Key Points
CMP is an effective induction regimen for transplant-ineligible MM patients.
The CMP regimen is safe and well tolerated with a notable lack of peripheral neuropathy.
Abstract
This phase 1/2 dose-escalation study investigated the combination of carfilzomib with melphalan and prednisone (CMP) in patients aged >65 years with newly diagnosed multiple myeloma (MM). Melphalan and prednisone were administered orally on days 1 to 4; carfilzomib was IV administered on days 1, 2, 8, 9, 22, 23, 29, and 30 of a 42-day cycle. Patients received up to 9 cycles of CMP. In the phase 1 dose-escalation portion, the primary objectives were to determine the incidence of dose-limiting toxicities during the first cycle of CMP treatment to define the maximal tolerated dose (MTD) of carfilzomib. In the phase 2 portion, the primary objective was to evaluate the overall response rate (ORR) of CMP. In the phase 1 portion of the study, 24 patients received CMP at carfilzomib dosing levels of 20 mg/m2, 27 mg/m2, 36 mg/m2, and 45 mg/m2. The MTD was established as 36 mg/m2. In the phase 2 portion of the study, 44 patients were enrolled at the MTD. Among 50 efficacy-evaluable patients treated at the MTD, the ORR was 90%. The projected 3-year overall survival rate was 80%. The combination of CMP was observed to be effective in elderly patients with newly diagnosed MM. This trial was registered at www.clinicaltrials.gov as #NCT01279694 (Eudract identifier 2010-019462-92).
Introduction
Prior to 2007, frontline chemotherapy with melphalan and prednisone (MP) was considered a standard of care in the treatment of elderly patients with multiple myeloma (MM) who are ineligible for stem cell transplantation.1 Since then, however, several prospective, randomized, phase 3 studies comparing MP with or without novel agents such as bortezomib (VMP regimen) or thalidomide (MPT regimen) have demonstrated that MPT and VMP are superior to MP in terms of overall response rate (ORR), time to progression (TTP) or progression-free survival (PFS), and overall survival (OS).2 In the VISTA trial that compared VMP and MP, median TTP was 24 months in the VMP arm vs 16.6 months in the MP arm, and median OS was also significantly increased in the VMP arm of the study.3 In the Intergroupe Francophone du Myélome (IFM) 99-06 trial, the median PFS was 27.5 months with MPT vs 18 months with MP, and the median OS was 52 months vs 33 months, respectively.4 These studies were the pivotal trials upon which the approval of these 2 therapeutic regimens for the treatment of patients with MM was based. Both MPT and VMP are now considered standard-of-care regimens in patients with newly diagnosed MM who are older than 65 years or not eligible for autologous stem cell transplantation. However, one of the major toxicity concerns of these 2 regimens is peripheral neuropathy (PN), which was observed in 13% and 6% (grade 3-4) of the patients in the VISTA and the IFM 99-06 trials, respectively.3,4
The combination of MP and lenalidomide has also been evaluated in MM, but this combination has not yet been approved by regulatory authorities.5 Recently, the MM020 trial prospectively compared outcomes of MPT vs lenalidomide and low-dose dexamethasone (Len-Dex), and in this trial, treatment with Len-Dex until disease progression showed improved PFS and OS compared with MPT.6
Carfilzomib is a selective proteasome inhibitor that has demonstrated robust and durable activity and a favorable safety and tolerability profile as a single agent in heavily pretreated patients with relapsed and/or refractory MM.7 Grade 3 to 4 adverse events (AEs) observed in phase 2 carfilzomib studies were mostly hematologic and are thought to be manageable with supportive measures and/or dose modifications. Carfilzomib is approved in the United States to treat patients with MM who have received at least 2 prior lines of therapy, including bortezomib and an immunomodulatory agent, and who have experienced disease progression during or within 60 days of completing their last therapy.8 In relapsed and/or refractory MM, carfilzomib has also been evaluated in 3 prospective, randomized, phase 3 trials: the ASPIRE,9 ENDEAVOR (clinicaltrials.gov #NCT01080391), and FOCUS (#NCT01568866) studies. The interim analysis of the ASPIRE trial showed that in patients with relapsed MM, the addition of carfilzomib to lenalidomide and dexamethasone resulted in significantly improved PFS compared with lenalidomide and dexamethasone.9 The results of ENDEAVOR and FOCUS have not yet been published.
The favorable toxicity profile of carfilzomib, including a lower incidence of PN compared with previous reports for bortezomib,10 together with its impressive activity resulting in rapid and durable antitumor responses, makes it an attractive candidate for inclusion in a combination regimen with MP (CMP) for the frontline treatment of elderly patients with MM. Herein, we report results from the first prospective phase 1/2 study of the CMP regimen in patients aged >65 years with newly diagnosed MM.
Patients and methods
Patients
Patients aged >65 years with a diagnosis of symptomatic, measurable, newly diagnosed MM were eligible for enrollment. Adequate organ function (defined as absolute neutrophil count >1.0 × 109/L, platelet count >50 × 109/L, aspartate transaminase and alanine transaminase <3 times the upper limit of normal, total bilirubin less than twofold of the upper limit of normal, and creatinine clearance >30 mL/min), and Eastern Cooperative Oncology Group performance status of 0 to 2 at study entry were required. Exclusion criteria were the presence of another cancer, amyloidosis, severe ongoing infection, or any other serious medical condition or psychiatric disease. All patients provided written, informed consent in accordance with the Declaration of Helsinki.
Study design and treatment
Patients in this phase 1/2, multicenter, single-arm, open-label, dose-escalation study of CMP (www.clinicaltrials.gov identifier NCT01279694, Eudract identifier 2010-019462-92) were enrolled from 8 study centers in France between October 2010 and October 2012. The primary objectives of the phase 1 portion of the study were to determine the incidence of dose-limiting toxicities (DLTs) during the first cycle of carfilzomib (at a given dose level) in combination with MP to define the maximum tolerated dose (MTD) of carfilzomib; the primary objective of the phase 2 portion was to evaluate the ORR (defined as the percentage of patients with complete response [CR], very good partial response [VGPR], and partial response) of CMP. Secondary objectives were to evaluate the safety and tolerability of CMP and to assess the efficacy of CMP in terms of PFS (defined as the time from enrollment until disease progression or death from any cause) and OS (defined as the time from enrollment until the date of death or the date the patient was last known to be alive). The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by a single institutional review board/independent ethics committee (Tours, France) for all of the study centers involved, according to French law.
A standard 6 + 6 dose-escalation scheme was used to determine the MTD of carfilzomib in the CMP regimen. Six patients were treated at each carfilzomib dosing level; if ≤1 DLT was observed at a dosing level, an additional 6 patients were subsequently enrolled at the next-highest dosing level. If ≥2 DLTs were observed at a single dosing level, the previous dosing level was identified as the MTD. After the MTD was defined in the phase 1 portion of the study, 44 additional patients were enrolled in the phase 2 portion at the MTD to further evaluate the safety profile and to estimate the efficacy of CMP (phase 2).
As assessed in the first cycle of treatment, DLTs were defined as any hematologic toxicity of grade 4 intensity or preventing the administration of ≥2 of the 8 carfilzomib doses of the first treatment cycle (except for grade 4 thrombocytopenia without bleeding lasting ≤7 days or for grade 4 neutropenia lasting ≤7 days), grade ≥3 febrile neutropenia, grade ≥3 gastrointestinal toxicities (except for grade ≥3 nausea/vomiting if the patient had not received adequate antiemetic prophylaxis), any other grade ≥3 nonhematologic toxicity considered related to carfilzomib, or grade ≥3 PN persisting for >3 weeks after discontinuation of study drugs. Administration of granulocyte colony-stimulating factor was not permitted during the first cycle of therapy.
In the phase 1 portion of the study, treatment consisted of oral melphalan (9 mg/m2) and oral prednisone (60 mg/m2), both administered on days 1 to 4 in combination with escalating doses of carfilzomib (administered IV on days 1, 2, 8, 9, 22, 23, 29, and 30) within a 42-day cycle. Patients received up to 9 cycles of treatment.
Carfilzomib was administered at 20 mg/m2 on days 1 and 2 of the first cycle and at 20, 27, or 36 mg/m2 thereafter. The dosage of carfilzomib in dosing cohort 3 (ie, 36 mg/m2) had been anticipated as the highest tolerable dose. However, in November 2008, no DLT had yet been observed at the carfilzomib dose of 36 mg/m2 in combination with MP, and the protocol was subsequently amended (amendment 1) to open a fourth dosing cohort at 45 mg/m2. In the phase 2 portion of the trial, patients received 9 42-day cycles of CMP at the MTD.
Assessments
Assessments of efficacy and safety were conducted every 6 weeks or more frequently, if clinically required. Response and progressive disease were evaluated using the International Uniform Response Criteria for Multiple Myeloma.11 All AEs were assessed at each patient visit and were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0).12
Statistical analysis
Following the phase 1 portion of the study (6 + 6 design) and the definition of the MTD, the sample size of the phase 2 portion was defined as 44 patients, in order to study a population of 50 patients at the MTD level. This sample size was based on the results of a similar phase 1/2 study evaluating MP plus bortezomib in the same population of patients, in which 12 patients were enrolled in the phase 1 portion and 48 in the phase 2 portion at the MTD level.13 We assumed an ORR π0 of 0.65, 0.70, or 0.75, under which further study of the CMP combination would not be justified, and an ORR π1 of 0.80, 0.85, 0.90, or 0.95 that, if observed, could justify additional tests of this combination.14 We used reasonable ranges for π0 and π1 instead of a priori fixed values, which are usually used for calculations prior to study initiation. When using these values and a 2-sided test with type I error of 0.05, if the observed ORR is ≥85% in a sample of 50 patients receiving the MTD, the power will be at least 0.85 and the trial could be considered positive. Kaplan-Meier curves for PFS and OS were plotted.15
Results
Patient characteristics
A total of 72 patients with newly diagnosed MM were enrolled in the study. Four patients were excluded for protocol violations: 3 patients did not receive the first dose of carfilzomib because of an exclusion criterion not having been followed (severe ongoing infection [n = 1], preexisting solid tumor [n = 1], and preexisting cardiac failure [n = 1]), and 1 patient received the first 2 doses of carfilzomib (day 1 and 2 of cycle 1) despite a preexisting, severe ongoing infection at study entry and died of sepsis on day 5. Sixty-eight patients who received at least 1 dose of carfilzomib and who fulfilled all inclusion and exclusion criteria were assessable for toxicity.
Baseline demographics and disease characteristics are shown in Table 1. The overall median age was 72 years (range, 66 to 86 years), and 35% of the patients had International Staging System stage 3 disease at study entry. Eighteen percent of the patients had adverse cytogenetic features at enrollment.
Patient and disease characteristics at baseline
| Characteristic . | Patients (n = 68) . |
|---|---|
| Sex, n (%) | |
| Male | 35 (51) |
| Female | 33 (49) |
| Median age, years (range) | 72 (66-86) |
| ECOG performance status, n (%) | |
| 0 | 25 (37) |
| 1 | 28 (41) |
| 2 | 15 (22) |
| ISS stage, n (%) | |
| 1 | 23 (34) |
| 2 | 21 (31) |
| 3 | 24 (35) |
| Median creatinine level, μM/L (range) | 88 (43-250) |
| Patients with creatinine clearance <60 mL/min (%) | 7 (10) |
| Median calcium level (mM/L) (range) | 2.34 (2.01-2.99) |
| Patients with calcium level >2.75 mM/L (%) | 4 (6) |
| Median hemoglobin level (g/dL) (range) | 10.8 (7.4-14.3) |
| Patients with hemoglobin level <10 g/dL (%) | 19 (27.9) |
| Cytogenetics (FISH), n (missing) | 59 (9 missing data) |
| t(4;14), n (%) | 5 (8) |
| del17p, n (%) | 6 (10) |
| no t(4;14); no del17p, n (%) | 48 (82) |
| Characteristic . | Patients (n = 68) . |
|---|---|
| Sex, n (%) | |
| Male | 35 (51) |
| Female | 33 (49) |
| Median age, years (range) | 72 (66-86) |
| ECOG performance status, n (%) | |
| 0 | 25 (37) |
| 1 | 28 (41) |
| 2 | 15 (22) |
| ISS stage, n (%) | |
| 1 | 23 (34) |
| 2 | 21 (31) |
| 3 | 24 (35) |
| Median creatinine level, μM/L (range) | 88 (43-250) |
| Patients with creatinine clearance <60 mL/min (%) | 7 (10) |
| Median calcium level (mM/L) (range) | 2.34 (2.01-2.99) |
| Patients with calcium level >2.75 mM/L (%) | 4 (6) |
| Median hemoglobin level (g/dL) (range) | 10.8 (7.4-14.3) |
| Patients with hemoglobin level <10 g/dL (%) | 19 (27.9) |
| Cytogenetics (FISH), n (missing) | 59 (9 missing data) |
| t(4;14), n (%) | 5 (8) |
| del17p, n (%) | 6 (10) |
| no t(4;14); no del17p, n (%) | 48 (82) |
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; ISS, International Staging System.
DLTs and the definition of the MTD
The phase 1 portion of the study included a total of 24 patients, 6 in each carfilzomib dosing-level cohort. One DLT was observed at the 20-mg/m2 dosing level (grade 3 deep vein thrombosis), 1 DLT was observed at the 27-mg/m2 dosing level (grade 3 febrile neutropenia), 1 DLT was observed at the 36-mg/m2 dosing level (grade 3 febrile neutropenia), and 2 DLTs were observed at the 45-mg/m2 dosing level (fever and hypotension during carfilzomib infusion). Therefore, the dose of 36 mg/m2 was considered to be the MTD of carfilzomib in the CMP regimen. All DLTs had a favorable outcome. The 4 patients enrolled at the 45-mg/m2 carfilzomib dosing level who did not experience a DLT remained on study and received the 36-mg/m2 carfilzomib dose during subsequent cycles.
Efficacy
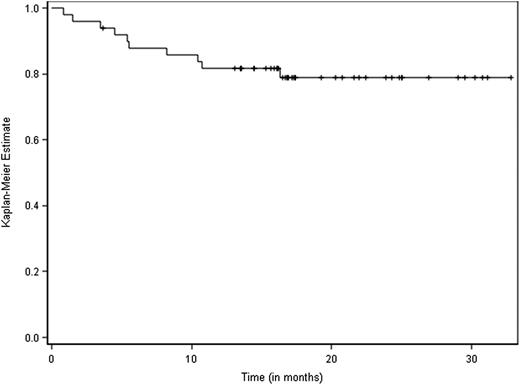
At the cutoff date of April 14, 2014, the median follow-up for all patients was 22 months. Fifty patients were assessable for efficacy at the MTD level of 36 mg/m2: 6 in the phase 1 portion of the study and 44 in the phase 2 portion. Patients received a median number of 9 cycles (range, 1-9) of CMP, and 37 patients (74%) completed all 9 cycles. The ORR was 90% (95% confidence interval [CI] [77.8; 96.6]), including 58% of patients with VGPR or better, and 12% of patients achieving a CR (Table 2). No patient had progressive disease on therapy. The median time to response among patients who had a partial response or better was 42 days (range, 42-126 days). For this group of 50 patients, the projected 3-year OS rate was 80% (95% CI [67.8; 90]) (Figure 1) and the median PFS was 21 months (95% CI [18.2; 23.1]) (Figure 2).
Response rates
| Best overall response . | Patients treated at the MTD of 36 mg/m2 (n = 50) . |
|---|---|
| CR | 6 (12) |
| VGPR | 23 (46) |
| Partial response | 16 (32) |
| Stable disease | 5 (10) |
| Progressive disease | 0 (0) |
| ORR | 45 (90) |
| Best overall response . | Patients treated at the MTD of 36 mg/m2 (n = 50) . |
|---|---|
| CR | 6 (12) |
| VGPR | 23 (46) |
| Partial response | 16 (32) |
| Stable disease | 5 (10) |
| Progressive disease | 0 (0) |
| ORR | 45 (90) |
Data are presented as n (%) of patients.
Safety
For the entire safety population (n = 68, including all cycles of CMP received), 3104 AEs of any grade were collected, including 572 (18.4%) grade 3 to 4 AEs. The most frequent grade 3 to 4 AEs (occurring in >10% of patients) were neutropenia, thrombocytopenia, and anemia (Table 3). Other grade 3 to 4 AEs accounted for <6% of the total number of AEs experienced by patients in the study (Table 3). No specific cardiac toxicity was observed. Three cases of grade 3 congestive heart failure were reported in 3 patients with a history of hypertension. Each case was related to excess hydration and was reversible with the administration of IV diuretics. Two patients experienced reversible grade 3 elevated creatinine during infection. None of these grade 3 AEs led to treatment discontinuation. Only 1 patient with preexisting diabetes developed grade 3 PN. Three additional patients developed grade 2 PN on study.
Adverse events
| Adverse event, n (%) . | Patients (n = 68) . | |
|---|---|---|
| Any grade AE . | Grade 3 to 4 AEs . | |
| Thrombocytopenia | 58 (85, [74.6; 92.7]) | 19 (28, [17.7; 40.1]) |
| Anemia | 56 (82, [71.2; 90.6]) | 24 (35, [24.1; 47.8]) |
| Fatigue | 53 (78, [66.2; 87.1]) | 2 (3, [0.4; 10.2]) |
| Neutropenia | 44 (65, [52.2; 75.9]) | 26 (38, [26.7; 50.8]) |
| Infections | 36 (53, [39.0; 63.8]) | 5 (7, [2.4; 16.3]) |
| Nausea | 33 (48, [37.6; 62.4]) | 4 (6, [1.6; 14.4]) |
| Elevated liver enzymes | 20 (29, [19; 41.7]) | 3 (4, [0.9; 12.3]) |
| Peripheral neuropathy | 17 (25, [15.9; 37]) | 1 (1.5, [0.04; 7.9]) |
| Peripheral edema | 15 (22, [14.1; 35.4]) | 0 (0, [0; 5.2]) |
| Elevated creatinine | 14 (21, [12.6; 31.2]) | 2 (3, [0.4; 10.2]) |
| Diarrhea | 12 (18, [8.4; 27.1]) | 0 (0, [0; 5.2]) |
| Deep vein thrombosis | 4 (6, [1.6; 14.4]) | 1 (1.5, [0.04; 7.9]) |
| Congestive heart failure | 4 (6, [1.6; 14.4]) | 3 (4, [0.9; 12.3]) |
| Atrial fibrillation | 3 (4, [0.9; 12.3]) | 1 (1.5, [0.04; 7.9]) |
| Hypertension | 2 (3, [0.4; 10.2]) | 2 (3, [0.4; 10.2]) |
| Adverse event, n (%) . | Patients (n = 68) . | |
|---|---|---|
| Any grade AE . | Grade 3 to 4 AEs . | |
| Thrombocytopenia | 58 (85, [74.6; 92.7]) | 19 (28, [17.7; 40.1]) |
| Anemia | 56 (82, [71.2; 90.6]) | 24 (35, [24.1; 47.8]) |
| Fatigue | 53 (78, [66.2; 87.1]) | 2 (3, [0.4; 10.2]) |
| Neutropenia | 44 (65, [52.2; 75.9]) | 26 (38, [26.7; 50.8]) |
| Infections | 36 (53, [39.0; 63.8]) | 5 (7, [2.4; 16.3]) |
| Nausea | 33 (48, [37.6; 62.4]) | 4 (6, [1.6; 14.4]) |
| Elevated liver enzymes | 20 (29, [19; 41.7]) | 3 (4, [0.9; 12.3]) |
| Peripheral neuropathy | 17 (25, [15.9; 37]) | 1 (1.5, [0.04; 7.9]) |
| Peripheral edema | 15 (22, [14.1; 35.4]) | 0 (0, [0; 5.2]) |
| Elevated creatinine | 14 (21, [12.6; 31.2]) | 2 (3, [0.4; 10.2]) |
| Diarrhea | 12 (18, [8.4; 27.1]) | 0 (0, [0; 5.2]) |
| Deep vein thrombosis | 4 (6, [1.6; 14.4]) | 1 (1.5, [0.04; 7.9]) |
| Congestive heart failure | 4 (6, [1.6; 14.4]) | 3 (4, [0.9; 12.3]) |
| Atrial fibrillation | 3 (4, [0.9; 12.3]) | 1 (1.5, [0.04; 7.9]) |
| Hypertension | 2 (3, [0.4; 10.2]) | 2 (3, [0.4; 10.2]) |
Data are presented as n (%, [95% CI]).
Twelve patients had died at the time of this analysis. Deaths occurred due to progressive disease (n = 7), infections (n = 2), cardiac failure (n = 1), respiratory distress (n = 1), and metastatic urothelial carcinoma (n = 1). The latter patient died of metastatic urothelial carcinoma that was diagnosed during cycle 3. At that time, the cancer had already disseminated, and its diagnosis was likely missed at study entry. The case was thus not considered to be a second primary malignancy.
Discussion
This trial is the first to combine the proteasome inhibitor carfilzomib with melphalan and prednisone as part of frontline therapy in patients over 65 years of age with symptomatic MM. We demonstrate that the MTD of carfilzomib incorporated into the CMP regimen is 36 mg/m2. We also show that the safety profile of CMP includes few grade 3 to 4 infections, only 1 case of grade 3 deep vein thrombosis, no adverse cardiac toxicity signals, and no cases of second primary malignancies. A single patient experienced grade 3 PN; these results compared favorably with those observed with VMP or MPT in similar patient populations for whom PN is the most significant toxicity (in these studies, grade ≥3 PN was observed in 6% to 13% of patients).2-4 Management strategies for PN have improved over the last decade and include dose reduction of bortezomib and thalidomide and weekly or subcutaneous administration of bortezomib.16,17 Despite these improvements, PN still remains an important issue for patients treated with VMP or MPT, and the favorable toxicity profile of carfilzomib relative to bortezomib or thalidomide in this regard is an interesting advantage of the CMP regimen.
Lastly, the CMP regimen was able to induce high rates of response, with an ORR and a VGPR-or-better rate of 90% and 58%, respectively, at the MTD. The 12% CR rate observed may be underestimated, as bone marrow aspiration was not performed in some patients with both normal electrophoresis and negative immunofixation. Responses were rapid, and the majority of patients were able to complete the planned 9 cycles of treatment, reflecting not only the efficacy but also the tolerability of CMP in these patients.
Although caution is required when comparing results of phase 2 vs phase 3 trials, the observed 90% ORR compares favorably with other novel-agent–based regimens used as part of frontline therapy in elderly patients ineligible for stem cell transplantation. For example, in the phase 1/2 study of VMP using a very similar schedule (ie, 9 6-week cycles), the ORR was 89% in 53 patients,13 whereas in the phase 3 VISTA study, the ORR in the VMP arm was 71%.3 The ORR of MPT ranged from 59% in a meta-analysis of all MPT vs MP trials reported by Fayers et al18 to 62% in the recent MM020 study comparing Len-Dex to MPT6 to up to 76% in the IFM 99-06 pivotal trial upon which the approval of MPT was based.4 The doublet combination of Len-Dex, which is not yet approved in Europe but is widely used in the United States, was associated with an ORR of 76% in the Eastern Cooperative Oncology Group trial19 and an ORR of 73% and 75% in the 18-month Len-Dex arm and the Len-Dex treatment to progression arm, respectively, in the recent MM020 study.6
In the present study, the 21-month median PFS at the MTD was deemed satisfactory by the study investigators, and the projected 80% OS rate at 36 months suggested that patients progressing on CMP can easily be salvaged at the time of progression. Nevertheless, the PFS curve showed a continuous drop from about month 15, when the carry-over effect of CMP appeared to be exhausted, suggesting that a prolonged therapy with additional cycles of CMP beyond the 9 cycles or maintenance with carfilzomib could result in a significant benefit. These survival data should be cautiously compared with those described in the recent phase 3 MM020 trial in a similar population of patients, in which the MPT arm was associated with a median PFS of 21.2 months and Len-Dex administered for either 18 months or until progression was associated with a median PFS of 20.7 and 25.5 months, respectively.6 Similarly, the VMP arm of the VISTA study was associated with a median time-to-progression of 24 months.3 Although our study provides promising preliminary results, robust data for both OS and PFS with the CMP regimen will require prospective analyses from phase 3 trials. CMP, administered per the schedule described herein, is the basis of the ongoing phase 3 multicenter international CLARION trial (#NCT01818752), which was recently completed and has enrolled a similar population of patients in a prospective comparison with VMP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Support for third-party editorial assistance for this manuscript was provided by BlueMomentum, a division of KnowledgePoint 360 Group, an Ashfield company, and funded by Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Authorship
Contribution: P.M. and T.F. designed the research, and all authors performed the research; J.-Y.M. performed the statistical analysis; P.M., C.T., J.-Y.M., and T.F. analyzed and interpreted the data; P.M. wrote the initial draft of the manuscript and edited the final draft; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: P.M., M.A., X.L., C.H., H.A.-L., and T.F. have served as consultants or advisors for and received honoraria from Amgen, Celgene, Janssen, Millennium, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Philippe Moreau, Hematology Department, University Hospital Hôtel-Dieu, Pl Ricordeau, 44093 Nantes, France; e-mail: philippe.moreau@chu-nantes.fr.