In this issue of Blood, Xiang et al identify a novel mechanism, involving activation of the polyol pathway and repression of microRNA-24 (miR-24), through which hyperglycemia augments von Willebrand factor (VWF) expression and secretion.1
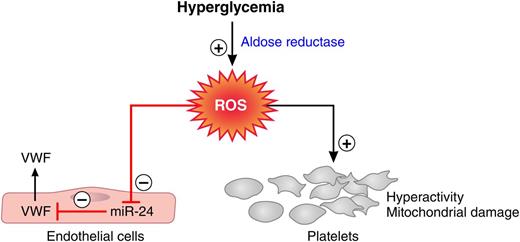
Aldose reductase-mediated effects of hyperglycemia on the coagulation system. Flux of glucose via aldose reductase into the polyol pathway generates ROS. ROS formation depresses miR-24, which releases this miRNA’s block on VWF secretion and results in elevated VWF plasma levels. Aldose reductase-mediated ROS formation also causes platelet hyperreactivity and mitochondrial damage. Red lines, negative regulation; black arrows, positive regulation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Aldose reductase-mediated effects of hyperglycemia on the coagulation system. Flux of glucose via aldose reductase into the polyol pathway generates ROS. ROS formation depresses miR-24, which releases this miRNA’s block on VWF secretion and results in elevated VWF plasma levels. Aldose reductase-mediated ROS formation also causes platelet hyperreactivity and mitochondrial damage. Red lines, negative regulation; black arrows, positive regulation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Hyperglycemia alters glucose metabolism profoundly. Increased flux of glucose into the polyol pathway, when the capacity of the glycolytic pathway is saturated, is one of the major metabolic changes that has been associated with the development of diabetic complications.2 The enzymes aldose reductase and sorbitol dehydrogenase generate sorbitol and fructose in processes that use nicotinamide adenosine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NAD+) as cofactors. As increasing amounts of glucose enter the polyol pathway, 2 major mechanisms lead to an increase in oxidant stress: (1) the augmented formation of NADH leads to the production of reactive oxygen species (ROS) by NADH oxidase, and (2) the depletion of NADPH causes reduction of cytosolic glutathione levels, impairing the antioxidant defense system of the cell. Xiang et al discover that polyol pathway-mediated oxidant stress represses miR-24, which leads to elevated plasma VWF concentrations in hyperglycemia (see figure).1 Previous work conducted in the same laboratory showed that polyol-mediated oxidant stress also contributes to platelet hyperreactivity and platelet mitochondrial damage in diabetic patients (see figure).3,4
Mature VWF mediates platelet adhesion and aggregation and stabilizes blood coagulation factor VIII.5 Elevated VWF levels have been associated with arterial thrombosis6 and poor glycemic control.7 However, whether and how high glucose might cause upregulation of VWF remain unknown.
Xiang et al hypothesized that miRNAs might be involved in the regulation of VWF and screened 29 candidate miRNAs in reporter gene assays using either the human or the mouse VWF 3′ untranslated region (3′UTR). Although miR-24 was not the only hit, it was the miRNA with the most pronounced effects on both mouse and human VWF 3′UTR activity. As expected, circulating miR-24 levels were reduced and plasma VWF levels elevated in patients with type 1 and type 2 diabetes compared with age- and sex-matched healthy controls.7,8 Importantly, mouse biology recapitulated the observations in humans faithfully. Three diabetic mouse models showed decreased miR-24 and increased VWF levels.
Xiang et al exploited the conservation of miR-24 to conduct a series of loss- and gain-of-function experiments in mice using anti-miRNAs and miRNA mimics. These experiments revealed the causal links among repression of miR-24, increase in VWF expression, and propensity for thrombosis. Experiments in cells showed that miR-24 regulated multiple events in VWF biosynthesis, maturation, and secretion, which may all contribute to the increase in VWF plasma levels when miR-24 is repressed. Repression of miR-24 by high glucose was reversed by an inhibitor of the rate-limiting enzyme in the polyol pathway: aldose reductase. Experiments with the aldose reductase inhibitor also revealed that c-Myc was activated by glucose flux through the polyol pathway and knockdown of c-Myc in turn increased miR-24 levels with remarkable specificity.
These findings reveal a mechanism for the elevated levels of high-molecular-weight VWF observed in diabetic patients and possibly also for the increased risk of thrombotic events. Further work will be required to assess whether miR-24 and VWF levels can be manipulated in humans (eg, with miR-24 mimics) and whether such interventions have potential as antithrombotic therapies in patients with diabetes mellitus. Targeting the polyol pathway seems conceptually attractive because of the coincident activities on platelets3,4 and VWF1 (see figure), although it is not the only source of oxidant stress in diabetes. Indeed, several aldose reductase inhibitors have been developed and undergone clinical testing primarily for diabetic neuropathy, but there remains uncertainty regarding their efficacy and safety. One of the problems in diabetic neuropathy is the imprecision of biomarkers and measures of efficacy. However, the impact of aldose reductase inhibitors on VWF concentrations and platelet function may be more quantitatively assessable.
These novel results on the molecular mechanisms contributing to thrombosis in diabetic patients may lead to new opportunities for therapeutic intervention.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal