Key Points
Mn stimulates macrophages via Dectin-2 to induce donor Th17 differentiation after allogeneic bone marrow transplantation.
Mn-induced Th17 cells accumulate in the lungs to cause pulmonary GVHD.
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative therapy for various hematopoietic disorders. Graft-versus-host disease (GVHD) and infections are the major obstacles of HSCT, and their close relationship has been suggested. Although roles of bacterial and viral infections in the pathophysiology of GVHD are well described, impacts of fungal infection on GVHD remain to be elucidated. In mouse models of GVHD, injection of α-mannan (Mn), a major component of fungal cell wall, or heat-killed Candida albicans exacerbated GVHD, particularly in the lung. Mn-induced donor T-cell polarization toward Th17 and lung-specific chemokine environment in GVHD led to accumulation of Th17 cells in the lung. The detrimental effects of Mn on GVHD depended on donor IL-17A production and host C-type lectin receptor Dectin-2. These results suggest a previously unrecognized link between pulmonary GVHD and fungal infection after allogeneic HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective therapy for hematologic malignant disorders, bone marrow failure, or inherited immunodeficiency syndromes, however, graft-versus-host disease (GVHD) and related microbial infections remain major obstacles to perform HSCT. It has been well demonstrated that both bacterial and viral infections play a significant role in amplifying GVHD by stimulating innate immune responses via pattern recognition receptors to produce inflammatory cytokines that upregulate expressions of major histocompatibility complex (MHC) and co-stimulatory molecules, and therefore enhance allogeneic T-cell responses.1 Clinical data also suggest that bacterial and viral infections lead to the exacerbation of acute GVHD.2,3
Mycosis is also a serious and frequent complication after allogeneic HSCT, however, its effects on GVHD remain to be elucidated. A previous study demonstrated that prophylactic administration of fluconazole was associated with a reduced incidence of severe acute GVHD,4 but its mechanism is yet to be defined.
C-type lectin receptors recognize polysaccharide chain on fungal cell wall, which is mainly composed of multiple layers of carbohydrates, including Mn (polymers of mannose), β-glucan (polymers of d-glucose linked by β-glycosidic bonds), and chitins (polymers of N-acetyl-d-glucosamine).5,6 Mn and β-d-glucan are recognized by Dectin-2 and Dectin-1 receptors, respectively, on host cells to activate macrophages and dendritic cells as antigen-presenting cells, which forces them to produce inflammatory cytokines and drives naïve T-cell differentiation toward Th17 to eliminate fungi.7-10 Host defense against various fungi is dependent on the corresponding receptors to specific fungal species; recognition of Mn by Dectin-2 and subsequent Th17 responses are essential for candida elimination in mice.11
In this study, we have evaluated whether fungal infection could affect severity of acute GVHD using Mn and heat-killed Candida albicans in mouse models of bone marrow transplantation (BMT), and we found a previously unrecognized association between antifungal immunity and pulmonary GVHD.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), B6-Ptprca (H-2b, CD45.1+), B6D2F1 (H-2b/d, CD45.2+), BALB/c (H-2d), and C3H.Sw (H-2b) mice were purchased from Charles River Japan, KBT Oriental, or Japan SLC. IL-17A deficient (IL-17A−/−), Dectin-2 deficient (Dectin-2−/−) mice, and IL-17A−yellow fluorescent protein (YFP) reporter mice (Il17aCreR26ReYFP) with the B6 background were generated as previously reported.11,12 All animal experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee.
BMT
Mice were transplanted as previously described.13 In brief, B6D2F1 and B6 recipients were given x-ray total body irradiation with doses of 12.5 Gy and 10.0 Gy, respectively. TBI was split into 2 doses with a 4-hour interval to reduce gastrointestinal toxicities, followed by intravenous injection with 4 × 106 T-cell-depleted BM (TCD-BM) cells plus 4 × 106 splenic T cells on day 0. Isolation of T cells and T-cell depletion were performed using the Pan-T cell isolation kit and anti-CD90-MicroBeads, respectively, and the AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Mice were intraperitoneally injected with Mn (Sigma-Aldrich, St. Louis, MO) diluted in phosphate-buffered saline (PBS) or PBS control on day 1. Mice were maintained in specific pathogen-free condition and received normal chow and autoclaved hyperchlorinated water for the first 3 weeks post-BMT and filtered water thereafter. Survival after BMT was monitored daily and the degree of clinical GVHD was assessed weekly by a scoring system, as previously described.14 To determine oxygen saturation levels of peripheral artery, a pulse oxygen sensor (2000SL) was attached to the left rear paw and monitored by 9847V monitor (Nonin Medical Inc., Plymouth, MI).15
C albicans culture and injection
C albicans (NBRC1385) was obtained from NITE Biological Resource Center (Chiba, Japan) and grown at 30°C on potato dextrose agar plates (Eiken Kizai, Tokyo, Japan). Yeast form of C albicans was harvested and killed with heat (65°C, 2 h) as previously shown.11 Groups of recipients were intraperitoneally injected with heat-killed C albicans at doses from 105 to 107 per mouse on the day of BMT.
Histologic analysis
Paraffin sections of the lung, liver, and small intestine were stained with hematoxylin and eosin. Acute GVHD was assessed by detailed histopathological analysis using a semiquantitative scoring system in a blinded fashion.14,16 Pictures from tissue sections were taken at room temperature using a digital camera (DP72; Olympus, Tokyo, Japan) mounted on a microscope (BX51; Olympus).
Isolation of leukocytes from tissues
Tissue-localized leukocytes were isolated after perfusion with 10 mL of cold PBS from the left ventricle of the heart as previously described.17,18 The lungs were digested with 100 U/mL collagenase D (Roche Diagnostics, Indianapolis, IN) and 1 μg/mL DNase (Sigma-Aldrich) in complete media that consisted of RPMI 1640 with 10% fetal calf serum (Gibco, Tokyo, Japan), 100 U penicillin/streptomycin (Gibco), 50 μM 2-mercaptoethanol (Sigma-Aldrich), and 20 mM l-glutamine (Gibco) for 1 hour at 37°C. The homogenized lungs were suspended in 4 mL of 40% Percoll (GE Healthcare, Tokyo Japan) and layered onto 4 mL of 70% Percoll, and centrifuged (2400 rpm at 4°C for 30 minutes). The cells were collected from the Percoll interface.
Cell cultures
The 5 × 104 T cells were cultured with 1 × 105 peritoneal macrophages in complete media and stimulated with 0.5 μg/mL of anti-CD3 (2C11) and 10 ng/mL transforming growth factor (TGF)-β (PeproTech, Rocky Hill, NJ) in the presence or absence of 100 μg/mL Mn. Ninety-six hours later, cytokine levels in culture supernatants were determined with a BDCytometric Bead Array (BD Pharmingen, San Diego, CA) by following the manufacturer’s instructions.
Protein extraction from lung tissues
Lung tissues were homogenized in 5 µL/mg wet tissue weight of lysis buffer, consisting of 10 mM Tris-HCl (pH 8.0), 1% NP-40 (Calbiochem, La Jolla, CA), 5 mM EDTA, and Protease inhibitor cocktail (Sigma-Aldrich), using Tissueruptor (Qiagen). After 1-hour incubation at 4°C, the lysates were centrifuged and the supernatants were collected. Cytokine levels in lung extracts were determined by using a BD Cytometric Bead Array according to the manufacturer’s instructions.
Intracellular cytokine staining and cytokine analysis
Lymphocytes were stimulated in vitro with 50 ng/mL of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 100 ng/mL of ionomycin (Sigma-Aldrich) at 37°C for 3 hours. Cells were then incubated with GolgiStop (BD Pharmingen) for an additional 2 hours. Then the cultured cells were stained with surface markers and intracellular cytokines. Monoconal antibodies conjugated with fluorescein isothiocyanate, phycoerythrin, peridinin-chlorophyll protein complexes, allophycocyanin, or allophycocyanin-Cy7 were purchased from BD Pharmingen or eBioscience (San Diego, CA). The 7-aminoactinomycin D (BD Pharmingen) was used for the exclusion of dead cells. Cells were analyzed on fluorescence-activated cell sorters (FACSCalibur and FACSCanto II flow cytometer; BD Biosciences, San Jose, CA) and FlowJo software (TreeStar, Ashland, OR). Cell sorting was performed using an FACSAria III sorter (BD Biosciences).
Quantitative real-time PCR analysis
Total RNA from pulmonary lymphocytes or frozen tissues was extracted using ISOGEN II (Nippon Gene, Tokyo, Japan). Reverse transcription was done with a QuantiTect reverse transcription kit (Qiagen, Venlo, Netherlands) and specific primer and probe sets (Applied Biosystems, Foster, CA; Sigma-Aldrich). Quantitative real-time polymerase chain reaction (PCR) was performed on ABI 7300 (Applied Biosystems) with TaqMan Universal PCR Master Mix (Applied Biosystems). The 18S ribosomal RNA was separately amplified in the same plate as an internal control for variation in the amount of complementary DNA in PCR. The collected data were analyzed using the sequence detector software (Applied Biosystems). Relative amounts of the messenger RNA individual genes were calculated by the comparative ΔC (t) method.
Statistical analysis
Mann-Whitney U tests were used to compare data, the Kaplan-Meier product limit method was used to obtain survival probability, and the log-rank test was applied to compare survival curves. Analyses were performed using JMP Pro Version 10.0.2 (SAS, Cary, NC). P < .05 was considered statistically significant.
Results
Administration of Mn increases mortality and morbidity of GVHD
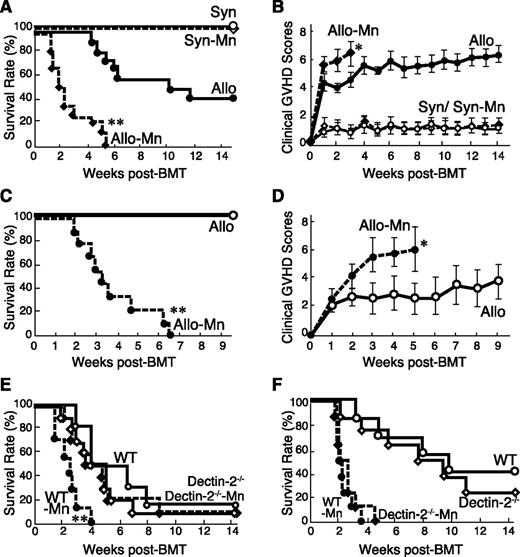
To evaluate the effects of Mn administration on acute GVHD, B6D2F1 mice were lethally irradiated with 12.5 Gy TBI and intraventricularly injected with 4 × 106 purified T cells plus 4 × 106 TCD-BM from MHC-haploidentical C57BL/6 (B6) or syngeneic donors on day 0. Mice were intraperitoneally injected with 1 mg/g body weight of Mn or diluent on day 1. All syngeneic mice survived, whereas 60% of allogeneic control mice died by 14 week after BMT (Figure 1A). Injection of Mn neither induced mortality nor increased clinical scores in syngeneic animals. In contrast, injection of Mn significantly increased mortality and morbidity of GVHD, resulting in increased clinical GVHD scores and 100% mortality in 6 weeks (Figure 1A-B). The effect of Mn was dose-dependent (supplemental Figure 1, available on the Blood Web site). We also confirmed the similar deteriorating effects of Mn on GVHD in a MHC compatible but minor histocompatibility antigen–mismatched C3H.Sw → B6 BMT model (Figure 1C-D) and in a MHC fully mismatched BALB/c → B6 model (Figure 1E). The effects of Mn administration were abrogated when recipient mice lacked a corresponding receptor, Dectin-2 (Figure 1E). In contrast, the recipients transplanted with bone marrow and T cells from Dectin-2−/− donor showed the comparable aggravation of GVHD to the recipients of wild-type (WT) donors after Mn injection (Figure 1F). These results indicated the critical role of Dectin-2 signaling in host cells for Mn-induced GVHD exacerbation.
Administration of Mn exacerbates acute GVHD after allogeneic BMT. (A-B) Lethally irradiated B6D2F1 mice were transplanted with 4 × 106 TCD-BM cells plus 4 × 106 T cells from allogeneic (Allo) B6 donors or syngeneic (Syn) donors on day 0, followed by single intaperitoneally injection of Mn (1 mg/g body weight) or PBS. Survival (A) and clinical GVHD scores (B, means ± standard error) of Syn controls (open circles), Mn-treated Syn (open diamonds), Allo controls (closed circles), and Mn-treated Allo (closed diamonds) from 3 independent experiments were combined (n = 12-15 per group). (C-D) Lethally irradiated B6 mice were transplanted from C3H.Sw donors. Survival (C) and clinical GVHD scores (D, means ± standard error) of Allo controls (open circles) and Mn-treated Allo (closed circles) from 3 independent experiments were combined (n = 9 per group). (E) Lethally irradiated WT or Dectin-2−/− B6 mice were transplanted with cells from BALB/c donors. Survival of WT controls (open circles), Dectin-2−/− controls (open diamonds), Mn-treated WT mice (closed circles), and Mn-treated Dectin-2−/− mice (closed diamonds) from 3 independent experiments were combined (n = 6-10 per group). (F) Lethally irradiated B6D2F1 mice were transplanted with BM and T cells from WT or Dectin-2−/− B6 donors. Survival of Allo controls with WT donors (open circles), Allo controls with Dectin-2−/− donors (open diamonds), Mn-treated mice with WT donors (closed circles), and Mn-treated mice with Dectin-2−/− donors (closed diamonds) from 2 independent experiments were combined (n = 5-10 per group). *P < .05; **P < .01.
Administration of Mn exacerbates acute GVHD after allogeneic BMT. (A-B) Lethally irradiated B6D2F1 mice were transplanted with 4 × 106 TCD-BM cells plus 4 × 106 T cells from allogeneic (Allo) B6 donors or syngeneic (Syn) donors on day 0, followed by single intaperitoneally injection of Mn (1 mg/g body weight) or PBS. Survival (A) and clinical GVHD scores (B, means ± standard error) of Syn controls (open circles), Mn-treated Syn (open diamonds), Allo controls (closed circles), and Mn-treated Allo (closed diamonds) from 3 independent experiments were combined (n = 12-15 per group). (C-D) Lethally irradiated B6 mice were transplanted from C3H.Sw donors. Survival (C) and clinical GVHD scores (D, means ± standard error) of Allo controls (open circles) and Mn-treated Allo (closed circles) from 3 independent experiments were combined (n = 9 per group). (E) Lethally irradiated WT or Dectin-2−/− B6 mice were transplanted with cells from BALB/c donors. Survival of WT controls (open circles), Dectin-2−/− controls (open diamonds), Mn-treated WT mice (closed circles), and Mn-treated Dectin-2−/− mice (closed diamonds) from 3 independent experiments were combined (n = 6-10 per group). (F) Lethally irradiated B6D2F1 mice were transplanted with BM and T cells from WT or Dectin-2−/− B6 donors. Survival of Allo controls with WT donors (open circles), Allo controls with Dectin-2−/− donors (open diamonds), Mn-treated mice with WT donors (closed circles), and Mn-treated mice with Dectin-2−/− donors (closed diamonds) from 2 independent experiments were combined (n = 5-10 per group). *P < .05; **P < .01.
Mn and C albicans exacerbate pulmonary GVHD pathology
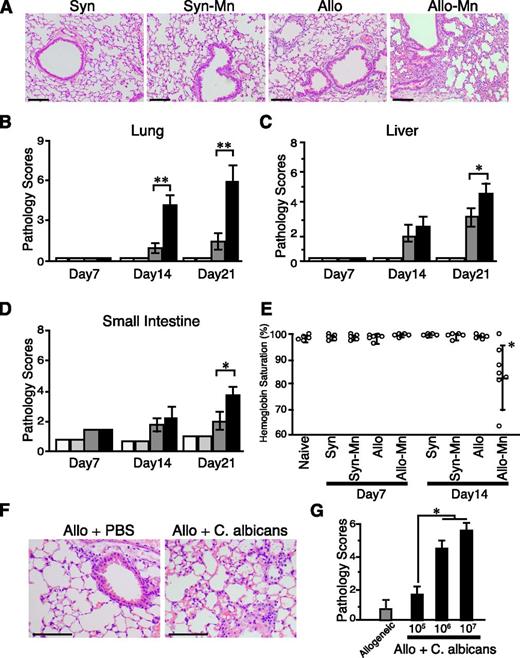
Next, we performed histologic assessment of the lung, liver, and gut on days 7, 14, and 21 after BMT, respectively. In allogeneic control mice, pathology scores of all evaluated organs were significantly higher than those of syngeneic animals on days 14 and 21 after BMT. Mn administration dramatically exacerbated GVHD pathology in the lung after allogeneic BMT, whereas the pathology scores of the liver and small intestine were only modestly increased (Figure 2A-D). The pulmonary lesions were characterized by perivascular cuffing, vasculitis, peribronchiolar cuffing, and alveolar hemorrhage, which were consistent with acute pulmonary GVHD (Figure 2A).14,19,20 The peripheral oxygen levels determined by using a pulse oxygen sensor were not changed on days 7 and 14 after syngeneic or allogeneic BMT (Figure 2E). Mn administration significantly decreased peripheral oxygen levels 14 days after allogeneic BMT. These results indicated that Mn administration accelerates GVHD with most prominent changes in the lung, leading to the respiratory failure. Importantly, Mn administration did not show any detrimental effects after syngeneic transplantation. To test the clinical relevance of these findings, the recipient mice were treated with 1 × 105, 1 × 106, or 1 × 107 of heat-killed C albicans after BMT. The C albicans significantly exaggerated lung GVHD in a dose-dependent manner, suggesting a causative role of fungal infection in lung GVHD (Figure 2F-G).
Mn and C albicans exaggerated GVHD pathology. BMT were performed as shown in Figure 1A. (A) The representative images of the lungs stained with hematoxylin and eosin on day 21 after syngeneic (Syn) and allogeneic (Allo) BMT with or without Mn treatment are shown. Pathology scores of the lung (B), liver (C), and small intestine (D) from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) of 3 independent experiments were combined (n = 5 per group, means ± standard error). (E) Peripheral arterial oxygen saturation levels from 2 independent experiments are combined and means ± standard error are shown. (F-G) Allogeneic recipient mice were intraperitoneally injected with 1 × 105, 1 × 106, or 1 × 107 of heat-killed C albicans on the day of BMT. (F) The representative images of the lungs stained with hematoxylin and eosin on day 14 after allogeneic (Allo) BMT with or without the injection of 1 × 107C albicans are shown. (G) Bar graph shows pathology scores of the lung from Allo controls (dark gray bars) and Allo mice treated with indicated doses of C albicans (black bars) are shown. Data from 1 of 2 independent experiments were shown (n = 3-5 per group, means ± standard error). Original magnification ×200. Scale bars represent 100 μm (A,F). *P < .05; **P < .01.
Mn and C albicans exaggerated GVHD pathology. BMT were performed as shown in Figure 1A. (A) The representative images of the lungs stained with hematoxylin and eosin on day 21 after syngeneic (Syn) and allogeneic (Allo) BMT with or without Mn treatment are shown. Pathology scores of the lung (B), liver (C), and small intestine (D) from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) of 3 independent experiments were combined (n = 5 per group, means ± standard error). (E) Peripheral arterial oxygen saturation levels from 2 independent experiments are combined and means ± standard error are shown. (F-G) Allogeneic recipient mice were intraperitoneally injected with 1 × 105, 1 × 106, or 1 × 107 of heat-killed C albicans on the day of BMT. (F) The representative images of the lungs stained with hematoxylin and eosin on day 14 after allogeneic (Allo) BMT with or without the injection of 1 × 107C albicans are shown. (G) Bar graph shows pathology scores of the lung from Allo controls (dark gray bars) and Allo mice treated with indicated doses of C albicans (black bars) are shown. Data from 1 of 2 independent experiments were shown (n = 3-5 per group, means ± standard error). Original magnification ×200. Scale bars represent 100 μm (A,F). *P < .05; **P < .01.
Mn and C albicans promote accumulation of Th17 cells in the lung
Next, we characterized donor cells infiltrated in the lung on day 14 after BMT. Absolute numbers of donor CD4+ T cells, CD8+ T cells, and neutrophils were significantly increased in the lungs of allogeneic animals than those in syngeneic animals (Figure 3A). Mn administration further increased infiltration of these cells only in allogeneic animals (Figure 3A). CD4+ T cells were isolated from the lungs and expression levels of Ifng, Il4, Foxp3, and Il17a, the representative cytokines and transcription factor associated with Th1, Th2, Treg, and Th17 were evaluated, respectively. Expression levels of Ifng and Il4 were significantly greater, whereas Foxp3 expression was dramatically lower in allogeneic animals compared with those in syngeneic animals (Figure 3B). Although expression of Il17a was not detected in allogeneic animals, administration of Mn significantly enhanced Il17a expression, but not Ifng, Il4, or Foxp3 (Figure 3B). Expression levels of Rorc and Tgfb3 were also significantly enhanced in Mn-treated allogeneic animals, further confirming Th17 differentiation (Figure 3B). These Th17-related changes were not observed in Mn-treated syngeneic animals. Intracellular cytokine staining demonstrated that Mn injection increased frequencies and absolute numbers of IL-17A+ CD4+ T cells (Figure 3C-E), but not IFN-γ+ CD4 T cells in the lung (Figure 3F-G). Furthermore, similar results were obtained when C albicans was administered after allogeneic BMT (Figure 3H-I). Altogether, fungal components of C albicans and Mn selectively promoted accumulation of Th17 cells in the lungs of allogeneic animals.
Mn administration increases infiltration of Th17 cells in the lung. BMT were performed as shown in Figure 1A. (A) Numbers of donor CD4+ T cells, CD8+ T cells, and CD11b+Ly6G+ neutrophils per lung were enumerated on day 14 after after BMT and shown as means ± SE. (n = 4-5 per group). (B) CD3+ CD4+ cells were sorted and their expression of Ifng, Il4, Foxp3, Il17a, Rorc, and Tgfb3 messenger RNA were determined by quantitative PCR method. (C-G) Mononuclear cells harvested on day 14 after BMT were stained for CD3, CD4, CD8, and intracellular IFN-γ and IL-17A. The gating strategies of staining (C), frequencies (D), and absolute numbers (E) of CD4+ IL-17A+ T cells, and frequencies (F) and absolute numbers (G) of IFN-γ+ CD4+ T cells are shown. Data of Syn controls (white bars), Mn-treated Syn (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo (black bars) from one of 5 to 6 independent experiments are shown as means ± standard error. (H,I) Allogeneic recipient mice were intraperitoneally injected with 1 × 107 of heat-killed C albicans on the day of BMT. The frequencies (H) and absolute numbers (I) of IL-17A+ CD4+ T cells in Allo controls (dark gray bars) and C albicans-treated Allo (black bars) mice are shown. Data from 1 of 2 independent experiments are shown as means ± standard error. *P < .05; **P < .01. n.d., not detected.
Mn administration increases infiltration of Th17 cells in the lung. BMT were performed as shown in Figure 1A. (A) Numbers of donor CD4+ T cells, CD8+ T cells, and CD11b+Ly6G+ neutrophils per lung were enumerated on day 14 after after BMT and shown as means ± SE. (n = 4-5 per group). (B) CD3+ CD4+ cells were sorted and their expression of Ifng, Il4, Foxp3, Il17a, Rorc, and Tgfb3 messenger RNA were determined by quantitative PCR method. (C-G) Mononuclear cells harvested on day 14 after BMT were stained for CD3, CD4, CD8, and intracellular IFN-γ and IL-17A. The gating strategies of staining (C), frequencies (D), and absolute numbers (E) of CD4+ IL-17A+ T cells, and frequencies (F) and absolute numbers (G) of IFN-γ+ CD4+ T cells are shown. Data of Syn controls (white bars), Mn-treated Syn (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo (black bars) from one of 5 to 6 independent experiments are shown as means ± standard error. (H,I) Allogeneic recipient mice were intraperitoneally injected with 1 × 107 of heat-killed C albicans on the day of BMT. The frequencies (H) and absolute numbers (I) of IL-17A+ CD4+ T cells in Allo controls (dark gray bars) and C albicans-treated Allo (black bars) mice are shown. Data from 1 of 2 independent experiments are shown as means ± standard error. *P < .05; **P < .01. n.d., not detected.
Mn polarizes donor T cells toward Th17 via Dectin-2 signaling in macrophages
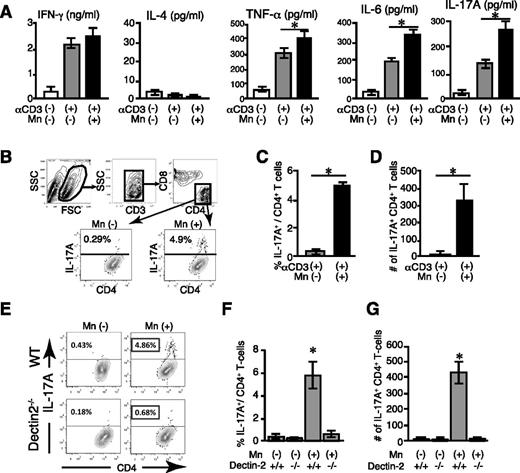
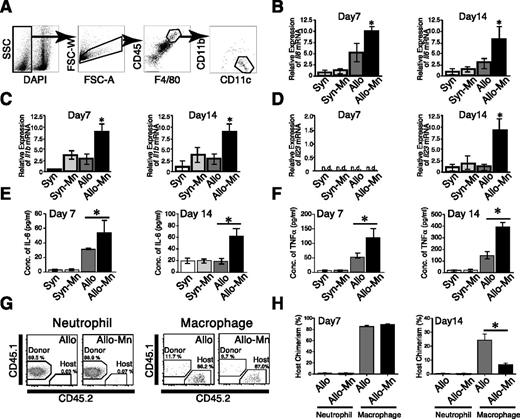
To further investigate the underlying mechanisms of the deteriorating effects of Mn on lung GVHD, we evaluated effects of Mn on T-cell responses in vitro. Given the abundant and specific expression of Dectin-2 (Clec4n) in macrophages (supplemental Figure 2),21 CD4+ CD25− T cells were cultured with peritoneal macrophages in the presence of anti-CD3 mAb and TGF-β for 5 days. Addition of Mn into the culture significantly increased the levels of IL-6, IL-17, and tumor necrosis factor (TNF)-α in the supernatants without changing IFN-γ and IL-4 levels (Figure 4A). Intracellular cytokine staining showed that Mn dramatically increased frequency and number of IL-17 producing CD4+ T cells, confirming Mn-induced Th17 differentiation (Figure 4B-D). This Th17 differentiation was abrogated when macrophages lacked Dectin-2, indicating the critical role of Dectin-2 on macrophages for Mn-induced donor Th17-differentiation (Figure 4E-G). To further examine the role of macrophages in Th17 differentiation in vivo, the cytokine expressions of macrophages isolated from the recipients on days 7 and 14 posttransplant were analyzed. Lung macrophages were sorted according to their expression of CD11b, CD11c, CD45, and F4/80 (Figure 5A),22 and their expression levels of Th17-driving cytokines, such as Il6, Il1b, and Il23 were examined by a quantitative PCR method. Expression of Il6 and Il1b was significantly upregulated in Mn-treated allogeneic animals compared with allogeneic controls by day 7 after BMT, whereas expression of Il23 were not detected on day 7 but upregulated on day 14 (Figure 5B-D). This sequential upregulation of cytokine expression reflects roles of IL-6 and IL-1β in the early phase of Th17 differentiation and role of IL-23 in later phase of Th17-induced pathology.23 The increased productions of Th17-related cytokines were further confirmed in the lung protein extracts; IL-6 and TNF-α were significantly increased in the lungs of allogeneic controls compared with those of syngeneic controls and Mn treatment further increased these cytokine production in allogeneic recipients, but not in syngeneic recipients (Figure 5 E-F). Given the critical role of host Dectin-2 in Mn-induced exacerbation of GVHD (Figure 1E), host macrophages should persist after allogeneic BMT to prime donor Th17 cells. We studied kinetics of macrophage turnover by transplanting CD45.1+ B6 cells into CD45.2+ B6D2F1 recipients. As expected, most of lung macrophages are host-derived (CD45.1−CD45.2+) on day 7 after BMT irrespective of Mn-treatment, whereas neutrophils and monocytes were completely converted to donor type (CD45.1+CD45.2−) at this time point (Figure 5G-H and data not shown). Host macrophages still persisted 14 days after allogeneic BMT, but were significantly less in Mn-treated animals compared with controls, likely reflecting exacerbated GVHD in Mn-treated recipients (24.3 ± 4.4% vs 6.7 ± 1.1%) (Figure 5H). These data, together with the critical role of Dectin-2 on macrophages (Figure 4E-G), show that Mn administration to allogeneic recipients promotes donor Th17 differentiation by inducing the productions of Th17-related cytokines, such as IL-6, IL-1β, TNF-α, and IL-23, from macrophages via Dectin-2 dependent manner.
Mn induces Th17 differentiation of donor T cells in a Dectin-2–dependent manner. (A-G) The 5 × 104 T cells were cultured with 1 × 105 12 Gy-irradiated macrophages in the presence of 0.5 μg/mL of CD3 monoconal antibodies and 10 ng/mL of TGF-β with or without 100 μg/mL of Mn for 5 days. (A) Levels of cytokines in supernatant from 1 of 3 independent experiments are shown as means ± standard error.(SE) (B) Representative dot plots of flow cytometric analysis of intracellular IL-17A staining of CD4+ T cells after 5-day culture are shown. Frequencies (C) and numbers (D) of IL-17A+CD4+ Th17 cells from 1 of 3 independent experiments are shown as means ± SE. (E-G) Intracellular IL-17A staining of T cells cultured with WT or Dectin-2−/− macrophages were performed. Representative dot plots (E) shows IL-17A production in CD4+ T cells after coculture with WT (top panels) or Dectin-2−/− (bottom panels) macrophages in the presence or absence of Mn. Frequencies (F) and numbers (G) of IL-17A+CD4+ Th17 cells from 1 of 3 independent experiments are shown as means ± SE. *P < .05.
Mn induces Th17 differentiation of donor T cells in a Dectin-2–dependent manner. (A-G) The 5 × 104 T cells were cultured with 1 × 105 12 Gy-irradiated macrophages in the presence of 0.5 μg/mL of CD3 monoconal antibodies and 10 ng/mL of TGF-β with or without 100 μg/mL of Mn for 5 days. (A) Levels of cytokines in supernatant from 1 of 3 independent experiments are shown as means ± standard error.(SE) (B) Representative dot plots of flow cytometric analysis of intracellular IL-17A staining of CD4+ T cells after 5-day culture are shown. Frequencies (C) and numbers (D) of IL-17A+CD4+ Th17 cells from 1 of 3 independent experiments are shown as means ± SE. (E-G) Intracellular IL-17A staining of T cells cultured with WT or Dectin-2−/− macrophages were performed. Representative dot plots (E) shows IL-17A production in CD4+ T cells after coculture with WT (top panels) or Dectin-2−/− (bottom panels) macrophages in the presence or absence of Mn. Frequencies (F) and numbers (G) of IL-17A+CD4+ Th17 cells from 1 of 3 independent experiments are shown as means ± SE. *P < .05.
Mn stimulates macrophage expression of cytokines related to Th17 differentiation. Lung macrophages were sorted as DAPI− F4/80+ CD45high CD11blow CD11chigh cells on days 7 and 14 after BMT. Sorting strategies (A) for lung macrophages are shown. Expressions of Il6 (B), Il1b (C), and Il23 (D) messenger RNA in macrophages sorted from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) were quantitated by real-time PCR method. Representative data from 3 independent experiments are shown as means ± standard error. (E-F) Levels of IL-6 and TNF-α in the protein extracts from recipients’ lungs on days 7 and 14 are shown as means ± standard error. (G-H) B6D2F1 mice were transplanted from B6-CD45.1+ mice. The donor (CD45.1+CD45.2−) and host (CD45.1−CD45.2+) neutrophils and macrophages from recipients’ lungs were analyzed on days 7 (G,H) and 14 (H), and representative dot plots (G) and host chimerism (H) are shown. (n = 3-5 per group). *P < .05. n.d., not detected.
Mn stimulates macrophage expression of cytokines related to Th17 differentiation. Lung macrophages were sorted as DAPI− F4/80+ CD45high CD11blow CD11chigh cells on days 7 and 14 after BMT. Sorting strategies (A) for lung macrophages are shown. Expressions of Il6 (B), Il1b (C), and Il23 (D) messenger RNA in macrophages sorted from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) were quantitated by real-time PCR method. Representative data from 3 independent experiments are shown as means ± standard error. (E-F) Levels of IL-6 and TNF-α in the protein extracts from recipients’ lungs on days 7 and 14 are shown as means ± standard error. (G-H) B6D2F1 mice were transplanted from B6-CD45.1+ mice. The donor (CD45.1+CD45.2−) and host (CD45.1−CD45.2+) neutrophils and macrophages from recipients’ lungs were analyzed on days 7 (G,H) and 14 (H), and representative dot plots (G) and host chimerism (H) are shown. (n = 3-5 per group). *P < .05. n.d., not detected.
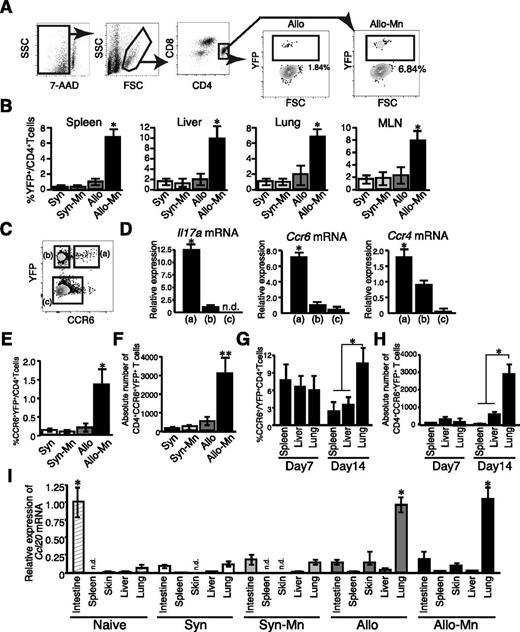
Mn administration promotes differentiation of the unique IL-17 producing CCR6+CCR4+ donor Th17 cells after allogeneic BMT
To further study the mechanisms by which Mn promoted the accumulations of donor Th17 cells in the lung, IL-17A−YFP-fate mapping mice, in which IL-17A producing cells and their progenies were labeled with YFP, were used as BMT donors.12 Mn treatment significantly expanded YFP+ CD4+ cells in the spleens, livers, lungs, and mesenteric lymph nodes 7 days after BMT, indicating that Mn promoted systemic Th17 differentiation early after allogeneic BMT (Figure 6A-B). Next, donor CD4+ T cells isolated from the lung on day 14 were divided into 3 populations according their expression of YFP and CCR6: YFP+ CCR6+ cells, YFP+ CCR6– cells, and YFP− cells, as previously described (Figure 6C).12 These subpopulations were sorted and messenger RNA was extracted from each population. YFP+ CCR6+ cells displayed significantly higher expression of Il17a, Ccr4, and Ccr6 compared with other populations (Figure 6D). This population is a mouse counterpart of human Th17 memory cells that produce a large amount of IL-17.24,25 Frequencies and absolute numbers of the YFP+CCR6+ subset in the lungs of Mn-treated allogeneic recipients were significantly increased, whereas this population was hardly detected in syngeneic or allogeneic controls 14 days after BMT (Figure 6E-F). Interestingly, this subset uniformly distributed in the spleen, liver, and lung of Mn-treated allogeneic recipients 7 days after BMT, but preferentially accumulated in the lung 14 days after BMT (Figure 6G-H). Indeed, Mn treatment dramatically increased the absolute numbers of CCR6+YFP+ cells in the lungs from allogeneic animals on day 14 compared with those on day 7 (Figure 6H). In contrast, the numbers of these cells in the spleen and liver were unchanged between 7 days and 14 days after BMT. The temporal change of the distribution of CCR6+YFP+ donor T cells, which also express a high amount of CCR4, prompted us to examine the expression of corresponding chemokines CCL20, CCL17, and CCL22 in GVHD–target organs. In naïve mice, CCL20, the ligand for CCR6, are highly expressed in the small intestine, whereas its expression was downregulated 14 days after both syngeneic and allogeneic BMT (Figure 6I). In sharp contrast, CCL20 expression was dramatically increased in the lungs after allogeneic BMT, irrespective of Mn treatment. Similarly, expression levels of CCL17 and CCL22, both ligands for CCR4, were high in the lung of allogeneic recipients, irrespective of the Mn treatment (supplemental Figure 3A-B). These results suggest that the lungs are vulnerable to Th17 infiltration in GVHD after allogeneic BMT.
Mn administration promotes differentiation of the unique CCR6+CCR4+ donor Th17 cells after allogeneic BMT. Lethally irradiated B6 or B6D2F1 mice were transplanted with 4 × 106 TCD-BM plus 4 × 106 T cells from B6-background IL-17A−YFP fate mapping mice. Representative figure (A) of flow cytometric analysis 7 days after BMT is shown. The frequency (B) of YFP+ cells in CD4+ T-cell fraction in the spleen, lung, liver, and mesenteric lymph nodes (MLNs) from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) are shown as means ± standard error (SE) (n = 3 per group). (C) Representative dot plot shows 3 distinctive cell populations defined as YFP+CCR6+ (a), YFP+CCR6− (b), and YFP−CCR6− (c), respectively. (D) Messenger RNA expression in each cell fraction from Mn-treated allogeneic mice are shown as means ± SE. Frequencies (E) and numbers (F) of YFP+CCR6+ in the lung tissue 14 days after BMT are shown as means ± SE. Frequencies (G) and absolute numbers (H) of CCR6+ CD4+ YFP+ cells from the spleen, liver, and lung from Mn-treated allogeneic recipients on day 7 and day 14 after BMT are shown as means ± SE (n = 3). (I) Expression levels of Ccl20 messenger RNA in different tissues 14 days after BMT are shown. Representative data from 3 independent experiments (means ± SE) are shown. *P < .05; **P < .01.
Mn administration promotes differentiation of the unique CCR6+CCR4+ donor Th17 cells after allogeneic BMT. Lethally irradiated B6 or B6D2F1 mice were transplanted with 4 × 106 TCD-BM plus 4 × 106 T cells from B6-background IL-17A−YFP fate mapping mice. Representative figure (A) of flow cytometric analysis 7 days after BMT is shown. The frequency (B) of YFP+ cells in CD4+ T-cell fraction in the spleen, lung, liver, and mesenteric lymph nodes (MLNs) from Syn controls (white bars), Mn-treated Syn mice (light gray bars), Allo controls (dark gray bars), and Mn-treated Allo mice (black bars) are shown as means ± standard error (SE) (n = 3 per group). (C) Representative dot plot shows 3 distinctive cell populations defined as YFP+CCR6+ (a), YFP+CCR6− (b), and YFP−CCR6− (c), respectively. (D) Messenger RNA expression in each cell fraction from Mn-treated allogeneic mice are shown as means ± SE. Frequencies (E) and numbers (F) of YFP+CCR6+ in the lung tissue 14 days after BMT are shown as means ± SE. Frequencies (G) and absolute numbers (H) of CCR6+ CD4+ YFP+ cells from the spleen, liver, and lung from Mn-treated allogeneic recipients on day 7 and day 14 after BMT are shown as means ± SE (n = 3). (I) Expression levels of Ccl20 messenger RNA in different tissues 14 days after BMT are shown. Representative data from 3 independent experiments (means ± SE) are shown. *P < .05; **P < .01.
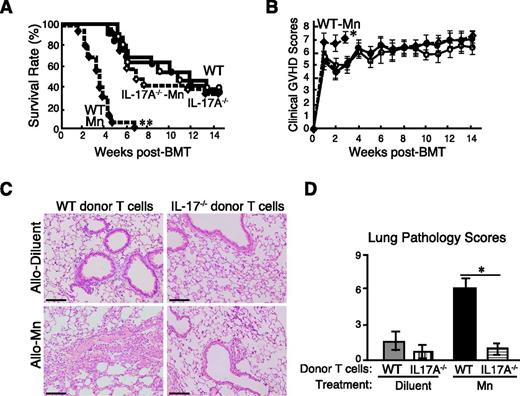
IL-17 production by donor T cells is critical for the development of lung injury in Mn-treated allogeneic recipients
Because Mn treatment specifically induced the differentiation and the pulmonary accumulation of IL-17–producing CCR6+ Th17 cells, we hypothesize that IL-17 itself plays a critical role in Mn-induced exacerbation of lung GVHD. To investigate the role of IL-17 produced by donor T cells, lethally irradiated recipient mice were transplanted with WT TCD-BM cells plus purified WT or IL-17A−/− T cells. IL-17A−/− T cells and WT T cells caused equivalently severe GVHD without Mn treatment (Figure 7A-B). However, the Mn-induced exacerbation of GVHD was completely abrogated in the recipients of IL-17A−/− T cells (Figure 7A-B). In contrast, the recipients of WT T cells plus either WT or IL-17A−/− TCD-BM, treated with Mn, presented comparable exacerbation of GVHD, further confirming that IL-17A production from donor T cells but not from other transplanted cells is critical for Mn-induced exacerbation of GVHD (data not shown). Histopathological examinations of the lungs on day 21 showed Mn-treatment in allogeneic recipients of IL-17A deficient T cells failed to show any enhancement of lung GVHD pathology compared with allogeneic controls, whereas Mn again showed drastic exacerbation of lung GVHD in the recipients of WT T cells, confirming the critical role of IL-17A production from donor T cells for the deteriorating effects of Mn on lung GVHD pathology (Figure 7C-D).
IL-17A production from donor T cells is required for the Mn-induced lung GVHD exacerbation. Lethally irradiated B6D2F1 mice were transplanted with 4 × 106 TCD-BM plus 4 × 106 T cells from WT or IL-17A−/− B6 mice. Survival (A) and clinical GVHD scores (B, means ± standard error) in WT controls (closed circles), IL-17A−/− controls (open circles), Mn-treated mice with WT donors (closed diamonds), and Mn-treated mice with IL-17A−/− donors (open diamonds) from 3 independent experiments were combined and shown (n = 12-15 per group). Representative lung sections were stained with hematoxylin and eosin (C) and the pathology scores of the lungs were analyzed 3 weeks after BMT from: (D) Allo controls with WT donors (gray bar), Allo controls with IL-17A−/− donors (vertical-striped bar), Mn-treated Allo with WT donors (black bar), and Mn-treated Allo with IL-17A−/− donors (horizontal-striped bar). Data from 3 independent experiments were combined and shown as means ± standard error. Original magnification ×200. Scale bars represent 100 μm (D). *P < .05; **P < .01.
IL-17A production from donor T cells is required for the Mn-induced lung GVHD exacerbation. Lethally irradiated B6D2F1 mice were transplanted with 4 × 106 TCD-BM plus 4 × 106 T cells from WT or IL-17A−/− B6 mice. Survival (A) and clinical GVHD scores (B, means ± standard error) in WT controls (closed circles), IL-17A−/− controls (open circles), Mn-treated mice with WT donors (closed diamonds), and Mn-treated mice with IL-17A−/− donors (open diamonds) from 3 independent experiments were combined and shown (n = 12-15 per group). Representative lung sections were stained with hematoxylin and eosin (C) and the pathology scores of the lungs were analyzed 3 weeks after BMT from: (D) Allo controls with WT donors (gray bar), Allo controls with IL-17A−/− donors (vertical-striped bar), Mn-treated Allo with WT donors (black bar), and Mn-treated Allo with IL-17A−/− donors (horizontal-striped bar). Data from 3 independent experiments were combined and shown as means ± standard error. Original magnification ×200. Scale bars represent 100 μm (D). *P < .05; **P < .01.
Discussion
It has been well-demonstrated that bacterial and viral infections are risks for GVHD by activating innate immunity. Experimental and clinical studies suggested that GVHD is reduced in germ-free mice or by the administration of oral antibiotics,1,2,13,26 and pattern recognition receptor polymorphisms are also risk factors for GVHD.27 Fungi, such as Candida, Saccharomyces, Aspergillus, and Penicillium, consist of normal flora in the oropharyngeal cavity, gastrointestinal tract, and vagina of humans.28 They are capable of causing opportunistic infection after disruption of the mucocutaneous barrier and a defect in host cellular immunity. Several clinical studies suggested a link between fungal infection and GVHD. Prolonged antifungal prophylaxis resulted in a reduction of severe acute GVHD and colonization with Candida species was associated to increased risk of acute GVHD.4,29 Interestingly, the polymorphism of Dectin-1, an innate receptor for fungi, was associated to colonization of C species in the human recipients and may increase the risk of acute GVHD.29-31 Although these data indicate a critical role of the fungal infection in the pathogenesis of GVHD, the mechanistic association between fungal infection and GVHD has yet to be clarified.
In vitro analysis showed that Mn polarized T cells toward Th17 in a Dectin-2 dependent manner, as also previously shown.10,11 Dectin-2 is expressed most abundantly in macrophages and at lesser levels in some dendritic cell subsets. In accordance with this high expression of Dectin-2, Mn acts on macrophages to enhance expression of Th17-related cytokines and mediates Th17 differentiation in vitro. Mn treatment induces neither expressions of IL-6 nor IL-23, and much less the level of IL-1β expression in syngeneic recipients, as compared with those in allogeneic recipients, suggesting that macrophages need to be primed by allogeneic T-cell responses, but not by irradiation, to secrete Th17-related cytokines in response to Mn, as allogeneic T-cell derived IFN-γ primes macrophages to enhance the responses to TLR ligands.32,33 However, it remains to be investigated if fungal infection could induce lung GVHD in BMT models after chemotherapy conditioning.
Macrophages activated by both donor T cells and Mn administration expressed high levels of IL-1β and IL-6, as early as day 7, whereas IL-23 production was delayed by day 14. This kinetics of cytokine expression fit the cytokine requirement for the differentiation and maintenance of pathogenic Th17 cells; IL-1β and IL-6 are critical for initial Th17 differentiation, and IL-23 is required for maintenance and induction of more pathogenic Th17 cells that are marked by TGF-β3 production.34-36 Previous reports show that early stage of Th17 cells in turn promote IL-23 secretion of macrophages.37
Our results showed that host Dectin-2 is critical for Mn-mediated donor Th17 differentiation and GVHD exaggeration. Dectin-1 also induces Th17 responses. Although the defense against C albicans is dependent on Dectin-2 signaling,11 Dectin-1 seems to be more responsible for the defense against Aspergillus and certain strains of C albicans.38,39 Thus, it is possible that not only Candida, but also Aspergillus may exaggerate GVHD. Furthermore both Dectin-1 and Dectin-2 are known to activate Nlrp3 inflammasome.40,41 Recently, host NLRP3 is shown to be critical for GVHD maximization through caspase-1 cleavage and productions of inflammatory cytokines.42 It is intriguing to study the role of host inflammasome in fungus-related lung GVHD by analyzing caspase-1 cleavage in the host macrophage in future studies. These findings altogether suggest more broad interaction between GVHD and various fungal infections may exist.
It should be noted that Mn-induced GVHD exacerbation was most prominent in the lungs, although we found that Mn administration exaggerated systemic acute GVHD to a much less extent. Mn-induced lung pathology was characterized by infiltrations of donor T cells surrounding vessels and bronchioles together with parenchymal injury and consistent with acute pulmonary GVHD.14,19,20 Lung acute GVHD typically developed later than gut GVHD in mice models.14 Mn-treatment systemically induced the differentiation of CCR6+ CCR4+ IL-17A producing Th17 cells in allogeneic mice, whereas this population was barely detected in syngeneic recipients regardless of Mn-treatment or in allogeneic control mice. The expressions of CCL17, CCL20, and CCL22, corresponding ligands to CCR6 and CCR4, were dramatically and specifically increased in the lungs of allogeneic recipients, irrespective of Mn-treatment by day 14 after BMT. The lung-specific upregulations of these chemokines resulted in the accumulation of CCR6+ CCR4+ IL17-producing donor T cells, the population induced only in Mn-treated allogeneic animals, in the lung. These results indicate GVHD-mediated inflammatory environment in the lung may be responsible for accumulation of Th17 cells, predominantly in the lung during GVHD after allogeneic BMT. This also has a clinically important implication; fungal infection in the tissues (outside of the lung) also induces lung-specific GVHD exaggeration via Mn-dependent systemic expansion of Th17 cells and induction of Th17-attracting chemokines in the lungs during GVHD.
Emerging evidence indicates the important roles of donor Th17 cells in GVHD, particularly in the skin and lung GVHD,43 although there are some controversies.44-47 These experiments produced conflicting results, possible due to the difference in model systems. We did not find any difference of morbidity and mortality after transplantation with IL-17A–deficient donor T cells and WT donor T cells. However, we found that Mn dramatically potentiate role of Th17 cells particularly in the lung. The role of Th17 cells in the lung GVHD was also demonstrated by transplanting with ex vivo generated Th17 cells.19 Given the fact that Th17 cells have several functions other than IL-17A secretion, such as secretion of IL-21, IL-22, TNF-α, and granulocyte macrophage–CSF, it is remarkable that IL-17A production from donor T cells alone was solely critical for Mn-induced GVHD exacerbation. We did not inquire about the mechanism of tissue damages caused by IL-17A, enhancement of antigen-specific cytotoxic T-cell development, and myeloid inflammatory cell recruitment that may contribute to the enhancement of GVHD.48,49 Indeed, we found that significantly increased numbers of donor neutrophils infiltrated into the lungs of Mn-treated allogeneic animals compared with controls. The critical role of neutrophils in the lung injury by producing proteases and reactive oxygen species is well described.50 More importantly, recipients’ neutrophils infiltrating in the gut early after transplantation contribute to GVHD and the depletion of host neutrophils early after BMT could ameliorate GVHD.51 Thus, it is it is possible that neutrophils also play a role in GVHD acceleration. Based on our results indicating that IL-17A–deficiency abrogates Mn-induced exacerbation of GVHD, IL-17 inhibitors can be beneficial to prevent lung GVHD after fungal infection in clinical allogeneic HSCT.52
Our findings uncover previously unrecognized mechanistic links between fungal infection and GVHD, and this may afford new insights to establish novel therapeutic strategies that can be used to prevent and treat Th17-driven GVHD. To the best our knowledge, this is the first study to demonstrate distinct mechanistic association between in vivo–induced Th17 by fungal derivative and GVHD. This may lead to improve the clinical outcome of allogeneic BMT.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Immunological Gene Project (ImmGen) for the data set of Clec4n gene expression in immune cells.
This work was supported by grants from the Grants-in-Aid for Scientific Research, Japan Society for the Promotion of Science, KAKENHI (26461438) (D.H.) and (25293217) (T.T.), the Promotion and Standardization of the Tenure-Track System (D.H.), the Health and Labor Science Research grants (T.T.), the Uehara Memorial Foundation (T.T.), and the North Japan Hematology Study Group.
Authorship
Contribution: H.U., D.H., and T.T. developed conceptual framework of study, designed experiments, conducted studies, analyzed data, and wrote the paper; K.K., E.H., S.M., S.T., R.O., H.I., T.M., and S.S. conducted experiments; and Y.M., Y.I., G.R.H., and K.A. supervised experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takanori Teshima, Department of Hematology, Hokkaido University Graduate School of Medicine, N15 W7, Kita-Ku, Sapporo 060-8638, Japan; e-mail: teshima@med.hokudai.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal