Abstract
Nucleotides and nucleosides—such as adenosine triphosphate (ATP) and adenosine—are famous for their intracellular roles as building blocks for the genetic code or cellular energy currencies. In contrast, their function in the extracellular space is different. Here, they are primarily known as signaling molecules via activation of purinergic receptors, classified as P1 receptors for adenosine or P2 receptors for ATP. Because extracellular ATP is rapidly converted to adenosine by ectonucleotidase, nucleotide-phosphohydrolysis is important for controlling the balance between P2 and P1 signaling. Gene-targeted mice for P1, P2 receptors, or ectonucleotidase exhibit only very mild phenotypic manifestations at baseline. However, they demonstrate alterations in disease susceptibilities when exposed to a variety of vascular or blood diseases. Examples of phenotypic manifestations include vascular barrier dysfunction, graft-vs-host disease, platelet activation, ischemia, and reperfusion injury or sickle cell disease. Many of these studies highlight that purinergic signaling events can be targeted therapeutically.
Introduction
Life on Earth revolves around information written into the genetic code.1 Indeed, the substrate of the genetic code, and thus the substrate of life itself, is present in the DNA of all cells.1 The genetic code is translated from DNA to proteins by means of RNA. Importantly, DNA and RNA are both composed of relatively simple molecules, which include the purines guanine and adenine, as well as the pyrimidines uracil, thiamine, and cytosine.1 Without these purine molecules, life might still be possible but it would be very different from life as we know it.1 Beyond their intracellular functions as critical building blocks for the genetic code or as central building blocks of universal biological energy currencies, purines have a “second life” in the extracellular space.1,2 An important step to discovering their functions within the extracellular compartment was taken in the year 1929 by Alan Drury and Albert Szent-Györgyi.3 As such, the scientists from the University of Cambridge, United Kingdom, pursued studies in which they injected extracts from cardiac tissues intravenously into an intact animal.3 Much to their surprise, they observed a very transient disturbance of the cardiac rhythm and concomitant slowing of the heart rate (bradycardia).3-5 Using several steps to purify the biological activity of the extract, they were able to attribute the heart rate–slowing effects to an “adenine compound.”3-5 Many years later, it became clear that the observed slowing of the heart rate was mediated by activation of adenosine receptors of the heart.6-8 The observation of Drury and Szent-Györgyi that purines can function as signaling molecules4 subsequently led to the discovery of extracellular receptors that mediate the signaling effects of purines.3,9-11 Particularly, the signaling effects of the nucleotide adenosine triphosphate (ATP) and the nucleoside adenosine have become very famous in regulating a wide range of disease outcomes.2,8 In the present review article, we describe the various pathways that control extracellular signaling events of purines and discuss a few examples of blood and vascular diseases in which extracellular nucleotide and nucleoside signaling affect disease outcomes.
Extracellular ATP release and signaling, conversion to adenosine, and termination of adenosine signaling
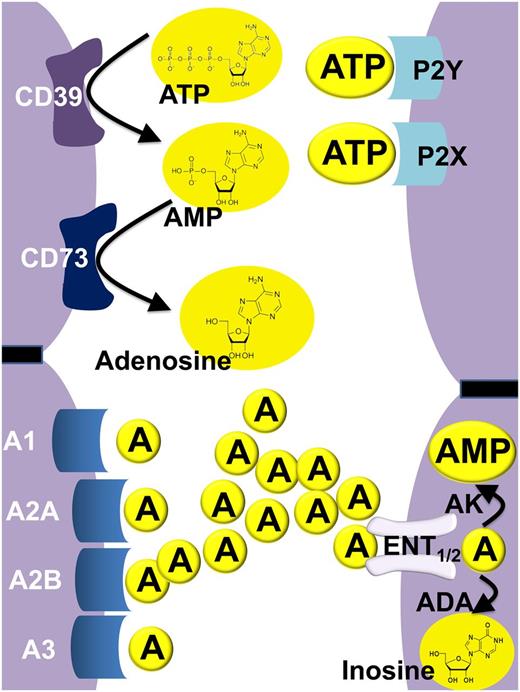
In many instances, purinergic signaling events are initiated by the release of the nucleotide ATP from the intracellular toward the extracellular compartment. It is important to keep in mind that intracellular ATP concentrations are very high—ranging from 4 to 8 mM.12 As such, it is not surprising that many forms of “cellular irritations” will cause the release of ATP from intracellular storage pools into the extracellular compartment. For example, ATP release occurs from multiple cell types during conditions of hypoxia10,13 or inflammation.13,14 Similarly, platelets are known to release nucleotides—particularly in the form of adenosine diphosphate (ADP) —via vesicular release.12,15,16 Although necrotic cells can spill ATP, apoptotic cells release ATP as a find-me signal for phagocytes.17 Recent studies have implicated pannexins channels as one of the primary known modes of nucleotide release from within cells into the extracellular compartment (eg, as a mechanism of ATP release from apoptotic cells).18 Interestingly, there are also several studies that have now implicated pannexins in inflammatory disease states.19 Once released into the extracellular compartment, ATP (and ADP) functions as agonist on purinergic P2 receptors. Although it is difficult to measure the concentration of extracellular nucleotides within extracellular space (particularly in a microenvironmental setting), this has important relevance for setting up gradients or activating specific nucleotide receptors. For example, when a cell lyses—as in necrosis—the amount of ATP released is high, whereas the same cell dying via apoptosis will release only 50 to 100 nM levels of ATP.17,20 P2 receptors can be classified into G-protein–coupled P2Y receptors (P2YR) or into ATP-gated ion channels—P2X receptors (P2XR) (Figure 1).2 After activation of ATP receptors, P2 signaling is terminated by diffusion of the ligand away from its receptors, and its subsequent extracellular phosphohydrolysis to adenosine. This process occurs in 2 separate steps, each of which is under the control of a specific ectonucleotidase. The first conversion step involves ATP/ADP phosphohydrolysis to adenosine monophosphate (AMP), which is catalyzed by the ecto-nucleoside-triphosphate-diphosphohydrolase 1 (CD39). In contrast to other ecto-nucleoside-triphosphate-diphosphohydrolases, CD39 is oriented as such that the catalytic side of the enzyme is facing the extracellular compartment and by its capacity to convert ATP or ADP completely to AMP.21-23 At present, there is no specific AMP receptor described24,25 ; however, some evidence indicates that AMP can directly activate adenosine receptors (eg, the Adora1).26 Importantly, there is compelling evidence that AMP serves as a metabolic nucleotide intermediate, which is rapidly converted to adenosine by the ecto-5′-nucleotidase CD73. CD73 has a similar orientation as CD39, with the enzymatic activity oriented toward the extracellular compartment. Although there are also alternative pathways for extracellular adenosine production,27,28 CD73-dependent adenosine production is frequently considered as the pacemaker reaction for extracellular adenosine generation during inflammatory or ischemic disease conditions.29-33 CD73-dependent adenosine liberation leads to the subsequent activation of adenosine receptors. At present, 4 different adenosine receptors have been described, including the Adora1, Adora2a, Adora2b, and Adora3 receptors (Figure 1).11 For example, the Adora1 has been shown to mediate the heart rate–slowing effects of adenosine,6 whereas the Adora2a is highly expressed on inflammatory cells and functions to dampen excessive immune cell activation.34-37 Signaling events through the Adora2b have been implicated in promoting ischemia tolerance and vascular protection.11 Adenosine signaling is terminated by uptake of adenosine by equilibrative nucleoside transporters (ENTs). These transporters allow adenosine to freely cross the cell membrane after its concentration gradient.38 Several studies have implicated ENT1 and ENT2 particularly in terminating extracellular adenosine signaling during inflammatory or hypoxic disease states.38-41 Once adenosine has reached the intracellular compartment, it is subjected to deamination by the adenosine deaminase42 or it can be phosphorylated to AMP by the adenosine kinase (Figure 1).43 Together, ENT-dependent adenosine uptake and intracellular metabolism are critical for the termination of adenosine signaling. The intimate relationship among ATP signaling, enzymatic adenosine generation, adenosine receptor activation, and termination of adenosine signaling highlighted the complexity of the signaling system and suggests that alterations on one end of the pathway have obvious implications on outcomes at the other end. For example, single-nucleotide polymorphisms44 or genetic deletion of CD3921-23,31,45,46 will be associated with attenuated ATP phosphohydrolysis and concomitant increases in P2 signaling, whereas adenosine production and P1 signaling will be attenuated. The following sections present some examples of how this relatively complex signaling system relates to disease outcomes with relevance to blood and vascular disease processes.
Overview of extracellular nucleotide and nucleoside signaling. In the extracellular compartment, the purinergic nucleotide ATP and its metabolic offspring—the nucleoside adenosine—are known to function as signaling molecules. During conditions of cellular stress (such as occurs during ischemia and reperfusion or vascular inflammation), multiple cell types release ATP (and ADP) into the extracellular compartment. Extracellular ATP can function as a signaling molecule via activation of purinergic P2 receptors specifically through the family of P2Y receptors—G-protein–coupled receptors—or through the family of P2X receptors, ligand-gated ion channels. In addition to its role as a direct activator of P2 receptors, ATP also functions as the main metabolic precursor for the extracellular generation of adenosine. For this purpose, ATP is metabolized by enzymatic phosphohydrolysis in a two-step process via CD39 conversion of ATP (or ADP) to AMP, and subsequent phosphohydrolysis of AMP to adenosine by CD73. Similar to ATP, adenosine functions as a direct activator of purinergic receptors, which are classified as P1 receptors. Currently, 4 distinct P1 adenosine receptors are known: Adora1 (A1), Adora2a (A2A), Adora2b (A2B), and Adora3 (A3). Adenosine signaling is terminated by uptake of adenosine from the extracellular toward the intracellular compartment by ENTs (particularly ENT1 and ENT2), followed by subsequent metabolism to inosine by the adenosine deaminase (ADA) or phosphorylation by the adenosine kinase (AK) to AMP.
Overview of extracellular nucleotide and nucleoside signaling. In the extracellular compartment, the purinergic nucleotide ATP and its metabolic offspring—the nucleoside adenosine—are known to function as signaling molecules. During conditions of cellular stress (such as occurs during ischemia and reperfusion or vascular inflammation), multiple cell types release ATP (and ADP) into the extracellular compartment. Extracellular ATP can function as a signaling molecule via activation of purinergic P2 receptors specifically through the family of P2Y receptors—G-protein–coupled receptors—or through the family of P2X receptors, ligand-gated ion channels. In addition to its role as a direct activator of P2 receptors, ATP also functions as the main metabolic precursor for the extracellular generation of adenosine. For this purpose, ATP is metabolized by enzymatic phosphohydrolysis in a two-step process via CD39 conversion of ATP (or ADP) to AMP, and subsequent phosphohydrolysis of AMP to adenosine by CD73. Similar to ATP, adenosine functions as a direct activator of purinergic receptors, which are classified as P1 receptors. Currently, 4 distinct P1 adenosine receptors are known: Adora1 (A1), Adora2a (A2A), Adora2b (A2B), and Adora3 (A3). Adenosine signaling is terminated by uptake of adenosine from the extracellular toward the intracellular compartment by ENTs (particularly ENT1 and ENT2), followed by subsequent metabolism to inosine by the adenosine deaminase (ADA) or phosphorylation by the adenosine kinase (AK) to AMP.
Endothelial barrier dysfunction during sepsis and inflammation
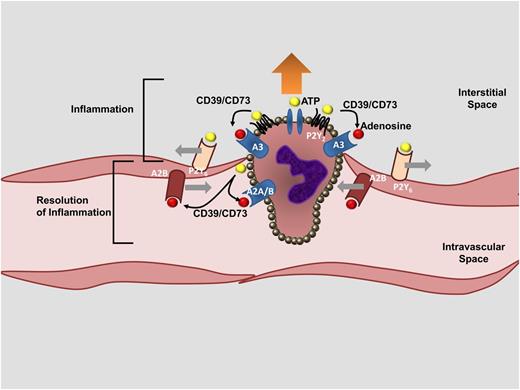
Tissue injuries of many different etiologies (eg, sepsis, ischemia, reperfusion injury) are frequently accompanied by increases in vascular permeability (vascular leakage to macromolecules), resulting in fluid loss into the interstitial space, which subsequently causes edema and organ dysfunction.47 For example, conditions of limited oxygen availability (hypoxia) are associated with decreased endothelial barrier function, fluid leakage into multiple organs, and elevations of circulating cytokine levels.48,49 Hypoxia-induced vascular inflammation can occur in the setting of high-altitude pulmonary edema, systemic inflammatory response syndrome, or sepsis.49 Indeed, one of the cardinal features of sepsis and septic shock is vascular leakage, where hypoxia and concomitant inflammatory responses cause dysfunction of the vascular endothelium, accompanied by cell death and loss of barrier integrity, giving rise to subcutaneous and body cavity edema.50 Purinergic signaling events have been significantly implicated in controlling the vascular barrier function and concomitant inflammatory events. Some evidence derives from studies of ATP signaling in models of inflammation and sepsis. Several studies implicate P2 signaling in promoting vascular inflammation and suggest a causal relationship for ATP signaling during hypoxia- or inflammation-associated endothelial dysfunction. For example, P2y6−/− mice are protected from inflammation and vascular leakage during intravascular lipopolysaccharide challenges.51 Additional evidence comes from studies of mice with deletion of enzymes responsible for the extracellular conversion of ATP to adenosine. Both cd39−/− and cd73−/− mice47,52 develop vascular leakage during conditions of limited oxygen availability, including pulmonary edema and generalized inflammation.53 Other studies addressed the functional role of adenosine signaling in endothelial barrier protection during conditions of hypoxia or inflammation. Genetic or pharmacologic studies provide evidence that adenosine signaling—particularly through the Adora2b—counter-regulates increases in vascular barrier function during inflammation or hypoxia.54,55 For example, the first description of Adora2b−/− mice highlights that these animals are prone to vascular inflammation56 and injury.57 As such, the effects of ATP and adenosine signaling are elegantly exemplified in the course of neutrophil-transepithelial migration. Neutrophils release ATP when they are transmigrating toward a chemotactic gradient of inflammatory mediators.12,52,58 Neutrophil-dependent ATP release on the leading edge of the polymorphonuclear neutrophil (PMN), in concert with activation of PMN-dependent P2Y2R, enhances neutrophil migration and is referred to as purinergic chemotaxis.59 Similarly, ATP receptor activation on vascular endothelial cells is associated with decreased endothelial barrier function, thereby promoting neutrophil-endothelial transmigration.52 At the same time, neutrophil-dependent ATP is rapidly converted by the CD39/CD73 pathway to adenosine. Activation of adenosine receptors—particularly of endothelial Adora2b—causes resealing of the endothelial barrier,54 whereas Adora2a signaling dampens inflammatory activation.60 Put differently, initial ATP signaling events open the door for neutrophils to transmigrate through the endothelial barrier, whereas subsequent adenosine signaling closes the gate behind them, thereby setting the stage for resolution of inflammation (Figure 2).
Role of purinergic signaling during neutrophil-endothelial transmigration. During neutrophil-endothelial transmigration, purinergic signaling events play a critical role in coordinating neutrophil movements toward a chemotactic gradient (purinergic chemotaxis), opening of the endothelial barrier, and subsequent resealing of the endothelial monolayer. ATP released from the leading edge of a neutrophil (yellow circle), activation of PMN-dependent ATP receptors (particularly the P2Y2R), conversion of ATP to adenosine (red circle) by the ectonucleotidase CD39 and CD73, and adenosine signaling through PMN-dependent A3 adenosine receptors are involved in promoting PMN movements toward a chemotactic gradient (“inflammation”). Similarly, purinergic signaling is involved in opening up the endothelial barrier function. For example, endothelial P2Y6Rs have been implicated in opening up the endothelial barrier function during inflammatory conditions. Interestingly, a subsequent set of purinergic signaling events is critical to resealing of the endothelial barrier function and in attenuating PMN-elicited inflammation, thereby initiating the “resolution of inflammation.” As such, activation of ADORA2B adenosine receptors (A2B) on vascular endothelial cells functions to increase the vascular barrier function, whereas ADORA2B and ADORA2A signaling (A2A, A2B) on PMN dampens neutrophil-elicited inflammation.
Role of purinergic signaling during neutrophil-endothelial transmigration. During neutrophil-endothelial transmigration, purinergic signaling events play a critical role in coordinating neutrophil movements toward a chemotactic gradient (purinergic chemotaxis), opening of the endothelial barrier, and subsequent resealing of the endothelial monolayer. ATP released from the leading edge of a neutrophil (yellow circle), activation of PMN-dependent ATP receptors (particularly the P2Y2R), conversion of ATP to adenosine (red circle) by the ectonucleotidase CD39 and CD73, and adenosine signaling through PMN-dependent A3 adenosine receptors are involved in promoting PMN movements toward a chemotactic gradient (“inflammation”). Similarly, purinergic signaling is involved in opening up the endothelial barrier function. For example, endothelial P2Y6Rs have been implicated in opening up the endothelial barrier function during inflammatory conditions. Interestingly, a subsequent set of purinergic signaling events is critical to resealing of the endothelial barrier function and in attenuating PMN-elicited inflammation, thereby initiating the “resolution of inflammation.” As such, activation of ADORA2B adenosine receptors (A2B) on vascular endothelial cells functions to increase the vascular barrier function, whereas ADORA2B and ADORA2A signaling (A2A, A2B) on PMN dampens neutrophil-elicited inflammation.
