Key Points
ST2 is independently associated with aGVHD after day 28 in cord blood transplantation recipients.
High ST2 levels predict for increased TRM in cord blood transplantation recipients.
Abstract
While cord blood transplantation (CBT) is an effective therapy for hematologic malignancies, acute graft-versus-host disease (aGVHD) is a leading cause of transplant-related mortality (TRM). We investigated if biomarkers could predict aGVHD and TRM after day 28 in CBT recipients. Day 28 samples from 113 CBT patients were analyzed. Suppressor of tumorigenicity 2 (ST2) was the only biomarker associated with grades II-IV and III-IV aGVHD and TRM. Day 180 grade III-IV aGVHD in patients with high ST2 levels was 30% (95% confidence interval [CI], 18-43) vs 13% (95% CI, 5-23) in patients with low levels (P = .024). The adverse effect of elevated ST2 was independent of HLA match. Moreover, high day 28 ST2 levels were associated with increased TRM with day 180 estimates of 23% (95% CI, 13-35) vs 5% (95% CI, 1-13) if levels were low (P = .001). GVHD was the most common cause of death in high ST2 patients. High concentrations of tumor necrosis factor receptor-1, interleukin-8, and regenerating islet-derived protein 3-α were also associated with TRM. Our results are consistent with those of adult donor allografts and warrant further prospective evaluation to facilitate future therapeutic intervention to ameliorate severe aGVHD and further improve survival after CBT.
Introduction
Unrelated donor cord blood (CB) is routinely used as an alternative hematopoietic stem cell (HSC) source for transplantation in patients with high-risk hematologic malignancies, and the use of double-unit grafts has greatly extended the application of CB transplantation (CBT) in adults.1,2 However, acute graft-versus-host disease (aGVHD) is common with an incidence of grade II-IV aGVHD of at least 50% in double-unit CBT (DCBT) recipients who received transplants with calcineurin inhibitor–based prophylaxis and no anti-thymocyte globulin.3-6 Moreover, approximately one-quarter of patients develop grade III-IV disease, and severe aGVHD is a leading source of morbidity and transplant-related mortality (TRM) after CBT.4,7
Plasma biomarkers have emerged as an important tool in the diagnosis of aGVHD after adult donor HSC transplantation. The biomarkers interleukin2 receptor α (IL2Rα), tumor necrosis factor receptor 1 (TNFR1), hepatocyte growth factor (HGF), interleukin-8 (IL-8), elafin, and regenerating islet-derived protein 3-α (REG3α) are associated with the diagnosis of aGVHD and are significantly associated with the subsequent risk of day 180 TRM in unmodified allograft recipients.8-12 Furthermore, levels of the biomarker suppressor of tumorigenicity 2 (ST2) obtained at the time of onset of aGVHD are associated with the risk of treatment-resistant aGVHD and 6-month TRM after aGVHD onset independent of aGVHD clinical grade.13
Whether GVHD biomarkers are informative in CBT recipients has not been investigated, and such biomarkers could have significant clinical utility. In a previous analysis at Memorial Sloan Kettering Cancer Center (MSKCC) of 115 recipients of DCBT, we found that the gastrointestinal (GI) tract is the organ most commonly affected in ∼80% of patients with grade II-IV aGVHD.5 Similarly, Alsultan et al also found that the gut was the predominant organ affected by aGVHD in CBT recipients.14 Accurate diagnosis of GVHD early after transplantation, however, can be complicated by preparative regimen toxicity, infection, and medication side-effects and tissue biopsy may sometimes have equivocal results after allogeneic transplantation.15-20 Biomarkers could potentially aid in early aGVHD diagnosis. Tailoring intensity of aGVHD therapy to the predicted severity of disease prior to clinical manifestations could also be greatly beneficial. Therefore, we investigated the clinical significance of day 28 peripheral blood biomarker levels in DCBT recipients who underwent transplantation at MSKCC. Our hypothesis was that elevated day 28 biomarker levels would be associated with the subsequent development of grade III-IV aGVHD.
Methods
Patients and graft characteristics
This analysis was performed on patients who received transplants at MSKCC between May 1, 2006 and May 31, 2012. All CBT recipients during this time period received double-unit grafts. Patients eligible for this analysis included all consecutive adult and pediatric recipients who achieved donor-derived neutrophil engraftment and had plasma or serum samples obtained at day 28 after DCBT. Of the 113 evaluable patients, 7 developed grade II-IV aGVHD ≤day 28 post-DCBT. These patients were excluded from aGVHD analyses but were evaluable for the TRM analysis. All patients provided written informed consent for transplantation and research specimen collection, and the analysis was approved by the MSKCC Institutional Review/Privacy Board. Research was conducted in accordance with the Declaration of Helsinki.
CB units were selected according to a 4-6/6 HLA-A, -B antigen, -DRB1 allele donor-recipient match, a cryopreserved total nucleated cell (TNC) dose of at least 1.5 × 107 per kg per unit, and the bank of origin.21 Unit-unit HLA match was not considered in CB unit selection. High-resolution HLA-A, -B, -C, -DRB1, -DQ allele typing of CB units was done routinely but usually did not influence unit selection. Units were thawed using an albumin-dextran dilution22 (n = 219) or thawed with wash (n = 7).
Conditioning regimen and GVHD prophylaxis
All patients were cared for in high-efficiency particulate air-filtered rooms and received similar supportive care. Pretransplant conditioning varied in intensity according to patient’s age, diagnosis, remission status, and comorbidities as previously described.5,7 Reduced-intensity regimens were functionally myeloablative. All patients received a calcineurin inhibitor (predominantly cyclosporine A) and mycophenolate mofetil (MMF) prophylaxis starting on day −3 IV as previously described5,7 and none received anti-thymocyte globulin (ATG).5 Granulocyte-colony-stimulating factor was given posttransplant to promote neutrophil recovery.
Peripheral blood samples
All blood samples (either serum or plasma) were prospectively collected at day 28 after DCBT and stored per institutional guidelines at MSKCC. The frozen samples were batch-shipped to the Paczesny Laboratory at the University of Indiana for analysis. A sequential enzyme-linked immunosorbent assay (ELISA) protocol was used to maximize the number of measured analytes per sample. This technique reuses the same aliquot consecutively in individual ELISA plates. Antibody pairs were purchased as follows: REG3α from MBL International (Ab-Match Assembly Human PAP1 kit and Ab-Match Universal kit), IL-8 OptEIA from Becton Dickinson/BD Biosciences, and all others from R&D Systems. Duoset kits were used for TNFR1, IL2Rα, elafin, and HGF, and quantikine kits for ST2 as they permit comparable measurements in plasma or serum. Measurement of ST2 levels by the Duoset kit as previously reported by Vander Lugt et al13 was not selected for this study because it only allows measurement of plasma samples. Samples were analyzed in duplicate as previously described.13 Laboratory investigators were blinded to all clinical information and transplant outcomes. Pipetting for the REG3α assay (384-well plate format) was performed using the EpMotion4500 liquid handling system (Eppendorf) and for other assays (96-well plate format) by multichannel. All washes were performed using the Aquamax 2000 plate washer (Molecular Devices). Absorbance was measured immediately after termination of the substrate reaction using a SpectraMax Plus plate reader (Molecular Devices), and results were calculated using SoftMax Pro, version 6.0 (Molecular Devices).
Study definitions and statistical analysis
GVHD was diagnosed clinically with histologic confirmation when appropriate. Grading was reviewed by a transplant clinician panel to reach a consensus of the maximum aGVHD grade by day 180. Acute and chronic GVHD were graded according to International Bone Marrow Transplant Registry23 and the National Institutes of Health consensus criteria,24 respectively. Relapse was defined as recurrence or progression of disease beyond the pretransplant baseline, whereas TRM was defined as death from any cause in continued remission. The primary cause of death was defined according to the algorithm of Copelan et al.25
Data on patient characteristics and transplant-related outcomes were obtained from the prospectively maintained MSKCC transplant database verified by primary source documentation. The cumulative incidence of aGVHD and TRM were estimated using cumulative incidence functions, with relapse or death in the absence of aGVHD and relapse considered competing risks for aGVHD and TRM, respectively. A landmark analysis was used for each outcome to account for the fact that selected biomarkers were evaluated at day 28 and not at the time of transplant. Patients were grouped according to being above or below the median day 28 value for each biomarker. Biomarker levels above the median are henceforth described as high whereas levels below the median were considered low. In addition, we generated receiver operating characteristic (ROC) curves to explore other ST2 thresholds as discriminators for grade III-IV aGVHD after day 28 and TRM.26 The area under the ROC curve (AUC) and true-positive and false-positive rates at select ST2 thresholds are presented. The Gray test was used to compare the incidence of aGVHD and TRM across biomarker groups. Univariate and multivariate Cox proportional hazard regression models were used to further examine the association between ST2 levels and the cause-specific hazard of aGVHD (after day 28) and TRM (all patients). An interaction term with ST2 and each patient and treatment characteristic was fit in a Cox regression model to explore potential modifiers of the ST2 association with GVHD and TRM. The concordance probability estimate (CPE) was analyzed for each multivariate model before and after the inclusion of the ST2 level to evaluate the marginal increase in predictive accuracy (concordance) after the ST2 level inclusion.27 HLA-A, -B, -DRB1 allele match of the dominant unit with the patient was used to analyze the association between HLA-match grade and aGVHD risk after day 28 as this has been shown to have a significant association with aGVHD in a prior analysis at our center.5 All analyses were performed using R 3.0.3 (R Development Core Team, 2011).
Results
Patient and graft demographics
Patient demographics are summarized in Table 1. One-hundred thirteen patients, predominantly adults (median age, 41 years) underwent transplantation. Approximately half the patients were male and cytomegalovirus (CMV) seropositive. The most common diagnosis was acute leukemia. The majority received myeloablative or reduced-intensity conditioning, and CB units were predominantly 4-5/6 HLA-A, -B antigen, -DRB1 allele matched to the recipient. Analysis of the distribution of the dominant unit-recipient HLA-A, -B, -DRB1 allele match demonstrated that 83 of 113 (73%) received better HLA-matched CB units (4-6/6 alleles) and the remainder had a very high degree of HLA mismatch when measured at high resolution.
Patient and graft demographics of 113 DCBT recipients and 226 units
| Characteristics . | Value . |
|---|---|
| Median age (range), y | 41 (1-69) |
| Male, N (%) | 61 (54) |
| Recipient CMV seropositive, N (%) | 61 (54) |
| Diagnosis, N (%) | |
| Acute leukemia | 58 (51) |
| MDS/CML/other MPD | 8 (7) |
| NHL/HL/CLL | 47 (42) |
| Conditioning, N (%) | |
| High-dose myeloablative | |
| Cy 120/Flu 75/TBI 1320-1375 cGy | 32 (28) |
| Clo 100/Mel 140/Thio 10 | 5 (4) |
| Reduced intensity | |
| Cy 50/Flu 150/Thio 10/TBI 400 cGy | 39 (35) |
| Mel 140/Flu 150 | 8 (7) |
| Nonmyeloablative | |
| Cy 50/Flu 150/TBI 200 cGy | 28 (25) |
| 6-TG, Flu 150/TBI 400 cGy | 1 (1) |
| Donor-recipient HLA-A, -B antigen, -DRB1 allele match, N (%) | |
| 6/6 | 9 (4) |
| 5/6 | 115 (51) |
| 4/6 | 102 (45) |
| Median infused TNC dose (range) | |
| Larger unit | 2.76 × 107/kg (1.46-7.19) |
| Smaller unit | 2.02 × 107/kg (1.13-4.51) |
| Median infused CD34+dose (range) | |
| Larger unit | 1.33 × 105/kg (0.41-4.51) |
| Smaller unit | 0.68 × 105/kg (0.13-2.0) |
| Characteristics . | Value . |
|---|---|
| Median age (range), y | 41 (1-69) |
| Male, N (%) | 61 (54) |
| Recipient CMV seropositive, N (%) | 61 (54) |
| Diagnosis, N (%) | |
| Acute leukemia | 58 (51) |
| MDS/CML/other MPD | 8 (7) |
| NHL/HL/CLL | 47 (42) |
| Conditioning, N (%) | |
| High-dose myeloablative | |
| Cy 120/Flu 75/TBI 1320-1375 cGy | 32 (28) |
| Clo 100/Mel 140/Thio 10 | 5 (4) |
| Reduced intensity | |
| Cy 50/Flu 150/Thio 10/TBI 400 cGy | 39 (35) |
| Mel 140/Flu 150 | 8 (7) |
| Nonmyeloablative | |
| Cy 50/Flu 150/TBI 200 cGy | 28 (25) |
| 6-TG, Flu 150/TBI 400 cGy | 1 (1) |
| Donor-recipient HLA-A, -B antigen, -DRB1 allele match, N (%) | |
| 6/6 | 9 (4) |
| 5/6 | 115 (51) |
| 4/6 | 102 (45) |
| Median infused TNC dose (range) | |
| Larger unit | 2.76 × 107/kg (1.46-7.19) |
| Smaller unit | 2.02 × 107/kg (1.13-4.51) |
| Median infused CD34+dose (range) | |
| Larger unit | 1.33 × 105/kg (0.41-4.51) |
| Smaller unit | 0.68 × 105/kg (0.13-2.0) |
6-TG, 6-thioguanine; CLL, chronic lymphocytic leukemia; Clo, clofarabine; CML, chronic myelogenous leukemia; CMV, cytomegalovirus; Cy, cyclophosphamide; Flu, fludarabine; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; Mel, melphalan; MPD, myeloproliferative disease; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; Thio, Thiotepa; TNC, total nucleated cell dose.
Day 180 aGVHD, 1-year chronic GVHD, and day 180 TRM (all patients)
Overall, 69 of the total 113 patient cohort developed grade II-IV aGVHD prior to day 180 for a day 180 cumulative incidence of 61% (95% confidence interval [CI], 51-69; median onset, 40 days; range, 17-161). Twenty-eight patients had grade III-IV disease (22 grade III, 6 grade IV) for a cumulative incidence of 25% (95% CI, 17-33) by day 180. The GI tract (either upper and/or lower tract) was the organ most commonly affected in 56 of 69 (81%) patients, followed by the skin (36 of 69, 52%), and the liver was the least commonly involved (9 of 69, 13%). Thirty-four patients had grade II gut aGVHD. Of these, 27 were treated with budesonide as sole treatment, and 23 of these patients responded to therapy with either complete or partial response by day 28 posttreatment. Of the 36 patients affected with skin aGVHD, 18 had stage 1-2, 18 had stage 3, and none had stage 4 disease. The incidence of chronic GVHD at 1 year was 14% (95% CI, 9-21).
The day 180 cumulative incidence of TRM was 14% (95% CI, 9-21). At day 180, 16 patients died of TRM (9 GVHD, 5 organ failure, 2 infection). By 2 years after transplant, the primary transplant-related causes of death included GVHD in 16, organ failure in 6, and infection in 2.
Association between day 28 ST2 levels and subsequent aGVHD risk
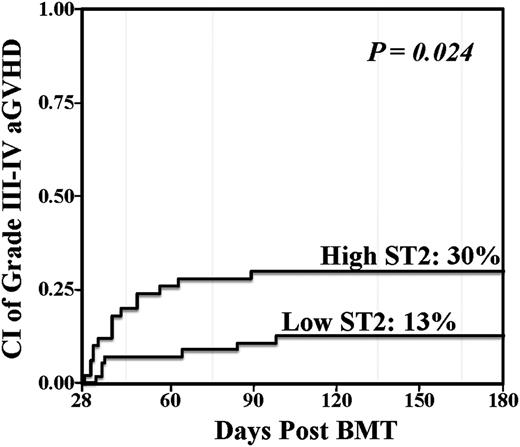
In the total group of 113 patients, the median ST2 level was 33.9 ng/mL (interquartile range [IQR], 18.3-75.5). This median value was used as the threshold to evaluate differences in patients with high vs low ST2 levels. The association between day 28 ST2 levels and aGVHD with an onset after day 28 was first evaluated in a total of 106 patients (the 7 patients whose aGVHD onset was ≤day 28 were excluded). Sixty-two in this 106 patient subset developed grade II-IV aGVHD after day 28 with a median onset of 42.5 days (range, 29-161). The cumulative incidence of day 180 grade II-IV aGVHD among patients with high ST2 levels was 66% (95% CI, 51-78) as compared with 52% (95% CI, 38-64) among patients with low ST2 levels (P = .048). Moreover, there was also a significant association between high day 28 ST2 levels and grade III-IV aGVHD when analyzed as above vs below the median (Figure 1, Table 2). The association between lower GI aGVHD involvement and ST2 levels was evaluated. High ST2 level patients had an incidence of grade II-IV aGVHD involving the lower gut at day 180 of 26% (95% CI, 15-39) whereas the incidence in low ST2 level patients was 16% (95% CI, 8-27) (P = .245).
Day 28 landmark analysis of grade III-IV aGVHD according to day 28 ST2 level. Patients who engrafted and were without grade II-IV aGVHD at day 28 were included in the analysis (n = 106). Elevated day 28 ST2 was associated with a significantly increased risk of subsequent severe aGVHD.
Day 28 landmark analysis of grade III-IV aGVHD according to day 28 ST2 level. Patients who engrafted and were without grade II-IV aGVHD at day 28 were included in the analysis (n = 106). Elevated day 28 ST2 was associated with a significantly increased risk of subsequent severe aGVHD.
Univariate analysis of factors potentially associated with grade III-IV aGVHD in 106 DCBT recipients
| Variable . | N . | Day 180 grade III-IV aGVHD, % (95% CI) . | P . |
|---|---|---|---|
| Age, y | |||
| 0-15 | 14 | 21 (5-46) | .921 |
| ≥16 | 92 | 21 (13-30) | |
| Conditioning | |||
| Myeloablative | 80 | 20 (12-29) | .764 |
| Nonmyeloablative | 26 | 23 (9-41) | |
| Infused total CD3+cell dose | |||
| ≤7.74 × 106/kg | 55 | 26 (15-38) | .170 |
| >7.74 × 106/kg | 51 | 16 (7-27) | |
| Dominant unit-recipient 6 allele HLA match | |||
| Worse HLA match: 2-3/6 | 28 | 32 (16-50) | .077 |
| Better HLA match: 4-6/6 | 78 | 17 (9-26) | |
| ST2 level, ng/mL | |||
| Low: ≤33.9 | 56 | 13 (5-23) | .024 |
| High: >33.9 | 50 | 30% (18-43) |
| Variable . | N . | Day 180 grade III-IV aGVHD, % (95% CI) . | P . |
|---|---|---|---|
| Age, y | |||
| 0-15 | 14 | 21 (5-46) | .921 |
| ≥16 | 92 | 21 (13-30) | |
| Conditioning | |||
| Myeloablative | 80 | 20 (12-29) | .764 |
| Nonmyeloablative | 26 | 23 (9-41) | |
| Infused total CD3+cell dose | |||
| ≤7.74 × 106/kg | 55 | 26 (15-38) | .170 |
| >7.74 × 106/kg | 51 | 16 (7-27) | |
| Dominant unit-recipient 6 allele HLA match | |||
| Worse HLA match: 2-3/6 | 28 | 32 (16-50) | .077 |
| Better HLA match: 4-6/6 | 78 | 17 (9-26) | |
| ST2 level, ng/mL | |||
| Low: ≤33.9 | 56 | 13 (5-23) | .024 |
| High: >33.9 | 50 | 30% (18-43) |
We evaluated the distribution of ST2 levels according to GVHD severity (grade 0-I aGVHD vs II-IV aGVHD). We found that the median ST2 levels were 27.5 ng/mL (IQR, 18.7-66.1) and 38.3 ng/mL (IQR, 17.5-67.7) in these patient groups, respectively, suggesting that the median value of 33.9 ng/mL for the entire patient cohort is an appropriate threshold for this analysis. The ROC curve for grade III-IV aGVHD was also generated to explore appropriate ST2 thresholds. The corresponding AUC for the ROC curve level was 0.59 (supplemental Figure 1A, available on the Blood Web site). The false-positive rate associated with the median ST2 level was 0.44 whereas the true-positive rate was 0.62.
We evaluated potential risk factors for grade III-IV aGVHD other than the ST2 level in the 106 aGVHD after day 28 patient cohort. We first evaluated the distribution of day 28 ST2 levels according to the conditioning regimen. Of the 106 patient cohort, 80 received myeloablative conditioning and had a median ST2 level of 34.0 ng/mL (IQR, 18.5-68.5) whereas the 26 recipients of nonmyeloablative conditioning had a median ST2 level of 26.2 ng/mL (IQR, 17.1-59.9) (P = .252). We then performed a univariate analysis to test the association between potential risk factors and day 180 grade III-IV aGVHD (Table 2). There was no association with patient age (0-15 vs ≥16 years), conditioning regimen intensity (myeloablative vs nonmyeloablative), or infused total CD3+ cell dose per kg. The dominant unit-recipient high-resolution HLA-A, -B, -DRB1 allele match (2-3/6 vs 4-6/6) showed a trend toward significance (P = .077). No patient or treatment characteristic significantly modified the ST2 association with GVHD (data not shown).
In a multivariate analysis including day 28 ST2 levels and dominant unit-recipient HLA-allele match, a high day 28 ST2 level was independently associated with an increased risk of subsequent grade III-IV aGVHD (hazard ratio, 2.62 [95% CI, 1.06-6.45], P = .036) whereas dominant unit-recipient HLA-allele match was no longer significant (Table 3). The CPE for HLA match alone was 0.57, and this increased to 0.65 when ST2 was added.
Multivariate analysis of the association between HLA match and day 28 ST2 level on grade III-IV aGVHD in 106 DCBT recipients
| Covariate . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Dominant unit-recipient 6 allele HLA match | ||
| Worse HLA match: 2-3/6 | 2.01 (0.86-4.72) | .109 |
| Better HLA match: 4-6/6 | Reference | |
| ST2 level, ng/mL | ||
| Low: ≤33.9 | Reference | .036 |
| High: >33.9 | 2.62 (1.06-6.45) |
| Covariate . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Dominant unit-recipient 6 allele HLA match | ||
| Worse HLA match: 2-3/6 | 2.01 (0.86-4.72) | .109 |
| Better HLA match: 4-6/6 | Reference | |
| ST2 level, ng/mL | ||
| Low: ≤33.9 | Reference | .036 |
| High: >33.9 | 2.62 (1.06-6.45) |
Association between day 28 ST2 levels and TRM
All 113 patients were assessed for the association between ST2 level and day 180 TRM. High day 28 ST2 levels were associated with substantially increased TRM with a cumulative incidence at day 180 post-DCBT of 23% (95% CI, 13-35) as compared with 5% (95% CI, 1-13) in the low ST2 level group (P = .001) (Table 4, Figure 2). The ROC curve had a corresponding AUC of 0.75. The false-positive and true-positive rates at the ST2 median value were 0.49 and 0.78, respectively (supplemental Figure 1B). Of the 19 of 56 patients with high day 28 ST2 levels who died of transplant-related causes, GVHD was the most common cause (12 of 19 [63%] deaths) with a median time to GVHD death of 173 days (range, 70-465). Of those, aGVHD developed in all patients and 2 had subsequent overlap syndrome (1 mild, 1 severe). Organ failure (exclusively pulmonary) was the second most common cause of death in this group (n = 6) and infection was the least common (n = 1). In contrast, during the period of the study only 5 of 57 patients with low day 28 ST2 levels died of transplant-related causes. Their primary causes of death were GVHD (n = 4) or infection (n = 1).
Univariate analysis of factors potentially associated with TRM including day 28 ST2 level in 113 DCBT recipients
| Variable . | N . | Day 180 TRM, % (95% CI) . | P . |
|---|---|---|---|
| Age, y | |||
| 0-15 | 15 | 0 (NA) | .034 |
| ≥16 | 98 | 16 (10-24) | |
| Recipient CMV serostatus | |||
| Seronegative | 52 | 2 (0-9) | .013 |
| Seropositive | 61 | 25 (15-36) | |
| Conditioning | |||
| Myeloablative | 84 | 13 (7-21) | .632 |
| Nonmyeloablative | 29 | 17 (6-33) | |
| Dominant unit TNC dose | |||
| <2.22 × 107/kg | 56 | 14 (7-25) | .654 |
| ≥2.22 × 107/kg | 57 | 14 (7-24) | |
| Dominant unit CD34+cell dose | |||
| <1 × 105/kg | 56 | 14 (7-25) | .363 |
| ≥1 × 105/kg | 57 | 14 (7-24) | |
| Dominant unit-recipient 6 allele HLA match | |||
| Worse HLA match: 2-3/6 | 30 | 20 (8-36) | .153 |
| Better HLA match: 4-6/6 | 83 | 12 (6-20) | |
| ST2 level, ng/mL | |||
| Low: ≤33.9 | 57 | 5 (1-13) | .001 |
| High: >33.9 | 56 | 23 (13-35) |
| Variable . | N . | Day 180 TRM, % (95% CI) . | P . |
|---|---|---|---|
| Age, y | |||
| 0-15 | 15 | 0 (NA) | .034 |
| ≥16 | 98 | 16 (10-24) | |
| Recipient CMV serostatus | |||
| Seronegative | 52 | 2 (0-9) | .013 |
| Seropositive | 61 | 25 (15-36) | |
| Conditioning | |||
| Myeloablative | 84 | 13 (7-21) | .632 |
| Nonmyeloablative | 29 | 17 (6-33) | |
| Dominant unit TNC dose | |||
| <2.22 × 107/kg | 56 | 14 (7-25) | .654 |
| ≥2.22 × 107/kg | 57 | 14 (7-24) | |
| Dominant unit CD34+cell dose | |||
| <1 × 105/kg | 56 | 14 (7-25) | .363 |
| ≥1 × 105/kg | 57 | 14 (7-24) | |
| Dominant unit-recipient 6 allele HLA match | |||
| Worse HLA match: 2-3/6 | 30 | 20 (8-36) | .153 |
| Better HLA match: 4-6/6 | 83 | 12 (6-20) | |
| ST2 level, ng/mL | |||
| Low: ≤33.9 | 57 | 5 (1-13) | .001 |
| High: >33.9 | 56 | 23 (13-35) |
Day 28 landmark analysis of TRM according to day 28 ST2 level in 113 DCBT recipients. DCBT recipients with elevated day 28 ST2 had a significantly increased risk of TRM.
Day 28 landmark analysis of TRM according to day 28 ST2 level in 113 DCBT recipients. DCBT recipients with elevated day 28 ST2 had a significantly increased risk of TRM.
Univariate analysis for other risk factors potentially associated with TRM showed that conditioning regimen (myeloablative vs nonmyeloablative), dominant unit TNC dose × 107/kg, dominant unit CD34+ cell dose × 105/kg, and dominant unit-recipient high-resolution HLA allele match (2-3/6 vs 4-6/6) were not significant (Table 4). In contrast, older patient age (≥16 years) and positive recipient CMV serology were associated with an increased TRM. There was no evidence that the ST2 association with TRM was modified by any patient or clinical factor (data not shown). Multivariate analysis revealed that both recipient CMV positivity (hazard ratio, 2.59 [95% CI, 1.02-6.59]) and a high day 28 ST2 level (hazard ratio, 4.21 [95% CI, 1.57-11.30]) were associated with an increased TRM risk whereas age was not (hazard ratio, 1.02 [95% CI, 1.00-1.05]) (Table 5). The CPE estimated for the multivariate model without ST2 was 0.68, and this increased to 0.74 with the inclusion of ST2.
Multivariate analysis of the association between age, recipient CMV serostatus, and ST2 level on TRM in 113 DCBT recipients
| Covariate . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Age* | 1.02 (1.00-1.05) | .096 |
| Recipient CMV serostatus | ||
| Seronegative | Reference | .046 |
| Seropositive | 2.59 (1.02-6.59) | |
| ST2 level, ng/mL | ||
| Low: ≤33.9 | Reference | .004 |
| High: >33.9 | 4.21 (1.57-11.30) |
| Covariate . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Age* | 1.02 (1.00-1.05) | .096 |
| Recipient CMV serostatus | ||
| Seronegative | Reference | .046 |
| Seropositive | 2.59 (1.02-6.59) | |
| ST2 level, ng/mL | ||
| Low: ≤33.9 | Reference | .004 |
| High: >33.9 | 4.21 (1.57-11.30) |
Continuous variable.
Other biomarkers
The association between other day 28 biomarkers (TNFR1, IL-8, REG3α, IL2Rα, elafin, and HGF) and transplant outcomes was also analyzed. Although biomarker concentrations greater than the median of TNFR1 (>4792 pg/mL), IL-8 (>51 pg/mL), and REG3α (>42 ng/mL) were not associated with differences in grade II-IV or III-IV aGVHD, they were significantly associated with increased TRM. Similar to high ST2 levels, GVHD was the predominant cause of transplant-related death in patients with high levels followed by pulmonary toxicity. In contrast, biomarker concentrations greater than the median of IL2Rα (>2426 pg/mL), elafin (>5155 pg/mL), and HGF (>940 pg/mL) were not associated with either grade II-IV and III-IV aGVHD or TRM (Table 6).
Association of TNFR1, IL-8, REG3α, IL2Rα, Elafin, and HGF with grade II-IV aGVHD, grade III-IV aGVHD, and TRM in 113 DCBT recipients
| Day 28 biomarker . | Day 180 Grade II-IV aGVHD, % (95% CI) . | P . | Day 180 Grade III-IV aGVHD, % (95% CI) . | P . | Day 180 TRM, % (95% CI) . | P . |
|---|---|---|---|---|---|---|
| TNFR1, pg/mL | ||||||
| ≤4792 | 60 (46-72) | .749 | 23 (12-35) | .645 | 5 (1-13) | .005 |
| >4792 | 57 (42-69) | 19 (10-31) | 23 (13-35) | |||
| IL-8, pg/mL | ||||||
| ≤51 | 59 (45-71) | .822 | 15 (7-26) | .147 | 5 (1-13) | .005 |
| >51 | 58 (43-70) | 27 (16-40) | 23 (13-35) | |||
| REG3α,ng/mL | ||||||
| ≤42 | 59 (44-71) | .922 | 25 (14-37) | .361 | 5 (1-13) | .032 |
| >42 | 59 (44-71) | 17 (8-28) | 24 (13-36) | |||
| IL2Rα, pg/mL | ||||||
| ≤2426 | 57 (42-69) | .830 | 21 (11-33) | .978 | 12 (5-22) | .081 |
| >2426 | 60 (46-72) | 21 (11-33) | 16 (8-27) | |||
| Elafin, pg/mL | ||||||
| ≤5155 | 56 (41-68) | .283 | 21 (11-33) | .996 | 11 (4-20) | .296 |
| >5155 | 61 (47-73) | 20 (11-32) | 18 (9-29) | |||
| HGF, pg/mL | ||||||
| ≤940 | 64 (49-76) | .368 | 25 (14-37) | .388 | 12 (5-22) | .919 |
| >940 | 53 (39-65) | 17 (8-28) | 16 (8-27) |
| Day 28 biomarker . | Day 180 Grade II-IV aGVHD, % (95% CI) . | P . | Day 180 Grade III-IV aGVHD, % (95% CI) . | P . | Day 180 TRM, % (95% CI) . | P . |
|---|---|---|---|---|---|---|
| TNFR1, pg/mL | ||||||
| ≤4792 | 60 (46-72) | .749 | 23 (12-35) | .645 | 5 (1-13) | .005 |
| >4792 | 57 (42-69) | 19 (10-31) | 23 (13-35) | |||
| IL-8, pg/mL | ||||||
| ≤51 | 59 (45-71) | .822 | 15 (7-26) | .147 | 5 (1-13) | .005 |
| >51 | 58 (43-70) | 27 (16-40) | 23 (13-35) | |||
| REG3α,ng/mL | ||||||
| ≤42 | 59 (44-71) | .922 | 25 (14-37) | .361 | 5 (1-13) | .032 |
| >42 | 59 (44-71) | 17 (8-28) | 24 (13-36) | |||
| IL2Rα, pg/mL | ||||||
| ≤2426 | 57 (42-69) | .830 | 21 (11-33) | .978 | 12 (5-22) | .081 |
| >2426 | 60 (46-72) | 21 (11-33) | 16 (8-27) | |||
| Elafin, pg/mL | ||||||
| ≤5155 | 56 (41-68) | .283 | 21 (11-33) | .996 | 11 (4-20) | .296 |
| >5155 | 61 (47-73) | 20 (11-32) | 18 (9-29) | |||
| HGF, pg/mL | ||||||
| ≤940 | 64 (49-76) | .368 | 25 (14-37) | .388 | 12 (5-22) | .919 |
| >940 | 53 (39-65) | 17 (8-28) | 16 (8-27) |
Discussion
CB allografts are routinely transplanted with very high levels of donor-recipient HLA mismatch5,28,29 and yet the incidence of GVHD after CBT is lower than expected for the degree of HLA mismatch. However, GVHD is a significant complication of CBT and although it can be abrogated by ATG, in vivo T-cell depletion has the disadvantage of the potential abrogation of graft-versus-leukemia effects after reduced-intensity allografts30 and a high risk of opportunistic infections.31-34 Our center has elected to pursue CBT with an ATG-free platform and although CBT GVHD can be highly corticosteroid responsive, a minority of patients will develop severe or lethal disease.5 Moreover, we have previously reported that it is acute disease that is the major challenge in CBT, and although some patients will evolve into an overlap syndrome, de novo classical chronic GVHD is very uncommon.5 Therefore, a peripheral blood assay that could provide both diagnosis and prognostication of aGVHD severity early after transplantation would be ideal for designing risk-adapted clinical trials and tailoring immunosuppression intensity in CBT recipients.
ST2 is the receptor of IL-33 and is present in 2 isoforms: a membrane form expressed on hematopoietic cells, especially T helper 2 (Th2) cells, and a soluble form secreted by endothelial cells, epithelial cells, and fibroblasts in response to inflammatory stimuli.35 Soluble ST2 acts as a decoy receptor for IL-33 limiting its access to Th2 cells and promoting the Th1 phenotype which has been associated in aGVHD pathophysiology.36,37 In this study, we show for the first time that an elevated ST2 level is independently associated with an increased risk of grade II-IV and III-IV aGVHD in recipients of double-unit CB grafts. It is well established that donor-recipient HLA match is a critical determinant of CBT outcome including an association with aGVHD and TRM.5,38,39 Nonetheless, not all recipients of highly mismatched CB grafts suffer severe aGVHD, and in this analysis elevated ST2 level for severe aGVHD added predictive value beyond dominant unit-recipient 6 allele HLA match. This finding is important as, although many centers are now appropriately selecting CB units based on high-resolution HLA match, some patients will still receive units with a high degree of mismatch. Therefore, our findings warrant further investigation in a larger cohort to be able to analyze the additional value of ST2 levels, especially in recipients of highly HLA-mismatched grafts.
Importantly, a high day 28 ST2 level was also predictive of increased TRM. Notably, the association with TRM is consistent with that of adult donor allograft recipients.13 That aGVHD was the predominant cause of death in patients with high day 28 ST2 levels reinforces the potential clinical utility of early detection of elevated levels and early therapeutic intervention especially as aGVHD onset is after day 28 in the majority of patients. However, the optimal time to measure biomarkers is yet to be determined and, in our analysis, 7 patients developed GVHD ≤day 28. Prospective evaluation of serial biomarker measurements from very early posttransplant and at the time of GVHD diagnosis is needed to refine the predictive value of these assays in CBT. In addition, although the median ST2 value had good overall performance relative to other potential thresholds, additional studies should further explore alternatives and elucidate the categorization with the highest clinical utility. Whether ST2 levels, for example, will also provide prognostic information independent of aGVHD grade also requires investigation. In the future, early or preemptive interventions based on high biomarker levels could be evaluated. Targeting higher therapeutic calcineurin inhibitor levels,40-43 delaying MMF taper,5 dosing MMF according to therapeutic trough levels,44,45 manipulating gut flora,46 tailoring the dose of systemic corticosteroids for aGVHD treatment, or treating with IL-2247 are all possible strategies.
We found that the ST2 values in this analysis were different from that reported by Vander Lugt et al.13 The likely explanation for this is the use of the more reliable ST2 quantikine kit in this analysis. This emphasizes the need to strictly standardize ST2 testing methodology in future studies. It is also possible that the clinically relevant threshold for this biomarker could differ among HSC sources. Therefore, algorithms to assign specific thresholds for intervention by HSC source will need to be established, ideally by prospective multicenter trials. Additionally, patients with high day 28 ST2 levels who died of organ toxicity had exclusively lung involvement. It would be beneficial to understand the mechanism of the association between ST2 elevation and pulmonary toxicity. One possible explanation is that the pulmonary toxicity is due to idiopathic pneumonia syndrome (IPS). This has been considered an aGVHD equivalent in the lungs by some investigators.48
Whether the other biomarkers (TNFR1, IL-8, REG3α, IL2Rα, elafin, and HGF) evaluated in this study have additional prognostic importance is unclear. A significant negative finding in this study was the lack of an association between elafin levels and aGVHD. This could be due to very few patients having stage 3 skin aGVHD and none having stage 4 disease. The corticosteroid responsiveness of skin aGVHD that has been observed in nearly all CBT recipients in our center may also be relevant. However, analysis in a larger population is needed to further investigate this question. It is notable that although elevated levels of TNFR1, IL-8, and REG3α were not associated with the incidence of grade II-IV and III-IV aGVHD, they were associated with aGVHD lethality. Thus, elevation of these biomarkers could further support the identification of a patient at significant TRM risk and further prospective evaluation addressing this question is indicated. It would be important to establish whether measurement of biomarkers earlier after CBT is also predictive of severe aGVHD and TRM given earlier intervention may be more effective than at 4 weeks posttransplantation. Additionally, understanding the role of ST2 levels in predicting treatment response or the subsequent evolution to overlap syndrome or development of chronic classic GVHD is required. The ultimate aim of these studies is to ameliorate severe GVHD and further improve survival in CBT recipients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the nursing staff, transplant coordinators, and the Hsu and Brentjens Laboratories who greatly contributed to this work.
S.P. was supported by the National Cancer Institute, National Institutes of Health R01CA174667, and the Lilly Physician Scientist Initiative Program. At MSKCC, this work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the Society of MSKCC (J.N.B. and S.G.), the MSKCC Translational and Integrative Medicine Research Program (J.N.B.), P01 CA23766 from the National Cancer Institute, National Institutes of Health (J.N.B., M.-A.P., M.R.M.v.d.B., and R.J.O.), and P30 CA008748 (P.H. and S.M.D.).
Authorship
Contribution: D.M.P. interpreted the data and wrote the manuscript; S.P. and J.N.B. designed the study, interpreted the data, and wrote the manuscript; P.H. and S.M.D. analyzed the data and wrote the manuscript; C.M. and M.L. collected the data; and S.G., J.D.G., A.H., K.H., R.J., M.-A.P., C.S., M.R.M.v.d.B., J.W.Y., R.B., N.A.K., S.E.P., R.J.O., and A.S. wrote the manuscript.
Conflict-of-interest disclosure: R.B. is a cofounder of, stockholder in, and consultant of Juno Therapeutics. S.P. has a patent on “Methods of detection of graft-versus-host disease” licensed to Viracor-IBT Laboratories. The remaining authors declare no competing financial interests.
Correspondence: Doris M. Ponce, Box 259, 1275 York Ave, New York, NY, 10065; e-mail: ponced@mskcc.org.
References
Author notes
S.P. and J.N.B. contributed equally to this study and are senior co-authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal