To the editor:
Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs), including polycythemia vera, essential thrombocythemia (ET), and primary myelofibrosis, are clonal hematopoietic stem cell disorders frequently associated with a somatic JAK2V617F mutation.1 The gain-of-function JAK2V617F mutation is associated with ligand-independent activation of cytokine signaling pathways. Tyrosine phosphorylation and activation of signal transducer and activator of transcription 5 (STAT5) and STAT3 appear to play a central role in MPN pathogenesis.2
Two independent studies reported recurrent mutations of the CALR gene in the majority of JAK2 wild-type ET and primary myelofibrosis patients.3,4 JAK2 and CALR mutations are often mutually exclusive, suggesting that the latter may also activate cytokine signaling. Indeed, expression of mutant CALR in Ba/F3 cells was reported to result in cytokine-independent proliferation with increased phospho-STAT5 expression and sensitivity to JAK2 inhibition.4 However, these data contrast with the observation that MARIMO cells, derived from an ET patient harboring an endogenous CALR mutation, contain very low levels of tyrosine-phosphorylated and unphosphorylated JAK2 and STAT5, and are insensitive to JAK2 inhibition.5
Rampal et al subsequently reported in this journal that a gene expression signature of JAK2 activation is shared by peripheral blood granulocytes from JAK2-mutant and CALR-mutant MPN patients.6 Microarray gene expression data for 93 MPN patients and 11 age-matched controls were described, providing a valuable resource for the MPN field.6 Gene set enrichment analysis (GSEA) was used to propose that similar JAK-STAT transcriptional profiles were induced in JAK2-mutant and CALR-mutant patients.6
GSEA is a widely used tool for analyzing microarray data. However, one drawback is that it relies on predefined gene sets often produced from published information or collected from other databases.7 The availability of biologically relevant data sets for GSEA is therefore a limitation to an otherwise powerful analytical tool. Rampal et al used RNA interference–mediated knockdown of JAK2 in the JAK2 homozygous mutant human erythroleukemia cell line to create a JAK2V617F short hairpin RNA knockdown signature, which was enriched to a similar extent in JAK2-mutant and CALR-mutant patients.6 Their conclusion that both groups of patients also shared a STAT signature was based on the use of a previously published data set of genes differentially expressed between in vitro–differentiated wild-type mouse embryonic stem cells and embryonic stem cells overexpressing STAT5A protein.8 Constitutively active STAT5A in this system is expressed from day 0 and therefore many of the expression differences identified by microarray analysis of day 5 cells may be due to indirect effects.8
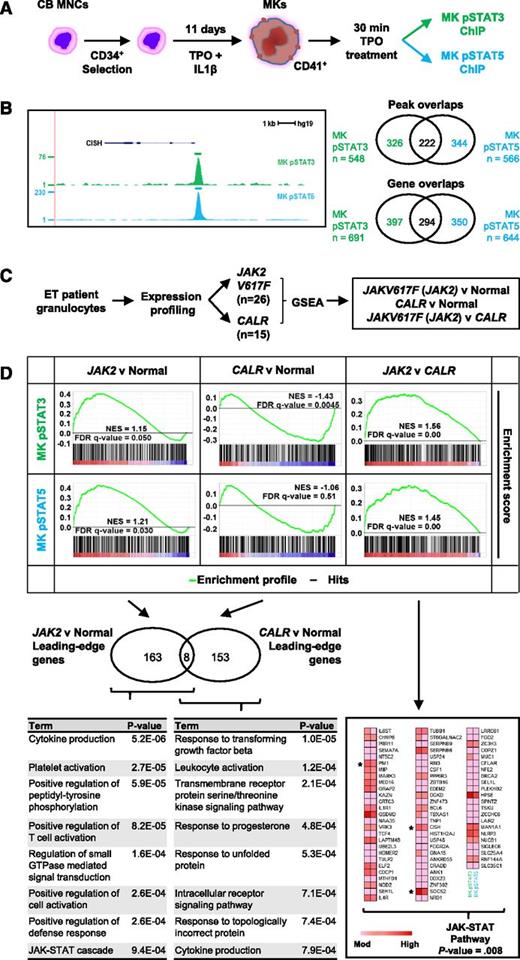
Recently, we have identified direct STAT targets that are activated in primary in vitro–cultured human megakaryocytes by performing genome-wide chromatin immunoprecipitation sequencing (ChIP-seq) analysis for phosphorylated STAT3 and STAT5 following thrombopoietin (TPO) stimulation (Figure 1A-B; unpublished data). This created an opportunity to use these gene sets, derived from a cell type central to MPN pathogenesis, to reevaluate the gene expression data presented by Rampal and colleagues (Figure 1C-D).
Megakaryocytic STAT targets are enriched in granulocytes from ET JAK2-mutant patients but not ET CALR-mutant patients. (A) Schematic of megakaryocyte (MK) cells derived from cord blood (CB) mononuclear cells (MNCs). After an 11-day culture in stem cell growth media (SCGM, Cell Genix) supplemented with 100 ng/mL TPO (Cell Genix) and 10 ng/mL interleukin-1β (IL-1β, Miltenyi), mature MK cells were treated with 50 ng/mL TPO for 30 minutes and used for ChIP with pSTAT3 and pSTAT5 antibodies (Cell Signaling Technologies). (B) Example of a density plot transformed from raw ChIP-seq data reads, displayed in the UCSC Genome Browser. CISH gene structure is shown above the tracks. Venn diagrams depict peak and gene overlaps from ChIP-seq data. Genomic coordinates of STAT-bound peaks were converted to gene lists using UCSC as the gene source. (For a complete list of STAT-bound peaks and genes, see supplementary Tables 1 and 2). (C) ET patient granulocytes were genotyped and expression-profiled by Rampal et al6 ; samples that were independently positive for JAK2V617F and CALR were processed in GSEA7 with pSTAT3 and pSTAT5 ChIP-Seq data sets from TPO-treated MK cells. (D) GSEA results showing activated STAT signatures enriched in ET patients with JAK2V617F (JAK2) mutation relative to both normal and ET patients positive for CALR mutation (CALR). All 3 GSEA comparisons (JAK2 vs normal, CALR vs normal and JAK2 vs CALR) were evaluated with leading-edge analysis (see supplementary Table 3) to determine which genes contributed to the normalized enrichment score (NES).7 Leading-edge genes are core-enriched genes that are ordered in a ranked gene list (as depicted as black and white bars below a GSEA profile) and appear at, or before, an automatically generated enrichment score threshold.7 Gene ontology analysis was performed for leading-edge genes using Enrichr9 and the Functional Annotation tool of the Database for Annotation, Visualization and Integrated Discovery10 , version 6.7 (david.abcc.ncifcrf.gov). Nonredundant gene ontology biological process terms with P values < .001 are shown below a Venn diagram of leading-edge genes enriched in JAK2-mutant patients compared with controls, and CALR-mutant patients compared with controls (for full gene ontology biological process table of terms, see supplementary Tables 4-6). For GSEA comparing JAK2 against CALR patients, the 76 leading-edge genes shared by both pSTAT ChIP-seq datasets in MK cells are also shown as a heat map indicating relative expression. Gene expression is denoted pink to red, indicating moderate (mod) to high expression. Gene ontology analysis of these 76 shared leading-edge genes was performed and the main Kyoto Encyclopedia of Genes and Genomes pathway is depicted. *Key canonical JAK-STAT target genes. All P values shown were evaluated by the modified Fisher’s exact test. FDR, false discovery rate; GTPase, guanosine triphosphatase. All supplementary tables are freely available at http://hscl.cimr.cam.ac.uk/genomic_supplementary.html.
Megakaryocytic STAT targets are enriched in granulocytes from ET JAK2-mutant patients but not ET CALR-mutant patients. (A) Schematic of megakaryocyte (MK) cells derived from cord blood (CB) mononuclear cells (MNCs). After an 11-day culture in stem cell growth media (SCGM, Cell Genix) supplemented with 100 ng/mL TPO (Cell Genix) and 10 ng/mL interleukin-1β (IL-1β, Miltenyi), mature MK cells were treated with 50 ng/mL TPO for 30 minutes and used for ChIP with pSTAT3 and pSTAT5 antibodies (Cell Signaling Technologies). (B) Example of a density plot transformed from raw ChIP-seq data reads, displayed in the UCSC Genome Browser. CISH gene structure is shown above the tracks. Venn diagrams depict peak and gene overlaps from ChIP-seq data. Genomic coordinates of STAT-bound peaks were converted to gene lists using UCSC as the gene source. (For a complete list of STAT-bound peaks and genes, see supplementary Tables 1 and 2). (C) ET patient granulocytes were genotyped and expression-profiled by Rampal et al6 ; samples that were independently positive for JAK2V617F and CALR were processed in GSEA7 with pSTAT3 and pSTAT5 ChIP-Seq data sets from TPO-treated MK cells. (D) GSEA results showing activated STAT signatures enriched in ET patients with JAK2V617F (JAK2) mutation relative to both normal and ET patients positive for CALR mutation (CALR). All 3 GSEA comparisons (JAK2 vs normal, CALR vs normal and JAK2 vs CALR) were evaluated with leading-edge analysis (see supplementary Table 3) to determine which genes contributed to the normalized enrichment score (NES).7 Leading-edge genes are core-enriched genes that are ordered in a ranked gene list (as depicted as black and white bars below a GSEA profile) and appear at, or before, an automatically generated enrichment score threshold.7 Gene ontology analysis was performed for leading-edge genes using Enrichr9 and the Functional Annotation tool of the Database for Annotation, Visualization and Integrated Discovery10 , version 6.7 (david.abcc.ncifcrf.gov). Nonredundant gene ontology biological process terms with P values < .001 are shown below a Venn diagram of leading-edge genes enriched in JAK2-mutant patients compared with controls, and CALR-mutant patients compared with controls (for full gene ontology biological process table of terms, see supplementary Tables 4-6). For GSEA comparing JAK2 against CALR patients, the 76 leading-edge genes shared by both pSTAT ChIP-seq datasets in MK cells are also shown as a heat map indicating relative expression. Gene expression is denoted pink to red, indicating moderate (mod) to high expression. Gene ontology analysis of these 76 shared leading-edge genes was performed and the main Kyoto Encyclopedia of Genes and Genomes pathway is depicted. *Key canonical JAK-STAT target genes. All P values shown were evaluated by the modified Fisher’s exact test. FDR, false discovery rate; GTPase, guanosine triphosphatase. All supplementary tables are freely available at http://hscl.cimr.cam.ac.uk/genomic_supplementary.html.
Using GSEA, we see markedly different STAT activation signatures between JAK2-mutant and CALR-mutant ET patients (Figure 1D). Overlapping subsets of direct STAT1 and STAT3 targets were induced in JAK2-, but not in CALR-mutant, patients when compared with normal controls. Gene ontology analysis of induced genes revealed contrasting biological processes, with the JAK-STAT cascade enriched in JAK2-mutant patients, whereas response to unfolded protein genes was enriched in CALR-mutant patients. To corroborate that genes enriched in JAK2-mutant patients, when compared with CALR, are JAK-activated STAT targets, we performed gene ontology analysis on the leading-edge genes shared between the 2 megakaryocyte phospho-STAT datasets used in GSEA, which identified JAK-STAT signaling as the top enriched pathway and included several paradigmatic JAK-STAT targets.
GSEA is critically dependent on the availability of biologically relevant gene sets. Our results demonstrate that direct STAT3 and STAT5 target genes, identified by ChIP-seq studies of a cell type central to ET pathogenesis (megakaryocytes), are upregulated in cells from JAK2- but not CALR-mutant ET patients. Therefore, although we concur with Rampal and colleagues that a JAK2 activation signal may be present in CALR-mutant ET granulocytes, our results indicate that downstream signaling mechanisms other than STAT3 and STAT5 are likely involved in the pathogenesis of CALR-mutant MPNs. Comparative analysis of normal and ET-derived primary megakaryocytes will likely be required to fully resolve potential differences in pathologic signaling between JAK2- and CALR-mutant MPNs.
Authorship
Acknowledgments: Samples were provided by the Cambridge Blood and Stem Cell Biobank, which is supported by the Cambridge National Institute for Health Research Biomedical Research Centre and the Cambridge Experimental Cancer Medicine Centre. Approval was obtained from Cambridge University Hospitals NHS Foundation Trust for these studies. Informed consent was provided according to the Declaration of Helsinki. Work in A.R.G.’s and B.G.’s laboratories is supported by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, Leukemia and Lymphoma Research, Cancer Research UK, the Leukemia and Lymphoma Society of America, the Cambridge National Institute for Health Research Biomedical Research Centre, and the Kay Kendall Leukaemia Fund (A.R.G.). Infrastructure funding was provided by the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute.
Contribution: W.W.Y.L. performed experiments, produced the figure and wrote the manuscript. W.W.Y.L. and R.H. performed data analysis. W.W.Y.L., B.G., and A.R.G. interpreted the data. B.G. and A.R.G. edited the manuscript. All authors read and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: B. Göttgens, Cambridge Institute for Medical Research, Hills Road, Cambridge, CB2 0XY, UK; e-mail: bg200@cam.ac.uk; and A. R. Green, Cambridge Institute for Medical Research, Hills Road, Cambridge, CB2 0XY, UK; e-mail: arg1000@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal