Key Points
miR-24 tightly regulates VWF expression, maturation, and secretion.
Hyperglycemia regulates VWF through aldose reductase, ROS, and c-Myc.
Abstract
An elevated level of von Willebrand factor (VWF) in diabetic patients is associated with increased risk of thrombotic cardiovascular events. The underlying mechanism of how VWF expression is upregulated in diabetes mellitus is poorly understood. We now report that hyperglycemia-induced repression of microRNA-24 (miR-24) increases VWF expression and secretion in diabetes mellitus. In diabetic patients and diabetic mouse models (streptozotocin/high-fat diet-induced and db/db mice), miR-24 is reduced in both tissues and plasma. Knockdown of miR-24 in mice leads to increased VWF mRNA and protein levels and enhanced platelet tethering (spontaneous thrombosis). miR-24 tightly controls VWF levels through pleiotropic effects, including direct binding to the 3′ untranslated region of VWF and targeting FURIN and the histamine H1 receptor, known regulators of VWF processing and secretion in endothelial cells. We present a novel mechanism for miR-24 downregulation through hyperglycemia-induced activation of aldose reductase, reactive oxygen species, and c-Myc. These findings support a critical role for hyperglycemic repression of miR-24 in VWF-induced pathology. miR-24 represents a novel therapeutic target to prevent adverse thrombotic events in patients with diabetes mellitus.
Introduction
In conjunction with the rising incidence of obesity, the prevalence of diabetes mellitus (DM) is rapidly increasing. Globally, the prevalence of DM is estimated to increase from 382 million individuals in 2013 to 592 million individuals by 2035 and is mainly attributable to type 2 DM (T2DM), which represents ∼90% to 95% of all cases.1 At present, >27.9 million Americans (11.8% of total population) have DM (diagnosed and undiagnosed), and >90 million (38.2%) have prediabetes (abnormal fasting glucose).2-5 Within the vasculature, DM impairs endothelial cell function and induces platelet hyperactivity. As such, DM serves as a major risk factor for cardiovascular disease and stroke, with more than half of all diabetic patients dying from cardiovascular-related thrombosis (acute coronary syndrome or cerebrovascular event).6,7 Despite such pervasiveness, the underlying mechanisms for the thrombotic complications in DM are not fully understood. von Willebrand factor (VWF) is a key blood component that initiates thrombosis and is highly predictive of adverse thrombotic cardiovascular events in DM patients.3,8-12
Expressed in endothelial cells and megakaryocytes (platelet precursor cells), VWF plays a crucial role in maintaining normal hemostasis and contributes to thrombotic disorders following endothelial and platelet dysfunction. VWF is a large multidomain plasma glycoprotein that is critical for normal platelet tethering during hemostasis.13 In response to blood shear forces, VWF unfolds from its inactive globular conformation into an active string-like form that can specifically recruit platelets.14-17 The multimeric size of VWF is a primary determinant of its platelet-tethering function and is proteolytically regulated by the plasma metalloprotease ADAMTS13,18,19 which is responsible for the degradation of large, thrombogenic VWF multimers.14-17,20 The importance of ADAMTS13 in maintaining the balance of VWF multimeric size is illustrated by its role in a number of hematologic disorders, including (1) the idiopathic form of thrombotic thrombocytopenic purpura, a blood-clotting disorder in which antibody-mediated inhibition or congenital deficiency of ADAMTS13 causes spontaneous platelet aggregation via accumulation of uncleaved ultralarge high-molecular-weight VWF multimers and (2) some cases of von Willebrand disease, type 2A, in which VWF is more rapidly cleaved by ADAMTS13, resulting in a bleeding phenotype. Given the importance of VWF in regulation of thrombosis, the molecular mechanism regulating VWF expression and secretion, particularly in DM patients, remains unexplored.
MicroRNAs (miRNAs) are small 19- to 23-nucleotide RNA molecules that negatively regulate the translation of their target mRNAs.21-23 miRNAs post-transcriptionally regulate the expression of thousands of genes in a broad range of organisms in both normal physiologic and disease contexts.24 In this study, we identify that reduction of miRNA-24 (miR-24) by hyperglycemia increases VWF biosynthesis and secretion. We provide new insights into VWF transcriptional and translational regulation by miRNAs in DM.
Materials and methods
Diabetic mouse model
All mouse studies were approved by Yale Institutional Animal Care and Use Committee. The diabetic mice model we applied was described previously.25 Wild-type (WT; C57BL/6J background) and diabetic mice (BKS.Cg-Dock7m+/+ Lepr d/b/j) were purchased from The Jackson Labs. To study the effects of hyperglycemia on endothelial miRNA and VWF expression, we also induced DM in mice using streptozotocin (STZ). Eight-week-old mice were divided into 2 groups; half were injected with STZ (50 mg/kg) intraperitoneally for 5 consecutive days to induce recurrent episodes of acute hyperglycemia (DM), and the other half were used as non-DM controls. Four weeks after STZ administration, DM and non-DM mice were maintained on a high-cholesterol diet for 12 weeks, fasted for 6 hours, and killed for blood sampling, and the whole lungs of mouse (N = 10 for DM and 6 for WT) were harvested. Glucose was measured from the tail-tip with a glucometer. The total RNA, including miRNAs, was extracted and purified using QIAzol lysis reagent and kits according to the manufacturer's instructions.
LNA synthesis and administration
Custom-made miRCURY locked nucleic acids (LNAs) for in vivo application were designed and synthesized as unconjugated and fully phosphorothiolated oligonucleotides by Exiqon. LNA-miR-24 inhibitor or negative control (scrambled LNA oligonucleotide) was intravenously delivered to C57BL/6J at a concentration of 10 mg/kg body weight in saline solution. Mice were euthanized 2 weeks after LNA administration for detailed assessment.
miRNA mimics delivery in vivo
The mirVana miR-24 mimic and mimic controls (Life Technologies) were complexed with Invivofectamine 2.0 reagent (Invitrogen) to form nanoparticles for in vivo applications. miRNA oligonucleotides (5 mg/mL in water, 250 μL) were mixed with manufacturer’s complexation buffer (250 μL), and then Invivofectamine 2.0 reagent was added (500 μL). After a 30-minute incubation at 50°C, filtration was performed in 15 mL phosphate-buffered saline (PBS) to remove excessive salts and solvents. The final concentration of miRNA oligonucleotides was 1.25 mg/mL and was intravenously delivered to db/db mice at a concentration of 5 mg/kg body weight. Mice were euthanized 2 weeks after in vivo delivery for detailed assessment.
Western blots and VWF enzyme-linked immunosorbent assays
Standard western blot analysis and VWF enzyme-linked immunosorbent assay (ELISA) were used. For details, including the antibodies used, please refer to the supplemental Methods available on the Blood Web site.
Human studies
Blood samples were drawn from consenting volunteers (healthy and DM subjects) at Yale University School of Medicine (Human Investigation Committee 1005006865). Written informed consent was obtained from all participating individuals. Eighty-two T2DM subjects (American Diabetic Association definition), 33 type 1 DM (T1DM; American Diabetic Association definition), and 112 healthy controls (HCs) were recruited for the studies (supplemental Table 1). Platelet-rich plasma (PRP) was prepared from blood by venipuncture into 3.8% trisodium citrate (w/v). PRP was obtained by centrifugation of blood at 250g (or 1200 rpm) at 25°C for 15 minutes. Platelet-poor plasma (PPP) was obtained by centrifugation of the PRP at 1400g at 25°C for 10 minutes (or 1800 rpm for 5 minutes). The supernatant PPP was stored at −80°C, and the platelet pellets (at the bottom of tube after removal of the PPP) were resuspended into platelet washing buffer (103 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 5 mmol/L glucose, 36 mmol/L citric acid, 3.5 mg/mL bovine serum albumin, pH 6.5) and stored at −80°C. PPP was used to determine levels of VWF and miR-24 as described above. Macrovascular thrombotic complications (acute coronary syndrome and cerebrovascular events; American Heart Association definitions) of the diabetic patients were recorded and analyzed.
Confocal microscopy and electron microscopy
For confocal microscopy, cultured endothelial cells were stimulated for 2 minutes with 100 μM histamine and then washed with PBS before fixing for 15 minutes with 4% paraformaldehyde in PBS. Cells were incubated with rabbit polyclonal antibodies to VWF (Dako; A0082) at 1:500 in PBS with 10% bovine serum albumin at 4°C overnight. Cells were washed and incubated with rabbit secondary Alexa-488 (1:1000; Invitrogen), and then incubated with 1 µg/mL 4,6 diamidino-2-phenylindole (DAPI; Life Technologies). Electron microscopy to visualize the Weibel-Palade bodies in endothelial cells was performed by Yale School of Medicine Center for Cellular and Molecular Imaging and processed as previously described.26
Immunohistochemistry of arterial sections
Mice were killed 2 weeks after LNA administration. The arteries were thoroughly perfused with 4% paraformaldehyde in PBS, excised, fixed with 4% paraformaldehyde in PBS for 4 hours at 4°C, rinsed with PBS, and incubated with 15% sucrose in PBS overnight at 4°C and then 30% sucrose in PBS overnight at 4°C. Then the fixed arteries were mounted in Tissue-Tek embedding optimum cutting temperature compound (Miles Inc.), snap-frozen, and subsequently stored at −80°C before sectioning. Sections were washed in PBS, blocked in 5% heat-inactivated goat serum in PBS for 1 hour at room temperature, and then probed with primary antibody VWF (rabbit polyclonal, A0082; 1:300; Dako) overnight at 4°C. After incubation, slides were washed with PBS, blocked for 1 hour at room temperature, and incubated with secondary antibodies and stained with DAPI overnight at 4°C.
Statistical analyses
All experiments in the study were performed at least in triplicate (unless otherwise specified) from ≥3 independent experiments, and data are presented as mean ± standard error of the mean. Statistical differences were assessed with an unpaired 2-tailed Student t test when only 2 groups were compared. Otherwise, statistical significance was determined using 1-way analysis of variance followed by Bonferroni's multiple comparison test. Relationships between variables were determined by the Pearson correlation coefficient. P < .05 was considered statistically significant.
Results
Our goal was to determine whether VWF is regulated by miRNA.
Elevated VWF and reduced miR-24 levels in diabetic patients
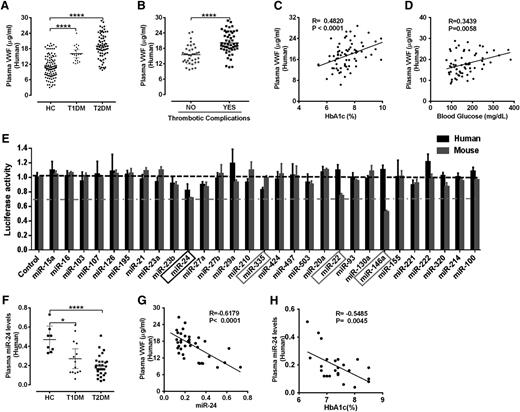
We initially recruited 227 consecutive consenting human subjects (112 HC, 33 T1DM, and 82 T2DM; supplemental Table 1) from Yale New Haven Hospital to determine whether VWF was increased in DM. Levels of mature VWF in T2DM was twofold higher and in T1DM was 1.6-fold higher compared with age- and sex-matched healthy controls (Figure 1A). Moreover, the increased VWF, particularly the most active ultralarge-high-molecular weight as determined by western blot analysis of uncleaved mature VWF monomers, was increased in DM patients (supplemental Figure 1). The clinical importance of these initial observations is that previous studies have correlated such high VWF levels with increased risk of adverse thrombotic cardiovascular events.27 Indeed, we also demonstrate increased thrombotic cardiovascular events (acute coronary syndrome and cerebrovascular events) associated with high VWF in our T2DM population (Figure 1B). Although the mean were significantly different, plasma concentrations of VWF in DM patients (and HCs) demonstrated wide variations. Recent studies suggested genetic factors may be a major determinant of plasma VWF levels, with 24% being related to ABO blood type.28 Nongenetic factors are also clearly important in VWF regulation. We then assessed several parameters recognized to be characteristic of DM control (including body mass index, lipids, hemoglobin A1c [HbA1c], and glucose) to determine potential associations with the increased VWF. We identified significant correlation of plasma VWF with fasting plasma glucose (R = 0.34, P = .0058) and HbA1c levels (R = 0.48, P < .0001; Figure 1C-D). Our initial results demonstrate a significant association between VWF and hyperglycemia and suggest that diabetic conditions may regulate VWF levels.
Elevated VWF and reduced miR-24 identified in diabetic plasma. (A) ELISA analysis and comparison of VWF levels in plasma of HCs (n = 112), T1DM (n = 33), and T2DM (n = 82); ****P < .0001. (B) Comparison of mature VWF levels in plasma of T2DM with or without thrombotic complications (acute coronary syndrome and cerebrovascular events). ****P < .0001. (C) Correlation between plasma VWF levels and HbA1c (%) levels. (D) Correlation between plasma VWF levels and blood glucose level. (E) A panel of 29 candidate VWF-targeting miRNAs (including 25 highly expressed miRNAs in endothelial cells and 4 miRNAs predicted by TargetScan) cotransfected with VWF 3′UTR Lenti-reporter-Luc vector into 293 T cells. The luciferase activity was measured and normalized to empty control reporter and empty miRNA control. miR-24, miR-335, miR-22 and miR-146a (highlighted) demonstrated a significant reduction in luciferase activity. These assays were performed in quadruplicate and repeated 3 times. (F) qPCR analysis of circulating miR-24 levels (normalized to cel-miR-238 spike-in) in plasma of healthy controls (n = 8), T1DM (n = 14), and T2DM (n = 30); ****P < .0001; *P < .05. (G) Correlation between plasma VWF levels and circulating miR-24 in DM (n = 37). (H) Correlation between circulating miR-24 and HbA1c (%) in DM (n = 25).
Elevated VWF and reduced miR-24 identified in diabetic plasma. (A) ELISA analysis and comparison of VWF levels in plasma of HCs (n = 112), T1DM (n = 33), and T2DM (n = 82); ****P < .0001. (B) Comparison of mature VWF levels in plasma of T2DM with or without thrombotic complications (acute coronary syndrome and cerebrovascular events). ****P < .0001. (C) Correlation between plasma VWF levels and HbA1c (%) levels. (D) Correlation between plasma VWF levels and blood glucose level. (E) A panel of 29 candidate VWF-targeting miRNAs (including 25 highly expressed miRNAs in endothelial cells and 4 miRNAs predicted by TargetScan) cotransfected with VWF 3′UTR Lenti-reporter-Luc vector into 293 T cells. The luciferase activity was measured and normalized to empty control reporter and empty miRNA control. miR-24, miR-335, miR-22 and miR-146a (highlighted) demonstrated a significant reduction in luciferase activity. These assays were performed in quadruplicate and repeated 3 times. (F) qPCR analysis of circulating miR-24 levels (normalized to cel-miR-238 spike-in) in plasma of healthy controls (n = 8), T1DM (n = 14), and T2DM (n = 30); ****P < .0001; *P < .05. (G) Correlation between plasma VWF levels and circulating miR-24 in DM (n = 37). (H) Correlation between circulating miR-24 and HbA1c (%) in DM (n = 25).
VWF is predominantly expressed in megakaryocytes and endothelial cells.29 Endothelial cells are known to be rich in miRNAs,30 which are capable of regulating hundreds of mRNAs and consequently can have substantial effects on a gene expression network.31 To identify whether VWF is targeted and regulated by miRNAs, we initially used a robust screening strategy that assessed the effects of the top 25 most abundant endothelial cell miRNAs30 and 4 miRNAs predicted to target VWF (TargetScan) (Figure 1E). miR-24 demonstrated a significant inhibitory effect on luciferase activity of both human and mouse VWF 3′untranslated region (UTR) vectors, whereas the other endothelial-derived miRNAs had minimal or differential effects (eg, miR-335 targeted human but not mouse VWF 3′UTR, miR-146a targeted mouse but not human VWF 3′UTR; Figure 1E). Thus, we chose to focus our efforts on miR-24 for further detailed mechanistic studies. Most miRNAs in extracellular fluids are surprisingly stable.32 We then extracted total circulating miRNA from the DM plasma samples of our recruited patients and quantified relative levels of miR-24 (age- and sex-matched controls). We observed that levels of miR-24 in plasma of T1DM and T2DM were significant lower than that in HCs (Figure 1F). Notably, plasma level of VWF inversely correlated with circulating miR-24 (R = −0.62, P < .0001; Figure 1G), and levels of miR-24 inversely correlated with HbA1c (R = −0.55, P = .0045; Figure 1H). Taken together, our DM patient studies support an association between high glucose levels, low miR-24 concentrations, and increased VWF.
Decreased miR-24 and increased VWF in diabetic mouse models
Next, we assessed whether high glucose increased VWF and thrombosis via miR-24 using mouse models. We initially defined the expression of miR-24 in different murine tissues. miR-24 was highly expressed in the lungs, heart, and kidneys and poorly expressed in the liver and muscle, as measured by quantitative real-time polymerase chain reaction (qPCR; Figure 2A). Such tissue distribution of miR-24 in mice is consistent with previous published reports.33 We then applied STZ as a model for T1DM, STZ/high-fat diet as a model for non–obesity-induced T2DM, and db/db mice as a model for T2DM. The highest fasting glucose levels (∼500 mg/dL compared with 120 mg/dL in WT controls) were found in the db/db mice (Figure 2B). Consistent with our human observations, miR-24 levels were decreased two- to threefold in STZ/high-fat diet and db/db diabetic mice lungs, and fivefold in db/db mice plasma compared with WT controls (Figure 2C-D). Correspondingly, VWF mRNA expression was increased ∼2-fold and plasma VWF protein levels increased 1.4-fold in STZ/high-fat diet diabetic mice compared with WT controls (Figure 2E-F). Taken together these results support the human data that glucose regulates VWF and miR-24 levels.
Decreased miR-24 and increased VWF in diabetic mice. (A) qPCR analysis of tissue miR-24 levels (normalized to U6) in WT mice (n = 8-12). (B) Comparison of fasting blood glucose levels in diabetic mouse models (n = 6-12); ***P < .001. (C) qPCR analysis of miR-24 levels in DM mice lung (normalized to U6; n = 6-12); ****P < .0001; **P < .01; *P < .05. (D) qPCR analysis and comparison of miR-24 levels in in lean control and db/db mice lung and kidney (normalized to U6) and plasma (normalized to cel-miR-238 spike-in; n = 6∼8); ***P < .001. (E) qPCR analysis and comparison of VWF mRNA level in WT and STZ/HFD diabetic mice lung (normalized to glyceraldehyde-3-phosphate dehydrogenase [GAPDH]; n = 8-10); ****P < .0001. (F) ELISA analysis and comparison of VWF levels in WT and STZ/HFD diabetic mice plasma (n = 8-10); ***P < .001.
Decreased miR-24 and increased VWF in diabetic mice. (A) qPCR analysis of tissue miR-24 levels (normalized to U6) in WT mice (n = 8-12). (B) Comparison of fasting blood glucose levels in diabetic mouse models (n = 6-12); ***P < .001. (C) qPCR analysis of miR-24 levels in DM mice lung (normalized to U6; n = 6-12); ****P < .0001; **P < .01; *P < .05. (D) qPCR analysis and comparison of miR-24 levels in in lean control and db/db mice lung and kidney (normalized to U6) and plasma (normalized to cel-miR-238 spike-in; n = 6∼8); ***P < .001. (E) qPCR analysis and comparison of VWF mRNA level in WT and STZ/HFD diabetic mice lung (normalized to glyceraldehyde-3-phosphate dehydrogenase [GAPDH]; n = 8-10); ****P < .0001. (F) ELISA analysis and comparison of VWF levels in WT and STZ/HFD diabetic mice plasma (n = 8-10); ***P < .001.
Modifying miR-24 levels regulates VWF expression in vivo
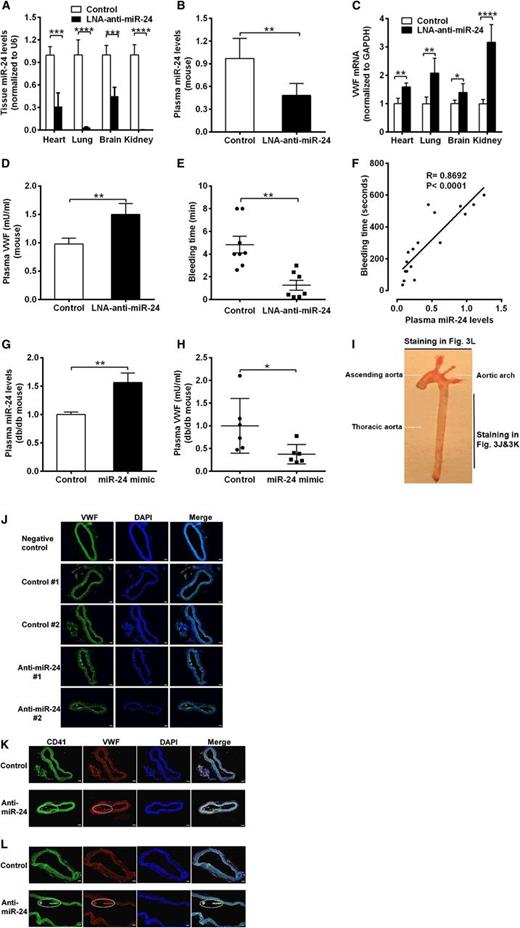
To confirm that the decreased miR-24 levels contribute to the elevated VWF levels in DM, we applied synthesized LNAs specifically targeting the seed sequence of miR-24. We directly introduced anti-miR-24 LNA or negative control (LNA scramble) into C57BL/6J mice using a jugular vein injection. qPCR analysis of miR-24 confirmed that anti-miR-24 LNA effectively and specifically silenced the miR-24 expression in mice (>90% reduction in kidney and lung, 60% reduction in heart, and 50% reduction in brain and plasma; Figure 3A-B; supplemental Figure 2). Application of anti-miR-24 significantly increased VWF mRNA levels in mice heart, lung, brain, and kidney (Figure 3C) and VWF protein levels in mice plasma (1.5-fold) in comparison with controls (Figure 3D). These 4 organs (lung, kidney, brain, and heart) are the main source of miR-24 and VWF in the body.33,34 Because VWF is an important coagulation factor, we tested the mice for bleeding time. We observed significantly reduced tail bleeding time, indicative of increased thrombosis, in LNA-treated mice compared with mice treated with negative control (Figure 3E). Analysis of bleeding time vs plasma miR-24 levels in these mice (control and LNA treated) demonstrated a strong positive association with increased miR-24 associated with increased bleeding time (Figure 3F). This is consistent with the results that elevated VWF levels in plasma leads to enhanced risk of arterial thrombosis (Figure 1B).3,8 In contrast, overexpression of miR-24 through in vivo nanoparticle delivery of miR-24 mimic into db/db mice significantly decreased plasma VWF levels (Figure 3G-H).We also found markedly increased VWF expression in the thoracic aorta in LNA-treated mice (Figure 3I-J). Notably, the increased VWF in LNA-treated murine arteries exhibited platelet aggregations (thrombus), as verified by CD41 (also known as integrin α2b and GPIIb, a transmembrane glycoprotein that is expressed by platelets) staining in the aorta (Figure 3I,K,L). These mouse studies confirm a critical role for miR-24 in regulating VWF and risk of arterial thrombosis. Further studies were required to determine the signaling mechanisms linking hyperglycemia to miR-24 and from miR-24 to VWF.
Response of VWF expression to knockdown of miR-24 in mice. (A) Anti-miR-24 LNA (n = 8) or negative control (n = 8) injected into C57BL/6J mice. qPCR analysis of miR-24 expression in mice in heart, lung, brain, and kidney (normalized to U6); ****P < .0001; ***P < .001. (B) qPCR analysis of miR-24 expression in plasma of C57BL/6J mice treated with negative control (n = 8) or treated with anti-miR-24 LNA (n = 6) for 2 weeks (normalized to cel-miR-238 spike-in); **P < .01. (C) qPCR analysis of VWF mRNA levels in C57BL/6J mice heart, lung, brain, and kidney (n = 8 in each group); ****P < .0001; **P < .01; *P < .05. (D) ELISA analysis of VWF levels in plasma of mice with LNA and control treatment (n = 6-8 in each group); **P < .01. (E) Bleeding times of individual mice treated with negative control (n = 8) or anti-miR-24 LNA (n = 7) for 2 weeks; **P < .01. (F) Correlation between mice tail bleeding time and plasma miR-24 in C57BL/6J mice (anti-miR-24 LNA: n = 8; negative scrambled control: n = 5; and saline control: n = 4). (G) qPCR analysis of miR-24 expression in plasma of db/db mice treated with mimic control (n = 6) or treated with miR-24 mimic (n = 6) for 2 weeks (normalized to cel-miR-238 spike-in); **P < .01. (H) ELISA analysis of VWF levels in plasma of db/db mice treated with mimic control (n = 6) or treated with miR-24 mimic (n = 6) for 2 weeks (n = 6-8); *P < .05. (I) A photograph of a freshly dissected mouse aorta demonstrating sites of sections obtained for staining in J-L. (J) Mice were killed 2 weeks after LNA administration, and the arteries were thoroughly perfused, excised, fixed with 4% paraformaldehyde in PBS, embedded with optimum cutting temperature compound, snap-frozen, sectioned, and stained with anti-VWF fluorescein isothiocyanate (FITC) antibody; representing 2 thoracic aortas from negative control (n = 8) and 2 thoracic aortas from anti-miR-24 LNA (n = 8); scale bars, 50 µm. (K) Staining with anti-CD41 FITC antibody and anti-VWF phycoerythrin antibody; representing thoracic aorta from negative control (n = 8) and from anti-miR-24 LNA (n = 8). White dashed line indicates VWF and platelet thrombus; scale bars, 50 µm. (L) Staining with anti-CD41 FITC antibody and anti-VWF phycoerythrin antibody; representing aortic arch from negative control (n = 8) and from anti-miR-24 LNA (n = 8) (diagonal section shown to highlight thrombus). White dashed line indicates VWF and platelet thrombus; scale bars, 50 µm.
Response of VWF expression to knockdown of miR-24 in mice. (A) Anti-miR-24 LNA (n = 8) or negative control (n = 8) injected into C57BL/6J mice. qPCR analysis of miR-24 expression in mice in heart, lung, brain, and kidney (normalized to U6); ****P < .0001; ***P < .001. (B) qPCR analysis of miR-24 expression in plasma of C57BL/6J mice treated with negative control (n = 8) or treated with anti-miR-24 LNA (n = 6) for 2 weeks (normalized to cel-miR-238 spike-in); **P < .01. (C) qPCR analysis of VWF mRNA levels in C57BL/6J mice heart, lung, brain, and kidney (n = 8 in each group); ****P < .0001; **P < .01; *P < .05. (D) ELISA analysis of VWF levels in plasma of mice with LNA and control treatment (n = 6-8 in each group); **P < .01. (E) Bleeding times of individual mice treated with negative control (n = 8) or anti-miR-24 LNA (n = 7) for 2 weeks; **P < .01. (F) Correlation between mice tail bleeding time and plasma miR-24 in C57BL/6J mice (anti-miR-24 LNA: n = 8; negative scrambled control: n = 5; and saline control: n = 4). (G) qPCR analysis of miR-24 expression in plasma of db/db mice treated with mimic control (n = 6) or treated with miR-24 mimic (n = 6) for 2 weeks (normalized to cel-miR-238 spike-in); **P < .01. (H) ELISA analysis of VWF levels in plasma of db/db mice treated with mimic control (n = 6) or treated with miR-24 mimic (n = 6) for 2 weeks (n = 6-8); *P < .05. (I) A photograph of a freshly dissected mouse aorta demonstrating sites of sections obtained for staining in J-L. (J) Mice were killed 2 weeks after LNA administration, and the arteries were thoroughly perfused, excised, fixed with 4% paraformaldehyde in PBS, embedded with optimum cutting temperature compound, snap-frozen, sectioned, and stained with anti-VWF fluorescein isothiocyanate (FITC) antibody; representing 2 thoracic aortas from negative control (n = 8) and 2 thoracic aortas from anti-miR-24 LNA (n = 8); scale bars, 50 µm. (K) Staining with anti-CD41 FITC antibody and anti-VWF phycoerythrin antibody; representing thoracic aorta from negative control (n = 8) and from anti-miR-24 LNA (n = 8). White dashed line indicates VWF and platelet thrombus; scale bars, 50 µm. (L) Staining with anti-CD41 FITC antibody and anti-VWF phycoerythrin antibody; representing aortic arch from negative control (n = 8) and from anti-miR-24 LNA (n = 8) (diagonal section shown to highlight thrombus). White dashed line indicates VWF and platelet thrombus; scale bars, 50 µm.
miR-24 directly targets the VWF 3′UTR to regulate VWF expression in endothelial cells
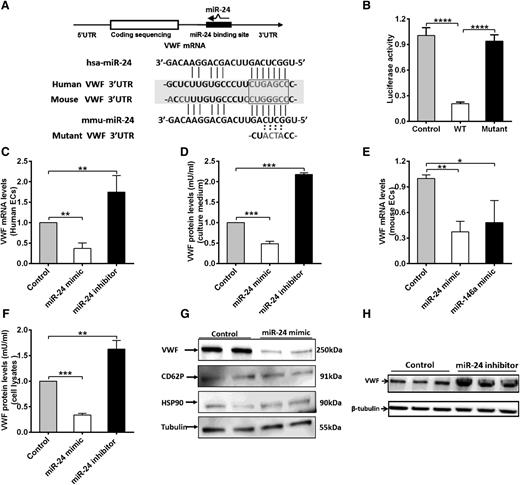
We next explored the mechanisms of how miR-24 regulates VWF levels. miR-24 is highly conserved in mammalian species (supplemental Figure 3). Our luciferase screening activity assays supported the presence of putative miR-24 binding sites within the 3′UTR region of VWF (human and mouse; Figure 1E). In silico analysis revealed that the miR-24 consensus binding motif shared sequence similarity to a region within the 3′UTR of the transcript for human VWF, extending between 90 and 106 bp (Figure 4A). In particular, the first 7-nt “seed” sequence of miR-24 can form base pairs that are complementary to an identical sense sequence of the VWF mRNA transcript, extending from nucleotides 100 to 106 within the VWF 3′UTR (Figure 4A). In the mouse VWF 3′UTR, however, a G substitutes an A, forming a G-U wobble base pair, which may also contribute to an interaction with the miR-24 seed sequence (Figure 4A; supplemental Figure 4), consistent with previous reports for miRNA-targeted base pairing.35 To confirm direct binding, we generated a reporter vector containing a mutant VWF 3′UTR (the sequence GAGC in the predicted seed sequences of VWF was mutated to ACTA; Figure 4A). Cotransfection of the miR-24 mimic with these constructs in HEK 293T cells markedly decreased the luciferase expression from the WT sequence but not the mutant construct (Figure 4B). We next sought to confirm the effect of miR-24 on VWF gene expression in endothelial cells. qPCR and western blot of endothelial cells (human umbilical vein endothelial cells [HUVECs]) transfected with miR-24 mimic or inhibitor were performed. The transfection efficiency of miR-24 mimic or inhibitor was evaluated by analysis of the intracellular miR-24 level by qPCR (supplemental Figure 5). We found both mRNA levels of VWF (Figure 4C), and the amount of VWF protein secreted into the culture media (Figure 4D), were downregulated with miR-24 overexpression by two- to threefold, whereas inhibiting miR-24 function led to upregulation of VWF mRNA by 1.8-fold (Figure 4C), and secreted VWF protein by twofold (Figure 4D). In addition, the inhibition of VWF expression of miR-24 was identified in isolated mouse lung endothelial cells transfected with miR-24 mimic (and miR-146a mimic; positive control shown in our screening assay [Figure 1E], to effectively reduce luciferase with mouse VWF sequences) (Figure 4E). For the intracellular stored VWF, overexpression of the miR-24 mimic led to a decrease in VWF expression by threefold (Figure 4F-G). Western blot quantification demonstrated that the miR-24 inhibitor increased VWF expression by 1.7-fold (Figure 4F,H). As controls to demonstrate specificity of miR-24 for VWF protein levels, we found that miR-24 had no effect on CD62P (also found in VWF-containing Weibel-Palade bodies), HSP90, or tubulin expression (Figure 4G). Taken together, these studies confirmed the reduction in expression and basal secretion of VWF by miR-24.
miR-24 inhibits VWF expression in 293T cells and endothelial cells. (A) Schematic of VWF mRNA and miR-24. miR-24 is partially complementary to a region in the human and mouse VWF 3′UTR. The sequence GAGC in the seed sequences of VWF was mutated to ACTA. (B) miR-24 inhibited luciferase activity of WT VWF 3′UTR but had no effect on luciferase activity of a mutant VWF 3′UTR. The assays were performed in triplicate; ****P < .0001. (C) VWF mRNA levels in cultured HUVEC lysates (harvested 48 hours after transfection with miR-24 mimics, inhibitors, or controls) were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control or inhibitor control). **P < .01. (D) VWF protein levels in cell culture medium (harvested 72 hours after transfection with miR-24 mimics, inhibitors, or controls) were analyzed by ELISA (normalized to the total amount of intracellular protein per well). ***P < .001. (E) VWF mRNA levels in cultured isolated mouse lung endothelial cell lysates (harvested 48 hours after transfection with miR-24 mimics or miR-146a mimics or controls) were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control or inhibitor control). **P < .01; *P < .05. (F) Total VWF protein levels in cell lysates (harvested 72 hours after transfection with miR-24 mimics or inhibitors or controls) were analyzed by ELISA (normalized to mimic control or inhibitor control). ***P < .001; **P < .01. (G) VWF protein level in cell lysates (harvested 72 hours after transfection with miR-24 mimics or controls) were analyzed by western blot; antibodies against CD62P, HSP90, and tubulin were applied as loading controls. (H) VWF protein level in cell lysates (harvested 72 hours after transfection with miR-24 inhibitors or controls) were analyzed by western blot.
miR-24 inhibits VWF expression in 293T cells and endothelial cells. (A) Schematic of VWF mRNA and miR-24. miR-24 is partially complementary to a region in the human and mouse VWF 3′UTR. The sequence GAGC in the seed sequences of VWF was mutated to ACTA. (B) miR-24 inhibited luciferase activity of WT VWF 3′UTR but had no effect on luciferase activity of a mutant VWF 3′UTR. The assays were performed in triplicate; ****P < .0001. (C) VWF mRNA levels in cultured HUVEC lysates (harvested 48 hours after transfection with miR-24 mimics, inhibitors, or controls) were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control or inhibitor control). **P < .01. (D) VWF protein levels in cell culture medium (harvested 72 hours after transfection with miR-24 mimics, inhibitors, or controls) were analyzed by ELISA (normalized to the total amount of intracellular protein per well). ***P < .001. (E) VWF mRNA levels in cultured isolated mouse lung endothelial cell lysates (harvested 48 hours after transfection with miR-24 mimics or miR-146a mimics or controls) were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control or inhibitor control). **P < .01; *P < .05. (F) Total VWF protein levels in cell lysates (harvested 72 hours after transfection with miR-24 mimics or inhibitors or controls) were analyzed by ELISA (normalized to mimic control or inhibitor control). ***P < .001; **P < .01. (G) VWF protein level in cell lysates (harvested 72 hours after transfection with miR-24 mimics or controls) were analyzed by western blot; antibodies against CD62P, HSP90, and tubulin were applied as loading controls. (H) VWF protein level in cell lysates (harvested 72 hours after transfection with miR-24 inhibitors or controls) were analyzed by western blot.
miR-24 also regulates VWF secretion through the histamine receptor 1
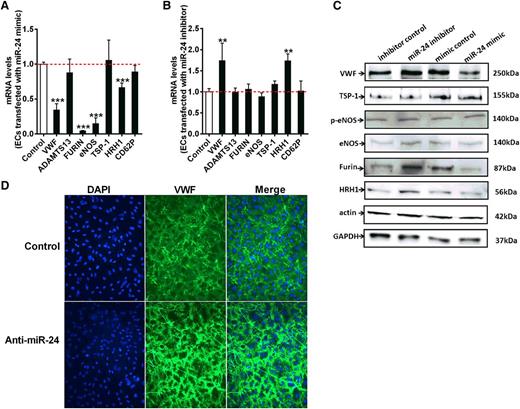
VWF biosynthesis, maturation, and secretion is a complex process involving C-terminal dimerization and disulfide bond formation in the endoplasmic reticulum, followed by furin-dependent cleavage of VWF pro-peptide and N-terminal multimerization in the Golgi complex.26 It has been proposed that ∼90% of newly synthesized VWF is constitutively secreted from endothelial cells, with only 10% (larger molecular VWF multimers) sorted to the Weibel-Palade bodies (storage granules).36-38 However, other studies have suggested that constitutive secretion of VWF from cultured endothelial cells is not significant because the high-molecular-weight form of endothelial VWF is released by regulated pathways.39 We showed that miR-24 downregulated basal VWF levels in culture medium (Figure 4D), indicating miR-24 decreases constitutive secretion of VWF. This is at least partially due to direct inhibition of VWF expression, but it is possible that miR-24 may target a number of specific enzymes or proteins involved in this complex pathway, indirectly contributing to VWF biosynthesis and secretion. Using qPCR, we further examined the relative expression level of protein disulfide isomerase, furin, thrombospondin-1 (TSP-1), integrin, ADAMTS13, P-selectin, histamine receptor (HRH1), and endothelial nitric oxide synthase (eNOS) in endothelial cells, which are all recognized to regulate VWF levels (Figure 5A-B). Interestingly, VWF, FURIN, ENOS, and HRH1 were all significantly reduced with miR-24 mimic; however, only VWF and HRH1 were significantly increased with miR-24 inhibitor (Figure 5A-B). For this reason and as histamine has recently been demonstrated to regulate VWF release,40 we set out to assess the relationship between miR-24 and HRH1. Examination of the 3′UTR of HRH1 demonstrated a highly conserved putative binding site for miR-24 (supplemental Figure 6). We found that both mRNA (Figure 5A) and protein levels (Figure 5C) of HRH1 were downregulated with miR-24 overexpression, whereas inhibiting miR-24 function caused upregulation of HRH1 mRNA (Figure 5B) and HRH1 protein (Figure 5C). We also used confocal microscopy to further visualize the effects of miR-24 on histamine-induced VWF secretion.40 We observed a reduction in newly secreted VWF from cultured endothelial cells transfected with miR-24 mimics, whereas increased secreted VWF was observed in cells transfected with miR-24 inhibitors (Figure 5D). This indicates that miR-24 also regulates the sensitivity of endothelial cells to histamine, coordinately regulating VWF secretion. Although mRNA for ENOS may also be regulated by miR-24 (Figure 5A), no significant effect on protein was observed (Figure 5C). miR-24 may also regulate FURIN mRNA and protein levels (Figure 5A,C; supplemental Figure 7). Taken together, this supports miR-24 as coordinately regulating multiple cellular components that controls VWF formation and secretion.
miR-24 regulates VWF maturation and secretion. (A) Endothelial cells were transfected with miR-24 mimic or control construct. The mRNA levels of ADAMTS13, FURIN, ENOS, TSP-1, HRH1, and CD62P in cell lysates were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control). *P < .05. (B) Endothelial cells transfected with miR-24 inhibitors or control constructs. The mRNA levels of ADAMTS13, FURIN, ENOS, TSP-1, HRH1, and CD62P in cell lysates were analyzed by qPCR (normalized to GAPDH and then normalized to inhibitor control); *P < .05. (C) VWF protein levels (VWF, TSP-1, p-eNOS, eNOS, Furin, and HRH1) in cell lysates determined by western blot. Actin and GAPDH were used as controls. (D) Endothelial cells transfected with miR-24 controls (upper) or inhibitors (lower). Cell surface attached VWF in endothelial cells was visualized by confocal microscopy (DAPI, blue; VWF, green) after 50 µM histamine stimulation (2 minutes before preparation for microscopy).
miR-24 regulates VWF maturation and secretion. (A) Endothelial cells were transfected with miR-24 mimic or control construct. The mRNA levels of ADAMTS13, FURIN, ENOS, TSP-1, HRH1, and CD62P in cell lysates were analyzed by qPCR (normalized to GAPDH and then normalized to mimic control). *P < .05. (B) Endothelial cells transfected with miR-24 inhibitors or control constructs. The mRNA levels of ADAMTS13, FURIN, ENOS, TSP-1, HRH1, and CD62P in cell lysates were analyzed by qPCR (normalized to GAPDH and then normalized to inhibitor control); *P < .05. (C) VWF protein levels (VWF, TSP-1, p-eNOS, eNOS, Furin, and HRH1) in cell lysates determined by western blot. Actin and GAPDH were used as controls. (D) Endothelial cells transfected with miR-24 controls (upper) or inhibitors (lower). Cell surface attached VWF in endothelial cells was visualized by confocal microscopy (DAPI, blue; VWF, green) after 50 µM histamine stimulation (2 minutes before preparation for microscopy).
Aldose reductase and c-Myc mediates hyperglycemia-regulated miR-24/VWF
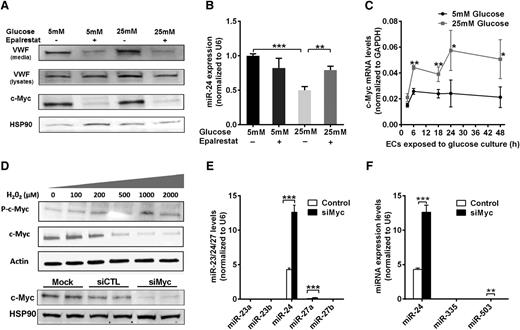
Hyperglycemia could influence regulation of miR-24 by treating HUVECs with high (25 mM) or normal (5 mM) concentrations of d-glucose, with d-mannitol as the control (for potential osmotic effects). We determined that hyperglycemia significantly downregulated the level of miR-24 both in the cells and their media (secreted miR-24) (supplemental Figure 8A-B). The relative amount of mature VWF in the cell pellets (including cell surface-bound VWF and Weibel-Palade body-stored VWF) from high glucose (25 mM)-treated HUVECs was 1.5-fold higher than that in the control (supplemental Figure 8C-D). Aldose reductase (AR) is the first and essential enzyme in the polyol pathway, converting glucose to sorbitol. The activity of AR is increased during hyperglycemia and results in increased production of reactive oxygen species (ROS).41 Interestingly, we found that the VWF level was significantly decreased in the cell pellets and medium of HUVECs cultured in high glucose when treated with an AR inhibitor (epalrestat) (Figure 6A). Epalrestat significantly increased the miR-24 level in endothelial cells cultured in high glucose (Figure 6B).
Mechanism of hyperglycemia-mediated downregulation of miR-24. (A) Western blot analysis of VWF and c-Myc expression in cell lysates under conditions of normal or high glucose (72-hour cultured HUVECs) in the presence or absence of an aldose reductase inhibitor (epalrestat). (B) qPCR quantitation of miR-24 expression in normal or high glucose (72-hour cultured HUVECs) with or without aldose reductase inhibitor (epalrestat); **P < .01; *P < .05. (C) Time course analysis of c-Myc mRNA expression in normal or high glucose (cultured HUVECs) using qPCR quantitation (normalized to GAPDH). (D) Endothelial cells were incubated with different concentrations of H2O2 (0 to 2000 μM) for 1 hour prior to analysis of cell lysates with western blot. (E) Western blot analysis of endothelial cell lysates when transfected with si-cMyc or si-Control followed by qPCR quantitation of miR-23/24/27 clusters expression; ***P < .001; **P < .01. (F) Western blot analysis of endothelial cell lysates when transfected with si-cMyc or si-Control followed by qPCR quantitation of miR-24/335/503 expression; ***P < .001; **P < .01.
Mechanism of hyperglycemia-mediated downregulation of miR-24. (A) Western blot analysis of VWF and c-Myc expression in cell lysates under conditions of normal or high glucose (72-hour cultured HUVECs) in the presence or absence of an aldose reductase inhibitor (epalrestat). (B) qPCR quantitation of miR-24 expression in normal or high glucose (72-hour cultured HUVECs) with or without aldose reductase inhibitor (epalrestat); **P < .01; *P < .05. (C) Time course analysis of c-Myc mRNA expression in normal or high glucose (cultured HUVECs) using qPCR quantitation (normalized to GAPDH). (D) Endothelial cells were incubated with different concentrations of H2O2 (0 to 2000 μM) for 1 hour prior to analysis of cell lysates with western blot. (E) Western blot analysis of endothelial cell lysates when transfected with si-cMyc or si-Control followed by qPCR quantitation of miR-23/24/27 clusters expression; ***P < .001; **P < .01. (F) Western blot analysis of endothelial cell lysates when transfected with si-cMyc or si-Control followed by qPCR quantitation of miR-24/335/503 expression; ***P < .001; **P < .01.
c-Myc, an oncogenic transcription factor known to regulate microRNAs,42-44 was recently reported to transcriptionally repress miR-23a and miR-23b in cancer cells.44 miR-24 is located in the same cluster along with miR-23a/24/27a and miR-23b/24/27b and thus may also be regulated by c-Myc in endothelial cells. We found hyperglycemia increased c-Myc mRNA in endothelial cells, and protein levels were blocked by epalrestat (Figure 6A,C). Moreover, endothelial cells treated with H2O2 mimicking hyperglycemia-induced high ROS (from AR activation), markedly increased the level of phosphorylated c-Myc levels (Figure 6D), which was associated with c-Myc activation, consistent with a previous report.45 This supports increased expression and activation of c-Myc by hyperglycemia through AR-induced ROS, leading to downregulation of miR-24 levels. We identified miR-24 is the most highly expressed miRNA among the miR-23a/24/27a and miR-23b/24/27b clusters in endothelial cells (Figure 6E). Knockdown of c-Myc significantly increased the miR-24 levels (Figure 6E). Although miR-27a increased by twofold, this was from negligible background levels (Figure 6E). This was also the case with miR-335 and miR-503, where the background levels were negligible in comparison with miR-24 for both control and c-Myc knockdown (Figure 6F). Collectively, our data implicate a pathway involving hyperglycemia/aldose reductase/ROS/c-Myc/miR-24 in regulating VWF expression and secretion (Figure 7).
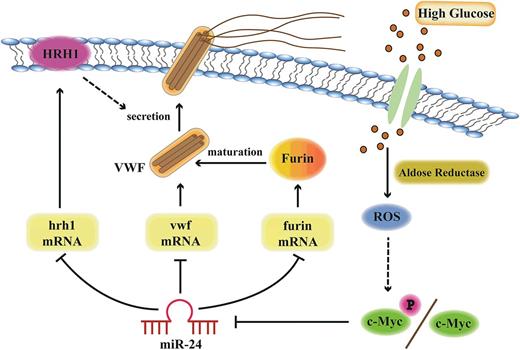
Model of miR-24-mediated regulation of VWF biosynthesis and secretion through the aldose reductase pathway. Hyperglycemia upregulates aldose reductase activity and increases ROS generation in endothelial cells. Increased ROS upregulates c-Myc/c-Myc phosphorylation, which downregulates miR-24, leading to increased expression of VWF, Furin, and HRH1. Therefore, the expression of VWF itself, Furin-dependent VWF maturation, and the histamine-regulated secretion pathways are coordinately upregulated with hyperglycemia.
Model of miR-24-mediated regulation of VWF biosynthesis and secretion through the aldose reductase pathway. Hyperglycemia upregulates aldose reductase activity and increases ROS generation in endothelial cells. Increased ROS upregulates c-Myc/c-Myc phosphorylation, which downregulates miR-24, leading to increased expression of VWF, Furin, and HRH1. Therefore, the expression of VWF itself, Furin-dependent VWF maturation, and the histamine-regulated secretion pathways are coordinately upregulated with hyperglycemia.
Model of regulation of VWF by hyperglycemia and miR-24
Based on our current studies and data in the literature, we present a model describing the importance of high glucose and miR-24 in regulating VWF expression and secretion (Figure 7). AR, ROS, and c-Myc play key roles in transducing high glucose to repression of miR-24. The reduction of miR-24 in essence removes the brakes from translation of VWF message and regulation of VWF through HRH1 and furin, resulting in enhanced risk of arterial thrombosis. These studies provide several potential signaling targets to combat enhanced thrombosis associated with diabetes mellitus.
Discussion
VWF plays an essential role in thrombus formation. High levels of circulating VWF are reflective of endothelial dysfunction, as well as an increased propensity toward thrombosis (via VWF-mediated platelet adhesion and activation of the coagulation cascade). Interestingly, in 1983, Mordes et al46 demonstrated that endothelial cells exposed in vitro to high glucose concentrations increased intracellular levels of VWF. However, no mechanistic insights were or have since been reported. We now provide the first report that VWF production and secretion is coordinately regulated by miR-24, tying hyperglycemia (as observed in diabetes mellitus) to increased VWF.
Recent studies have shown that miRNA expression disorders can contribute to the development of T2DM and its complications.47 There was a strong negative correlation between miR-126 and vascular complications in T2DM.30,48,52 Interestingly, miR-24 levels were also noted to be reduced in T2DM on miRNA screening.48 In addition, delivery of specific miRNAs by apoptotic bodies protects against atherosclerosis.49 miRNAs have also been reported to regulate vascularity after myocardial infarction.50 An increasing number of studies have reported differentially expressed miRNAs within diabetic patients, particularly circulating miRNAs, which are now being considered as possible novel biomarkers of disease.32 Through extensive screening, we identified and confirmed miR-24 as directly targeting the 3′UTR of the VWF gene. miR-24 is highly conserved in various species and is clustered with miR-23 and miR-27 on human chromosome 9 (miR-24-1) and 19 (miR-24-2). Both miR-24-1 and miR-24-2 generate the same mature product: miR-24. We found overexpression of miR-24 in endothelial cells downregulated VWF gene expression, whereas transfection of an anti-miR-24 upregulated VWF biosynthesis and secretion. miR-24 additionally targets multiple 3′UTR sites and genes, directly regulating VWF expression and specific enzymes or proteins involved in the process of VWF maturation and secretion. Our findings lend insight into miR-24-based regulation of VWF and provide a mechanism for the high VWF plasma levels and thrombotic events observed with T2DM.
Thrombotic cardiovascular diseases is the leading cause of mortality among diabetic patients, with >50% dying from such thrombotic events: acute coronary syndrome, stroke, and peripheral vascular disease.1 Of particular interest is miR-24. Several groups have reported contrasting results in mouse myocardial infarction models.50-53 Fiedler et al50 and Meloni et al51 suggested that inhibition of miR-24 had beneficial therapeutic effects on myocardial infarction, whereas Qian et al53 and Hu et al52 supported improved recovery after myocardial infarction with miR-24 mimic therapy. In line with these later studies, we identified decreased miR-24 in plasma and the heart under diabetic conditions. More recently, miR-24 was reported to attenuate vascular inflammation54 and correlate with atherosclerotic plaque stability,55 which are also consistent with our findings as VWF has long been established in promoting inflammation and atherosclerosis.56
We demonstrated that hyperglycemia is associated with elevated levels of VWF and decreased levels of miR-24 in both diabetic human and mouse blood samples and cultured endothelial cells. Under hyperglycemia and ROS, the formation of methionine sulfoxide at VWF (Met 1606) inhibits its cleavage by ADAMTS-13 by oxidizing VWF but concurrently enhances its cleavage by neutrophil proteases (elastase, cathepsin G).57-59 Direct oxidation modification of VWF alone cannot explain the mechanism of increased uncleaved VWF levels in DM. Recent studies have suggested that the AR pathway through ROS production mediates platelet hyperactivity associated with hyperglycemia,41 and the same pathway is responsible for increased thrombosis.60 We now report for the first time that ROS production from AR can lead to accelerated thrombus formation through activation of c-Myc, inhibition of miR-24, and enhanced VWF production and secretion. c-Myc can regulate transcription both positively and negatively in controlling genes in cells.61,62 Thus, we propose a model of coordinate regulation by hyperglycemia-induced repression of miR-24, as shown in Figure 7, arising from hyperglycemia and ROS production. Here we also established a link between levels of mature (high molecular weight) VWF and incidence of diabetic complications (thrombosis). Our study provides multiple mechanistic-based targets for antithrombotic therapy in patients with diabetes mellitus. Given the role of VWF in hemostasis and thrombosis, the miR-24 mimic may prove to be a novel miRNA-based drug for diabetic thrombotic diseases. Further clinical studies are required.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowlegements
The authors thank Silvio Inzucchi and Robert Sherwin for support in recruiting the patients.
These studies were supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants RO1 HL074190, R01HL115247, and U54 HL117798 (to J.H.).
Authorship
Contribution: Y. Xiang performed the research, analyzed data, and wrote the manuscript; Y. Xiang, J.S., G.A., J.D., W.H.T., S.H.L., G.S., Z.L., E.H., and R.I.H. recruited clinical patients and performed blood sample preparation; Y. Xiang, J.C., and J.L. performed miRNA screening experiments and analyzed data; Y. Xiang, D.W., X.H., and Y. Xie performed animal experiments and analyzed data; K.L. assisted in writing the manuscript; and J.H. and K.A.M. supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Hwa or Yaozu Xiang, Yale Cardiovascular Research Center, Yale School of Medicine, 300 George St, Rm 759H, New Haven, CT 06511; e-mail: john.hwa@yale.edu or yaozu.xiang@yale.edu.

![Figure 2. Decreased miR-24 and increased VWF in diabetic mice. (A) qPCR analysis of tissue miR-24 levels (normalized to U6) in WT mice (n = 8-12). (B) Comparison of fasting blood glucose levels in diabetic mouse models (n = 6-12); ***P < .001. (C) qPCR analysis of miR-24 levels in DM mice lung (normalized to U6; n = 6-12); ****P < .0001; **P < .01; *P < .05. (D) qPCR analysis and comparison of miR-24 levels in in lean control and db/db mice lung and kidney (normalized to U6) and plasma (normalized to cel-miR-238 spike-in; n = 6∼8); ***P < .001. (E) qPCR analysis and comparison of VWF mRNA level in WT and STZ/HFD diabetic mice lung (normalized to glyceraldehyde-3-phosphate dehydrogenase [GAPDH]; n = 8-10); ****P < .0001. (F) ELISA analysis and comparison of VWF levels in WT and STZ/HFD diabetic mice plasma (n = 8-10); ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/22/10.1182_blood-2015-01-620278/4/m_3377f2.jpeg?Expires=1769828574&Signature=SE8so7ZJmZ-ojm5mQj5MOBVCmBxv943TXkBBWNqQEOaLlTRRqx9I4NSuualV-OUxnRq8mxaXhvtdTJGm1VMLoyk8b3GaKpJ86rhrDGs~43xIzji6ce32DHKUR9XKGF2XLslacgATCbCfDAfops9dLelPhNymt56f0ttlJnvMhS~0TpYV5Y2Z~RbyDLBZGIZOmIWUDNZ4gWQ3OfyfO~sCIEpinqf6hxaVpnJUXrPOqi~gBnjxalfB9rnQQqvZXl0UTOeYUzacvgcAw9RgGymkBn25fvzEkWJth9j2MDZ9PhgtjWRu2AjA7wnRonIvCog~144hVOnxTl9eURI66PjnYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal