual lesion, also known as blooming, as seen in this case. Axial FLAIR (I) and coronal FLAIR images MR (J-K) of the brain demonstrates bilateral near symmetric hyperintense signal involving the parieto-occipital lobes with superior frontal involvement, in a distribution and pattern most consistent with PRES. There is both subcortical (I, K arrows) and cortical involvement. Axial DWI (L) demonstrates no corresponding increased signal to suggest for restricted diffusion. Transverse (M) and coronal (N), maximum intensity projection images from a phase contrast MRV. There is no signal within the right transverse sinus due to thrombosis. Axial T1-weighted MRI (O) demonstrates a small well-defined hyperintense focus within the right occipital white matter with hypointensity on axial GRE (P), consistent with hemorrhagic venous infarction secondary to thrombosis.
Multidisciplinary approach to management of a focal neurologic deficit in a child or adult with SCD
Acute care
In children or adults presenting with an acute focal neurologic deficit, initial supportive strategies should include: immediate evaluation by the hematology and neurology services with discussion of the assessment and plan for imaging of the brain, and initial laboratory evaluation to include at least complete blood count, reticulocyte count, type and cross match, HbS%, prothrombin time, activated partial thromboplastin time, and basic metabolic profile. Oxygen therapy should be initiated to keep oxygen saturation >95%, and if febrile, patient should be given antipyretics and started on empiric antibiotics. The diagnosis of an acute stroke and the decision to perform a timely exchange transfusion requires a multidisciplinary team, with input from the hematologist, neurologist, neuroradiologist, and transfusion medicine specialist.
To optimize imaging results, we have created a predefined MRI imaging sequence, modified from the hospital-wide acute stroke protocol. If the sequences of the MRI of the brain can be changed to detect cerebral hemorrhage,36 and done promptly, an MRI of the brain is the preferred strategy over a brain computed tomography (CT) scan37 to detect both hemorrhage and cerebral infarct. Further, using an MRI/DWI scan can determine whether the ischemic event occurred within the last 10 days.32,38-40 In a patient with focal neurologic deficit, distinguishing acute cerebral infarcts from old infarcts with an MRI is clinically relevant because the decision to perform acute exchange transfusion may require central line placement, multiple units of blood, and other associated risks, including the possibility of a stroke.41 In rare instances, children and adults with focal neurologic deficits may have a negative MRI/DWI scan, specifically negative DWIs, within 24 hours of the onset of symptoms.42,43 If MRI changes are absent, other diagnostic considerations include hemiplegic migraine, Todd’s paralysis following seizures, PRES,26,44,45 and CSVT.33,34 To decrease the likelihood of failing to diagnose CSVT, we recommend performing an MRV with the initial MRI. We do not initially perform a magnetic resonance angiogram (MRA) of the brain because the presence of cerebral vasculopathy does not alter the acute management.
Based on the established benefits of initial blood transfusion therapy in SCD, increasing Hb levels with increased oxygen delivery while simultaneously decreasing the HbS levels46 (both of which are associated with improvement of blood flow to the brain), our strategy is to perform an initial exchange transfusion.47 Additional indirect evidence is based on the observation that at the vast majority of hematology centers, an initial exchange transfusion with a prior simple transfusion or with an exchange alone is preferred over simple transfusion for children presenting with acute strokes.47 The optimal level to raise the Hb with an initial simple transfusion is not known, but we typically do not raise the Hb above 10 gm/dL unless the HbS% is <30% due to concerns for hyperviscosity and further neurologic injury.17,48,49 Table 1 provides the checklist we use when organizing an exchange transfusion therapy in an individual with focal neurologic deficit.50
Checklist for acute management of patients with SCD presenting with a focal neurologic deficit
| Checklist . |
|---|
| 1. Immediate assessment by the hematology and neurology service with shared discussion of the assessment and plan. |
| 2. Laboratory evaluation to include at least complete blood count with reticulocyte count, type and cross, prothrombin time, activated partial thromboplastin time, basic metabolic panel, and HbS quantification. |
| 3. Recommended imaging approach (may be institution specific): |
| CT scan of the brain to exclude cerebral hemorrhage, followed by MRI and MRV of the brain after the patient is stable (<6 h). |
| Alternatively, only an MRI and MRV of the brain may be performed if the images can be obtained within 60 min of evaluation and the scan sequence has been set up to detect cerebral hemorrhage. |
| Imaging may require sedation and support of the sedation team (this should be done after hematology and neurology service evaluation). |
| 4. Obtain vascular access and consultation with transfusion medicine team in preparation for blood transfusion. Obtain transfusion consent from patient and/or next of kin. |
| 5. Transfusion therapy approach: |
| Initiate emergent exchange blood transfusion therapy preferred. |
| Initial simple blood transfusion preferred if Hb is <10 gm/dL, followed by an exchange blood transfusion as soon as possible (requires assistance from intensivist for potential admission). |
| Simple transfusion or exchange blood transfusion may not be indicated if the Hb upon admission is >10 gm/dL or <50% of baseline, respectively. |
| 6. Supportive care measures |
| Oxygen administration to keep oxygen saturation >95%. |
| If febrile, blood culture, antipyretics, and antibiotics should be administered. |
| Checklist . |
|---|
| 1. Immediate assessment by the hematology and neurology service with shared discussion of the assessment and plan. |
| 2. Laboratory evaluation to include at least complete blood count with reticulocyte count, type and cross, prothrombin time, activated partial thromboplastin time, basic metabolic panel, and HbS quantification. |
| 3. Recommended imaging approach (may be institution specific): |
| CT scan of the brain to exclude cerebral hemorrhage, followed by MRI and MRV of the brain after the patient is stable (<6 h). |
| Alternatively, only an MRI and MRV of the brain may be performed if the images can be obtained within 60 min of evaluation and the scan sequence has been set up to detect cerebral hemorrhage. |
| Imaging may require sedation and support of the sedation team (this should be done after hematology and neurology service evaluation). |
| 4. Obtain vascular access and consultation with transfusion medicine team in preparation for blood transfusion. Obtain transfusion consent from patient and/or next of kin. |
| 5. Transfusion therapy approach: |
| Initiate emergent exchange blood transfusion therapy preferred. |
| Initial simple blood transfusion preferred if Hb is <10 gm/dL, followed by an exchange blood transfusion as soon as possible (requires assistance from intensivist for potential admission). |
| Simple transfusion or exchange blood transfusion may not be indicated if the Hb upon admission is >10 gm/dL or <50% of baseline, respectively. |
| 6. Supportive care measures |
| Oxygen administration to keep oxygen saturation >95%. |
| If febrile, blood culture, antipyretics, and antibiotics should be administered. |
Adapted from DeBaun50 with permission.
In adult patients with SCD presenting with acute ischemic stroke, the preferred initial approach remains an exchange transfusion because of the very high risk of recurrent cerebral infarcts. There is an ∼50% recurrence rate in the first 2 years if no treatment is initiated.2 Limited data exists regarding the use of thrombolysis in adults with SCD presenting with an acute stroke. The decision to treat patients with thrombolytics should be made on a case-by-case basis by a multidisciplinary team. Potential evidence that thrombolytic therapy may be considered in a patient with SCD presenting with an ischemic stroke is the presence of Hb SCD and comorbidity associated with strokes, particularly atrial fibrillation.51 However, more definitive data are needed to promote thrombolytic therapy for individuals with SCD.
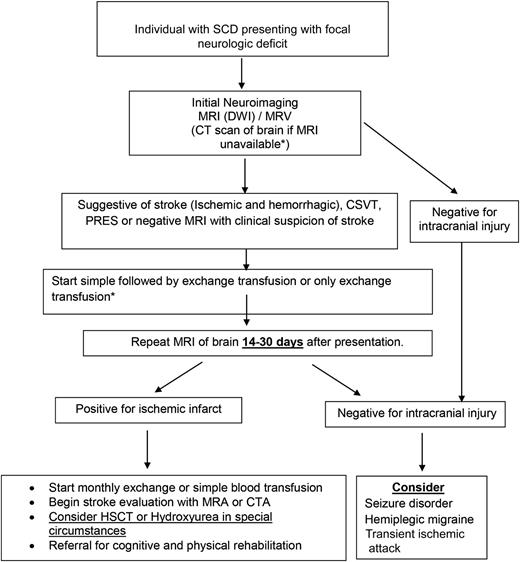
Given the lack of formal evidence on how to manage acute hemorrhagic strokes in children and adults with SCD, when feasible and appropriate, we refer to guidelines for the management of spontaneous intracerebral hemorrhage in the general population.52 Figure 3 shows the schema for the management of children and adults with SCD presenting with focal neurologic deficit.
Schema for the management of a patient with SCD presenting with focal neurologic deficit. Asterisk (*) denotes when an initial blood transfusion (simple followed quickly by an exchange transfusion) is recommended if the Hb is <10 gm/dL; an exchange transfusion is preferred when it can be expedited, or when Hb >10 gm/dL. CTA, computed tomography angiogram; HSCT, hematopoietic stem cell transplant.
Schema for the management of a patient with SCD presenting with focal neurologic deficit. Asterisk (*) denotes when an initial blood transfusion (simple followed quickly by an exchange transfusion) is recommended if the Hb is <10 gm/dL; an exchange transfusion is preferred when it can be expedited, or when Hb >10 gm/dL. CTA, computed tomography angiogram; HSCT, hematopoietic stem cell transplant.
Sub-acute care of a child or adult with SCD and an ischemic stroke
After initial stabilization and institution of transfusion therapy, we shift our focus to secondary stroke prevention. Following a stroke, the risk of recurrent stroke is estimated to be 60% to 90% without therapeutic intervention,2 thus a long-term strategy for secondary stroke prevention is needed. We commonly repeat the MRI of the brain within 14 to 30 days after the initial stroke, regardless of the cause, to ensure an appropriate baseline evaluation and for ongoing surveillance of the initial stroke, because the CT53,54 or MRI55 performed initially may not represent the true extent of the cerebral infarct.
In a prospective trial, despite receiving regular blood transfusion therapy for secondary prevention, 100% of participants had a cerebral infarct recurrence (overt and/or silent) when progressive vasculopathy was present.7 Based on these findings, we perform an MRA evaluation, typically with the first follow-up scan after the initial MRI.7
Screening for other comorbidities associated with strokes, such as hypertension, diabetes mellitus, or hyperlipidemia is important. Additionally, evaluation for a patent foramen ovale and other intracardiac shunts should be considered, as they are associated with strokes in children56 and in adults with SCD.57 Table 2 shows our checklist for sub-acute care of patients with SCD following an acute stroke.
Brief checklist of our sub-acute care strategy in patients with SCD following an acute stroke
| Steps . | Actions . |
|---|---|
| 1. | Discuss MRI results with the patient/family face to face in clinic |
| 2. | Refer to neurologist to confirm new baseline for any focal neurologic deficit |
| 3. | Refer to psychologist for testing to identify cognitive strengths and weaknesses, and determine the need for school/workplace accommodations. Domains to assess include: |
| General intellectual functioning | |
| Attention | |
| Memory | |
| Speed of information processing | |
| Executive function | |
| Visual-motor skills | |
| Academic achievement | |
| Behavioral and emotional adjustment | |
| 4. | Meet with patient and/or family to discuss results of #2, #3, and treatment recommendations when appropriate, based on patient’s/parents’ wishes, cognitive test scores, and school/work performance |
| Blood transfusion therapy | |
| Hydroxyurea in special circumstances | |
| Advocate for written IEP or workplace accommodations | |
| Adults are referred to vocational and/or neurologic rehabilitation programs | |
| 5. | Perform annual MRI/MRA to evaluate for new infarcts or progressive vasculopathy. If signs of progression are seen, then discuss about alternative therapies including SCT, if already started on regular blood transfusion therapy. |
| Steps . | Actions . |
|---|---|
| 1. | Discuss MRI results with the patient/family face to face in clinic |
| 2. | Refer to neurologist to confirm new baseline for any focal neurologic deficit |
| 3. | Refer to psychologist for testing to identify cognitive strengths and weaknesses, and determine the need for school/workplace accommodations. Domains to assess include: |
| General intellectual functioning | |
| Attention | |
| Memory | |
| Speed of information processing | |
| Executive function | |
| Visual-motor skills | |
| Academic achievement | |
| Behavioral and emotional adjustment | |
| 4. | Meet with patient and/or family to discuss results of #2, #3, and treatment recommendations when appropriate, based on patient’s/parents’ wishes, cognitive test scores, and school/work performance |
| Blood transfusion therapy | |
| Hydroxyurea in special circumstances | |
| Advocate for written IEP or workplace accommodations | |
| Adults are referred to vocational and/or neurologic rehabilitation programs | |
| 5. | Perform annual MRI/MRA to evaluate for new infarcts or progressive vasculopathy. If signs of progression are seen, then discuss about alternative therapies including SCT, if already started on regular blood transfusion therapy. |
Long-term management of stroke and sequelae
Regular blood transfusion therapy: simple vs exchange transfusion for secondary stroke prevention
For both children and adults with strokes, we employ the same strategy for secondary stroke prevention because our clinical experience, along with the literature, suggest that both children and adults with SCD have an increased rate of additional strokes after the initial ischemic stroke.2 Given the benefits of a less positive iron store with exchange transfusion (manual or automated), we strongly prefer this strategy to simple blood transfusions for regular blood transfusion therapy. At the very least, when possible, we perform a manual exchange,58,59 that is available to most hematology programs.58
Hydroxyurea therapy for secondary stroke prevention
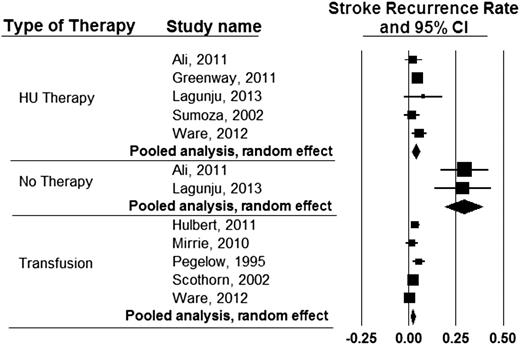
Unique circumstances may prohibit regular blood transfusion therapy as a strategy for secondary prevention of strokes. Although hydroxyurea therapy is not as effective as regular blood transfusion therapy for secondary stroke prevention,60 this is a reasonable alternative when compared with no therapy at all. To demonstrate the therapeutic benefit of hydroxyurea therapy, we pooled results of the most recent studies where patients were specifically evaluated for secondary stroke prevention with regular blood transfusion therapy,7,60-63 hydroxyurea therapy,60,64-67 or no therapy64,66 (Figure 4).
Incidence rates of recurrent stroke in individuals with SCD receiving either hydroxyurea therapy, blood transfusion therapy, or no therapy for secondary stroke prevention in published studies since 1995. The expected incidence rates of stroke recurrence while on regular blood transfusion therapy, hydroxyurea therapy, or no therapy were found to be 1.9 (95% CI, 1.0-2.9), 3.8 (95% CI, 1.9-5.7), and 29.1 (95% CI, 19.2-38.9) events per 100 patient years, respectively.
Incidence rates of recurrent stroke in individuals with SCD receiving either hydroxyurea therapy, blood transfusion therapy, or no therapy for secondary stroke prevention in published studies since 1995. The expected incidence rates of stroke recurrence while on regular blood transfusion therapy, hydroxyurea therapy, or no therapy were found to be 1.9 (95% CI, 1.0-2.9), 3.8 (95% CI, 1.9-5.7), and 29.1 (95% CI, 19.2-38.9) events per 100 patient years, respectively.
Matched related hematopoietic stem cell transplant (HSCT) for secondary stroke prevention
Based on the progressive nature of infarct recurrence, even with optimal blood transfusion therapy,7 we believe a matched, related HSCT is the preferred first line therapy for secondary prevention of overt strokes in children with SCD. This strategy is a departure from the National Heart, Lung, and Blood Institute guidelines for the management of acute stroke in SCD, where there was no mention of transplant as a therapeutic option for secondary stroke prevention.68 Our indication for HSCT is based on the preponderance of observational studies with sufficient follow-up time, suggesting that transplant appears more efficacious in secondary stroke prevention than regular blood transfusion therapy. Specifically, 4 studies69-72 with a minimum 5-year follow up, demonstrated the efficacy of HSCT for secondary prevention of strokes. In each HSCT study, the rate of infarct recurrence in patients with a prior stroke was much less than when compared with regular blood transfusion therapy, where you can expect almost 50% of children will have infarct recurrence over a mean follow-up of 5.5 years.7 Based on these studies, we believe that blood transfusion therapy for secondary stroke prevention is palliative, and HSCT offers both a chance for cure from the disease and is effective in secondary stroke prevention.
Experimental HSCT for secondary stroke prevention
Given the progressive nature of cerebral infarcts in children and adults, patients with no matched sibling donor should be offered alternate donor transplants in the setting of clinical trials. Unrelated donor transplants,73 umbilical cord blood,74 or haploidentical HSCT75 are increasingly being evaluated in clinical trials. Patients who meet the eligibility criteria should be offered these options in the context of clinical trials with a sufficient number of clinical centers enrolled to answer the research question.
Management of transfusion-related excessive iron stores
Even in the best of circumstances, after 2 years of regular blood transfusion therapy, most patients will require iron chelation to decrease excessive iron stores. We generally apply the following scoring system for hepatic iron assessment with Gradient-echo (GRE)/T2* MRI: a normal hepatic iron concentration (HIC) is <1.2 mg/g dry weight; an HIC of 3.2 to 7.0 mg/g dry weight indicates an optimal chelation range, an HIC of 7 to 15 mg/g dry weight indicates an elevated hepatic iron level (and a potential for organ toxicity), and an HIC >15 mg/g dry weight is considered significant (almost 10× the upper limit of normal) and is associated with a very high possibility of organ toxicity.76-78 Our experience is similar to that of Meloni et al,78 who demonstrated that after many years of blood transfusion and excessive iron stores, patients with SCD can develop myocardial dysfunction or liver dysfunction. This is in contrast to the perception that individuals with SCD do not have organ dysfunction associated with excessive iron stores.79
After 1 to 2 years of regular blood transfusion therapy, an increased HIC, and about 2.0 to 7.0 mg/g dry weight, we initially start with deferasirox (20 mg/kg per day). However, if the annual GRE/T2* MRI of the liver demonstrates an increase in the HIC for some patients because of either non-adherence or poor response to chelation, we increase the deferasirox dose to 40 mg/kg per day. If there is still a significant rise in HIC with the MRI of the liver at 6- or 12-month intervals, we add subcutaneous deferoxamine 40 mg/kg per day.
When the HIC >15 mg/g dry weight, we prefer to use inpatient IV deferoxamine plus deferasirox. The optimal dose of IV deferoxamine combined with deferasirox is unknown. The package insert for deforaxamine states that the standard dose for children is up to 40 mg/kg per day and for adults up to 60 mg/kg per day to be given via slow subcutaneous infusion over 8 to 12 hours. Based on studies in individuals with thalassemia demonstrating the efficacy of combination therapy of an IV deferoxamine dose of 35 to 50 mg/kg for 2 to 7 days, coupled with deferasirox of ∼20 mg/kg per day,80,81 we now use this regimen when applying dual chelation. Specifically, we use an IV deferoxamine dose of 50 mg/kg per day for our young patients, similar to Davis et al,82 coupled with deferasirox 20 to 40 mg/kg per day. If deferasirox is not tolerated, we then use IV deferoxamine alone at a dose of 100 mg/kg per day for up to 3 days (a level that is well below the threshold associated with pulmonary toxicity, 240 and 528 mg/kg per day,83,84 and even below previously tolerated IV deferoxamine in individuals with SCD with excessive iron stores, 360 mg/kg per day85 for 2 days and 150 mg/kg per day for 3 days).86 Ultimately, specific dual chelation trials are needed to identify optimal therapy regimens in individuals with SCD and excessive iron stores.
Physical rehabilitation and education remediation
Regardless of age, typically at the first outpatient visit, we refer the patient to a stroke rehabilitation service and arrange cognitive testing to assess the level of cognitive impairment. For children with strokes, we use the cognitive testing to inform the development of an Individualized Education Program (IEP),87 required by special education law to help children with disabilities meet academic standards. In some instances, third-party payers are unwilling to pay for cognitive testing; however, our experience is that the long-term benefits of cognitive testing, coupled with an IEP, often offset the short-term costs. For this reason, we use a variety of resources to obtain cognitive testing.
For adults with strokes, we use the cognitive testing to facilitate the referral to a rehabilitation program that offers a range of services, including vocational counseling and job assistance. In our experience, cognitive testing also helps establish the expectations for self-efficacy and family support. For instance, when the patient has a low IQ score and poor executive function, we often employ additional supportive strategies to facilitate appointments and adherence to medication.
Follow-up of case presentations
Case 1.
Patient was started on exchange transfusion every 28 days with the goal of keeping her maximum HbS <30% and an IEP was established after meeting with school personnel. Her HIC using GRE/T2* MRI was estimated to be 22.2 mg/g dry weight76 and increased to 23.6 mg/g dry weight after 1 year despite oral chelation therapy with deferasirox (40 mg/kg per day). Thus, we started dual chelation therapy with outpatient deferasirox (40 mg/kg per day) plus inpatient IV deferoxamine (50 mg/kg per day over 48 hours every 4 weeks).80,81
Given the multiple options of therapy for this patient, a family meeting was deemed critical to ensure a full understanding of the trade-offs for a secondary stroke prevention option. The possibility of stopping transfusion therapy was mentioned with the recognition that this would have the highest rate of recurrence.88,89 We also discussed the evidence that regular blood transfusion is not the definitive therapy, and we expect 45% of patients will have a new cerebral infarct7 over a course of 5.5 years. Based on the palliative nature of regular blood transfusion therapy, we offered the family the option of an Institutional Review Board approved HLA-haploidentical HSCT with posttransplant cyclophosphamide.75 The family declined this option.
Case 2.
Six months after the stroke, uncontrolled risk factors, such as hypertension and diabetes mellitus were better controlled with the addition of a primary care provider to his management team. We discussed with the family secondary stroke prevention strategies, such as regular blood transfusion therapy, hydroxyurea therapy, and the option of an experimental transplant (unrelated donor, umbilical cord blood, or haploidentical SCT). The patient opted for chronic transfusion therapy.
Expert commentary
The standard care for patients presenting with acute neurologic deficit is prompt blood transfusion therapy, preferably an exchange transfusion. For secondary stroke prevention, we believe that when an HLA-matched sibling donor is available, an HSCT should be done regardless of age. Prior to HSCT, regular transfusion, simple or exchange (manual or automated) should be immediately instituted. If no donor is available, regular exchange transfusion (simple or preferably) should be implemented with a goal of keeping the maximum HbS% <30%. In unique circumstances where regular blood transfusion therapy is not an acceptable option, hydroxyurea therapy is better than no therapy at all. We believe strongly that alternative donor transplants should only be completed in the context of a clinical trial with sufficient participant accrual goals, addressing the definitive question of efficacy of secondary stroke prevention. The presence of new infarcts detected on the annual surveillance MRI or progression of vasculopathy on MRA may compel consideration for alternative therapies, such as experimental transplant, encephaloduroarteriosynangiosis, encephalomyoarteriosynangiosis or more intensive regular blood transfusions; although, rigorous prospective evaluation of any of these additional strategies have not been published. For all children and adults with strokes, we recommend cognitive testing for appropriate educational support, vocational counseling, or both. Future investigation will require better stratification of those at risk for further neurologic injury in order for stepwise risk-based therapy to be initiated.
Acknowledgments
The authors thank the members of the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease for their helpful comments prior to submission.
This study was supported by the Health Resources and Services Administration (HRSA - U38MC22220) and the Burroughs Wellcome Fund.
Authorship
Contribution: A.A.K., N.A.G., S.P., and M.R.D. reviewed the literature, contributed to the manuscript, and reviewed its content prior to submission; A.A.K. and M.R.D. wrote the initial draft; N.A.G. performed the literature search to identify the critical articles for the pooled analysis; and S.P. identified the images describing the differential diagnosis of a focal neurologic deficit and the text that described the neuroimaging findings.
Conflict-of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt University School of Medicine, 2200 Children’s Way, 11206 DOT VCH, Nashville, TN 37232-9000; e-mail: m.debaun@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal