In this issue of Blood, Schneidawind et al1 demonstrate that the adoptive transfer of CD4+ invariant natural killer T (iNKT) cells from third-party mice protects from lethal graft-versus-host disease (GVHD) through expansion of donor regulatory T cells (Tregs) in a murine model of allogeneic hematopoietic cell transplantation (HCT).
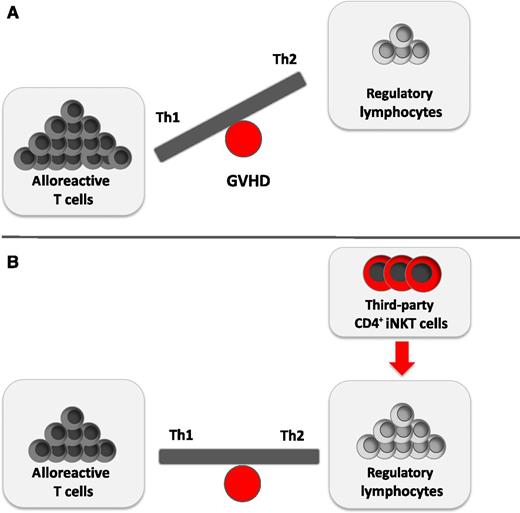
The balance between alloreactive donor T cells and regulatory lymphocytes is essential for GVHD prevention. (A) Allogeneic T cells are crucial in the development of GVHD. They secrete large amounts of proinflammatory Th1-biased cytokines such as IFN-γ, which results in further T-cell activation and expansion. In contrast, a Th2-directed immune polarization favors GVDH prevention. Accordingly, the imbalance of allogeneic T cells and immunomodulatory lymphocytes results in GVHD. (B) Schneidawind et al1 demonstrate how the adoptive transfer of low numbers of CD4+ iNKT cells expands donor Tregs and yields a cytokine bias in favor of Th2 cytokines. This balance of immunostimulatory and immunosuppressive cell subsets can dampen GVHD.
The balance between alloreactive donor T cells and regulatory lymphocytes is essential for GVHD prevention. (A) Allogeneic T cells are crucial in the development of GVHD. They secrete large amounts of proinflammatory Th1-biased cytokines such as IFN-γ, which results in further T-cell activation and expansion. In contrast, a Th2-directed immune polarization favors GVDH prevention. Accordingly, the imbalance of allogeneic T cells and immunomodulatory lymphocytes results in GVHD. (B) Schneidawind et al1 demonstrate how the adoptive transfer of low numbers of CD4+ iNKT cells expands donor Tregs and yields a cytokine bias in favor of Th2 cytokines. This balance of immunostimulatory and immunosuppressive cell subsets can dampen GVHD.
GVHD is one of the most serious complications after allogeneic HCT.2,3 Although advanced treatment options have been approved and new insight into cellular interactions and immune dysregulation has been gained, GVHD still develops in ∼40% to 60% of patients.2 T cells are essential for causing GVHD. These alloreactive donor T cells secrete large amounts of proinflammatory T helper (Th)1-biased cytokines such as interferon γ (IFN-γ), which results in further T-cell activation and expansion. In contrast, a Th2-directed immune polarization can favor GVDH prevention (see figure).2 Various attempts have been made to dampen GVHD, such as the use of Tregs that successfully reduce early expansion of alloreactive donor T cells.4 However, Tregs are rare in peripheral blood, and ex vivo expansion is complex and time-consuming.
NKT cells are a small subset of lymphocytes that bridge innate immune responses to adaptive immunity. They express several cell surface proteins characteristic for T and NK cells. In general, NKT cells are reactive to lipid antigens presented by CD1d major histocompatibility complex class I–like molecules.5 These CD1d-restricted NKT cells can be subclassified into at least 2 groups: one using a semi-invariant T-cell receptor (TCR) (iNKT or type I) and one expressing somewhat more diverse TCRs (type II NKT). Activation of iNKT cells stimulates the rapid release of distinct immunoregulatory cytokines that elicit both Th1 (IFN-γ) and Th2 (eg, interleukin [IL]-4, IL-10, and IL-13) responses. Of note, CD4+ iNKT cells seem to favor the release of Th2-biased cytokines.5
The proportion of immunoregulatory iNKT cells in human blood is highly variable and ranges from <0.1% to >2% of the entire T-cell pool. Remarkably, this proportion is thought to be genetically regulated.5 Some studies propose a correlation between low numbers of iNKT cells and the occurrence of autoimmune diseases, whereas transfer of NKT cells suppresses different autoimmune and alloimmune reactions in both animal models and humans.6 These observations have paved the way for clinical translation into the field of allogeneic transplantation and GVHD. For instance, higher amounts of CD4+ iNKT cells in the stem cell graft have been associated with a significantly lower risk of acute GVHD,7 and low peripheral blood iNKT/T-cell ratios after transplant have been identified as an independent risk factor for developing acute GVHD.8 Lastly, adoptive transfer of both donor and host iNKT cells has been described to suppress GVHD in mice.9,10 This effect mediated by iNKT-cell–secreted IL-4 drives Th2 polarization of conventional donor-derived T cells. Moreover, highly purified NKT-cell infusions protected from GVHD development in mice by limiting T cell–mediated secretion of proinflammatory cytokines such as IFN-γ and tumor necrosis factor α.10
Compared to Tregs, markedly lower numbers of CD4+ iNKT cells can prevent GVHD through inducing a Th2-biased immune response. In a previous study,9 Schneidawind and colleagues revealed the underlying mechanism of this effect by showing that transfer of donor CD4+ iNKT cells provokes a robust expansion of donor CD4+CD25+FoxP3+ Tregs. Of note, a ratio of <1:20 of CD4+ iNKT to conventional T cells was sufficient, whereas a ratio of ∼1:1 was needed when purified, expanded Tregs were transferred together with conventional T cells. Staining for Helios revealed that it was not inducible Tregs but naturally occurring Tregs contained within the graft that expanded on iNKT cell transfer. Although GVHD lethality was highly abrogated, graft-versus-tumor immune responses were retained because lymphoma growth did not accelerate during Treg expansion in 2 different models. These data underscore the therapeutic potential of iNKT cells for immunomodulation within the graft recipient.
The current study by Schneidawind et al1 uses third-party CD4+ iNKT cells instead of donor-derived iNKT cells (see figure), which extends the feasibility of the described approach and makes it even more useful for the clinic. Because CD1 molecules are nonpolymorphic, this immune-regulatory pathway can be targeted for development of non-HLA-dependent therapeutic approaches to T cell–mediated autoimmune diseases and GVHD. Accordingly, the use of third-party iNKT cells opens a huge source of availability and feasibility across major histocompatibility barriers. Although iNKT cells are a rare cell population, the transfer of low numbers of iNKT cells yields robust in vivo responses, which makes this approach interesting because only low amounts of blood from a third-party individual would be needed. However, dose-finding studies and careful observation of possible side effects will be essential in humans to determine the optimal dosage and timing of transferred cells. Finally, third-party iNKT cells are rejected early after allogeneic transplant, already around day +10, limiting the exposure of the recipient to these cells. However, iNKT cells have been proved to represent a highly potent immunoregulatory lymphocyte subset, and some studies have demonstrated that iNKT cells might under certain conditions also contribute to the pathogenesis of GVHD. Therefore, it would not be surprising to discover further yet unknown and maybe harmful effects of iNKT cells on additional immune and other healthy cells in the cellular interplay. Thus, further research is crucial for understanding GVHD and the concept of most optimally modulated adoptive iNKT cell therapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.