These findings have important implications for a wide range of vascular and inflammatory diseases, where control of excessive inflammation and preservation of the vascular barrier function are critical. For example, treatment with an Adora2b agonist (BAY 60-6583) has been shown to attenuate pulmonary edema during different forms of acute lung injury.39,54,55,61 Similarly, experimental studies in other inflammatory models, including inflammatory bowel disease62 or sepsis induced by cecal ligation and puncture,63 indicate protective effects of Adora2b adenosine receptor agonists. A critical step to advance these findings will be the development of clinical trials of Adora2b or Adora2a agonists in the treatment of vascular inflammatory disorders in humans. An ADORA2A agonist (regadenoson) has been recently approved for use in patients (eg, to be used for myocardial perfusion imaging in patients unable to undergo adequate exercise stress testing).64 Although Adora2b agonists such as BAY 60-6583 have been found to be highly effective in the treatment of vascular dysfunction in animal models,11 no such compound has yet been examined for its safety and efficiency in patients.15
Ischemia and reperfusion injury
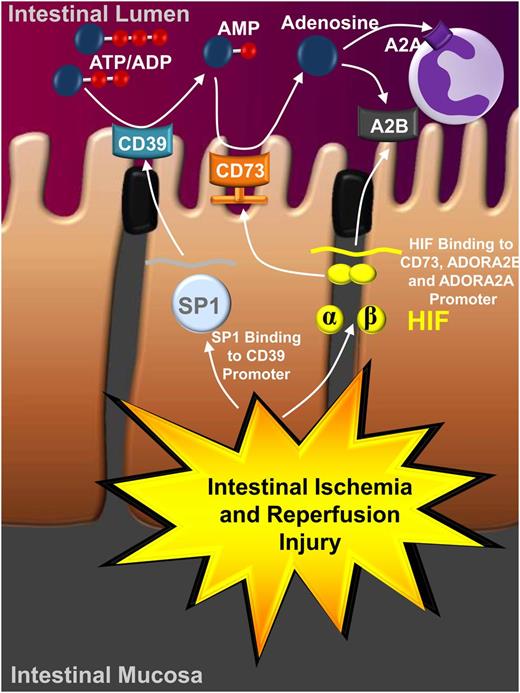
Ischemia and reperfusion is a disease state characterized by an initial restriction of blood supply to an organ followed by the subsequent restoration of perfusion and concomitant reoxygenation.65 Classically, occlusion of the arterial blood supply is caused by an embolus and results in severe imbalance of metabolic supply and demand, giving rise to severe tissue hypoxia.65 Somewhat counterintuitively, the restoration of blood flow and reintroduction of oxygenation is typically associated with an exacerbation of tissue injury and promotes a profound inflammatory response during the reperfusion period (reperfusion injury).66 Moreover, there are many instances where vascular responses play a critical role in the pathogenesis of ischemia and reperfusion.67 Because hypoxia has been shown to have a profound impact on purinergic signaling events,5,10,12,16 it is not surprising that signaling through P2 and P1 receptors is considered to play a central role during ischemia and reperfusion. Several studies implicate a causal role for ATP release and P2 signaling events in the generation of sterile inflammation during ischemia and reperfusion. For example, ATP released from necrotic cells can have pro-inflammatory functions (eg, by activating the Nlrp3 inflammasome), thereby generating an inflammatory microenvironment that alerts circulating neutrophils to adhere to the vasculature. Moreover, the generation of an intravascular chemokine gradient directs neutrophil migration through healthy tissue toward the area of tissue damage.68 An important mechanism to counterbalance ATP-mediated inflammation during ischemia and reperfusion is controlled by hypoxia-controlled transcription factors,69,70 including hypoxia-inducible factors HIF1A, HIF2A, and SP1.8,21,22,71-73 Activation of this transcriptional program has a profound impact on nucleotide metabolism and nucleoside signaling. As a first step, the transcription factor SP1 binds to the promoter of CD39 and dramatically increases CD39 transcript and protein levels, as well as its functional activity.21,22,52,53,58 Second, stabilization of HIF1A and its binding to the promoter of CD73 causes induction of CD73 transcript levels, protein, and function.14,33,47,74 The coordinated transcriptional induction of CD39 and CD73 is associated with a shift in the balance between P2 and P1 signaling, favoring ATP conversion to adenosine. This process is associated with increased nucleotide phosphohydrolysis and concomitant conversion of ATP to adenosine. In contrast to the detrimental effects of ATP signaling during ischemia and reperfusion, adenosine has been implicated via activation of the Adora2a and Adora2b in attenuating tissue inflammation and promoting ischemia tolerance (Figure 3).30,37,60,61,72,74-77 In addition, HIFs have been shown to dampen adenosine uptake via transcriptional repression of equilibrative adenosine transporters. A clinical trial examined the use of adenosine infusions as an adjunct to reperfusion in patients with myocardial infarction and ST-segment elevation.78 Interestingly, this study and a post hoc analysis79 reported that patients receiving higher concentrations of adenosine infusion (70 μg/kg per minute) had smaller myocardial infarct sizes, a finding that correlated with fewer adverse clinical events; and a subset that received adenosine within 3.17 hours had enhanced early and late survival and had reduced composite clinical end point of death at 6 months. Thus intravenous adenosine infusion is one of the very few molecules that has shown promise in a large randomized, controlled clinical trial to attenuate ischemia/reperfusion injury and improve “hard” clinical end points.79
Transcriptional control of purinergic signaling during ischemia and reperfusion. As part of ischemia and reperfusion injury (as shown here for intestinal ischemia and reperfusion injury), ischemic organs become severely hypoxic (eg, in the intestinal mucosa). Hypoxia results in the stabilization of hypoxia-dependent transcription factors, which in turn have a major effect on purinergic signaling events. For example, hypoxia-elicited activation of SP1 and subsequent binding of SP1 to the promoter of CD39 is associated with the transcriptional induction of CD39 expression and enzymatic activity. Similarly, hypoxia causes stabilization of the hypoxia-inducible transcription factor HIF, and binding of HIF to the promoter of CD73 promotes the transcriptional induction of CD73 transcript, protein, and enzyme activity. This coordinated response enhances the increased conversion of extracellular nucleotides (eg, ATP) to adenosine. Similarly, adenosine signaling events through adenosine receptors, for example the Adora2b (A2B) expressed on intestinal epithelial cells and the Adora2a (A2A) expressed on inflammatory cells, are increased by HIF. Together, these transcriptional alterations and concomitant changes in purinergic signaling are critical in attenuating inflammation and promoting ischemia tolerance during ischemia and reperfusion injury.
Transcriptional control of purinergic signaling during ischemia and reperfusion. As part of ischemia and reperfusion injury (as shown here for intestinal ischemia and reperfusion injury), ischemic organs become severely hypoxic (eg, in the intestinal mucosa). Hypoxia results in the stabilization of hypoxia-dependent transcription factors, which in turn have a major effect on purinergic signaling events. For example, hypoxia-elicited activation of SP1 and subsequent binding of SP1 to the promoter of CD39 is associated with the transcriptional induction of CD39 expression and enzymatic activity. Similarly, hypoxia causes stabilization of the hypoxia-inducible transcription factor HIF, and binding of HIF to the promoter of CD73 promotes the transcriptional induction of CD73 transcript, protein, and enzyme activity. This coordinated response enhances the increased conversion of extracellular nucleotides (eg, ATP) to adenosine. Similarly, adenosine signaling events through adenosine receptors, for example the Adora2b (A2B) expressed on intestinal epithelial cells and the Adora2a (A2A) expressed on inflammatory cells, are increased by HIF. Together, these transcriptional alterations and concomitant changes in purinergic signaling are critical in attenuating inflammation and promoting ischemia tolerance during ischemia and reperfusion injury.
Graft-versus-host disease
Allogeneic stem cell transplantation can be used clinically as a potentially curative treatment approach for hematologic malignancies that are refractory to conventional therapeutic approaches (eg, chemotherapy). However, the potentially lifesaving success of this therapeutic intervention can be limited by the occurrence of graft-vs-host disease (GVHD). GVHD represents one of the most significant adverse side effects of allogeneic stem cell transplantation, and causes significant morbidity and mortality. Interestingly, the systemic pathophysiologic responses that can be observed during GVHD show many similarities to those found in other systemic inflammatory disorders including sepsis, trauma, or ischemia and reperfusion. These findings suggest that endogenous nucleotides (particularly in the form of ATP—released from damages cells—and its metabolite adenosine) could function as common inflammatory mediators including GVHD.80 Therefore, it is not surprising that increased ATP levels can be found in GVHD target tissues, organs, and bodily fluids such as ascites of mice or humans experiencing GVHD.81 In addition, functional studies indicate that pharmacologic inhibition of ATP signaling reduces severity of acute GVHD in mice.81 Moreover, molecular studies provide experimental evidence that ATP signaling via activation of P2X7R on host dendritic cells leads to enhanced expression of co-stimulatory molecules such as CD80 and CD86 and consequently promotes activation and allogeneic priming of incoming donor T cells, thereby contributing to the immune responses in GVHD.81 In line with these findings, P2x7r−/− recipient mice show higher numbers of FoxP3+ regulatory T cells (Tregs) in in vivo models of GVHD. In contrast, in vitro studies demonstrate that alloantigen stimulation of P2x7r−/− dendritic cells results in STAT5 phosphorylation and FoxP3 expression in CD4+ T cells. These findings are important in the context of other recent studies that directly implicate Tregs in the prevention of GVHD.82 In contrast to P2 signaling, activation of P1 receptor subtypes by the ATP metabolite adenosine has been implicated in the attenuation of GVHD. Consistent with this notion, genetic deletion or pharmacologic inhibition of CD73—leading to attenuated ATP-dependent adenosine production—enhances acute GVHD. Moreover, pharmacologic treatment of wild-type animals with P1R antagonist mimics the increased GVHD severity observed in cd73−/− mice. Together, such studies indicate that the CD73-dependent generation of adenosine and signaling events through adenosine receptors (particularly the Adora2a) function to inhibit allogenic T cells, thereby limiting GVHD.80 These findings are in line with other studies implicating CD73-dependent adenosine production and signaling via Adora2a and Adora2b receptors in the immune-suppressive functions or Tregs.29,83,84 The protective role of adenosine signaling via the Adora2a in GVHD is also supported by studies demonstrating that treatment of animals with the selective Adora2a agonist ATL146e reduces cardinal features of GVHD by enhancing the number of donor-derived Tregs.85,86 In summary, these studies indicate that ATP signaling through the P2X7R is associated with enhanced generation of allogeneic donor T cells and suppression of Tregs, thereby contributing to the development of GVHD. In contrast, CD73-dependent adenosine generation and signaling through the Adora2a dampens GVHD via inhibition of allogeneic T cells and promotion of donor Tregs. Therefore, inhibition of P2X7R and concomitant enhancement of ADORA2A signaling resembles an experimental therapy for GVHD that has yet to be translated into patient treatments.
Sickle cell disease
Sickle cell disease is a genetic blood disorder characteristically associated with changes in the shape and rigidity of red blood cells (erythrocytes), referred to as “sickling.” Interestingly, sickling can be triggered by low concentrations of oxygen (local states of tissue hypoxia). This genetic disorder is caused by a point mutation in the β-globin chain of hemoglobin, converting a glutamic acid codon (GAG) to a valine codon (GTG), thus leading to the replacement of the hydrophilic amino acid glutamic acid by the hydrophobic amino acid valine in this position.87,88 During hypoxic conditions, this single nucleotide polymorphism promotes the polymerization of hemoglobin, causing red blood cells to undergo dramatic changes in their shape. Such shape changes include morphing from a round into a sickle-shaped form, with concomitant decreases in elasticity. Patients with sickle cell disease have periodic episodes of vaso-occlusive crisis, including pulmonary vaso-occlusion, which in some instances can be life threatening. In the past, microvascular occlusion was predominantly thought to be a function of the rigid sickled erythrocytes. More recently, however, ischemia and reperfusion injury with resultant activation of inflammatory cells has been implicated as an important contributor to the pathophysiology and disease mechanism of vascular occlusion during sickle cell disease.15,89 Sickle cell crisis is associated with the extracellular release of nucleotides, particularly of ATP and ADP. However, red blood cell–derived adenosine deaminase can lower adenosine levels in some compartments (eg, in the urine and kidneys), a process that may predispose to vaso-occlusive events.90 In fact, some evidence indicates that urinary adenosine levels in sickle cell patients are decreased during a crisis event.91 Several studies examined the role of purinergic signaling on ischemia and reperfusion injury during sickle cell crisis. Other studies examined the contributions of purinergic signaling pathways on their contributions to erythrocyte sickling. For example, a small randomized clinical trial in patients is suggestive of a detrimental role for P2Y12 signaling during sickle cell disease. Patients randomized to the P2Y12 agonist prasugrel had decreased platelet activation biomarkers and a trend toward decreased pain.92 The role of adenosine signaling during sickle cell disease is more complicated. On the one hand, experimental studies suggest that adenosine signaling via the Adora2a receptor can function to attenuate pulmonary inflammation and T-cell activation in the context of preventing hypoxia-reoxygenation–induced exacerbation of pulmonary injury.89 Moreover, a recently conducted phase 1 trial of the ADORA2A agonist regadenoson in adults with sickle cell disease suggests that infusional regadenoson administered during painful vaso-occlusive crises is associated with decreased activation of invariant natural killer T cells without toxicity.93
Although these studies indicate a protective role of Adora2a signaling during sickle cell disease via attenuating ischemia and reperfusion injury–associated inflammation, other studies suggest that the ADORA2B plays a functional role during sickle cell disease by promoting erythrocyte sickling. Using metabolomic profiling, this study provides evidence that the concentration of the nucleoside adenosine is elevated in the blood of a transgenic mouse model of sickle cell disease.94 Similarly, the authors of this study found elevated adenosine levels in blood samples of patients with sickle cell disease.94 Moreover, this study suggests that increased adenosine levels play a functional role in promoting sickling, hemolysis, and damage to multiple tissues. Molecular studies implicate signaling events through the Adora2b adenosine receptor in the induction of erythrocyte-dependent 2,3-diphosphoglycerate.94 This erythrocyte-specific metabolite is known to decrease the oxygen-binding affinity of hemoglobin and is critical for the induction of erythrocyte sickling by excess adenosine (Figure 4). Together together with the aforementioned studies, these findings indicate that the ideal adenosine-based therapy for sickle cell disease could combine an Adora2b antagonist to prevent sickling and an Adora2a agonist for the treatment of inflammation during vaso-occlusive crisis. However, such an approach may prove to be challenging from several aspects. First, the specificity of agonists or antagonists for the Adora2a and Adora2b may be offset at higher concentrations. Second, there are many studies implicating Adora2b signaling in tissue protection during ischemia and reperfusion injury.30,61,72,74-76 As such, Adora2b antagonists could potentially exacerbate ischemia and reperfusion injury–associated complications of sickle cell crisis.
Purinergic signaling during sickle cell disease. In patients with sickle cell disease, extracellular adenosine levels are elevated. As an important antiinflammatory signal, Adora2a signaling on inflammatory cells (eg, on natural killer T cells [NKT-cells]) suppresses the innate immune response and limits inflammation and cellular injury. This endogenous antiinflammatory mechanism can be targeted for the treatment of ischemia and reperfusion injury such as occurs during vaso-occlusive syndrome in patients with sickle cell disease (eg, by using an Adora2a agonist therapeutically). However, noting a complication in the role of adenosine signaling during sickle cell disease, other studies that demonstrate that activation of the Adora2b by adenosine on erythrocytes increases their 2,3-DPG levels, thereby promoting red blood sickling.
Purinergic signaling during sickle cell disease. In patients with sickle cell disease, extracellular adenosine levels are elevated. As an important antiinflammatory signal, Adora2a signaling on inflammatory cells (eg, on natural killer T cells [NKT-cells]) suppresses the innate immune response and limits inflammation and cellular injury. This endogenous antiinflammatory mechanism can be targeted for the treatment of ischemia and reperfusion injury such as occurs during vaso-occlusive syndrome in patients with sickle cell disease (eg, by using an Adora2a agonist therapeutically). However, noting a complication in the role of adenosine signaling during sickle cell disease, other studies that demonstrate that activation of the Adora2b by adenosine on erythrocytes increases their 2,3-DPG levels, thereby promoting red blood sickling.
Infectious diseases
Many studies implicate purinergic signaling events in the host defense against invading pathogens. Most studies suggest that ATP release and concomitant signaling events through P2 receptors are critical for mounting a protective inflammatory response that is critical to eliminating pathogens. For example, activation of the P2X7R on human macrophages induces killing of mycobacteria, and a recently described polymorphism in the P2X7 gene that dramatically reduces this killing is associated with markedly increased susceptibility to extrapulmonary tuberculosis in humans.95 Similarly, double-knockout mice for P2y1 and P2y2 show markedly increased disease susceptibility during intratracheal infections with Pseudomonas aeruginosa, including high rates of mortality in the context of an attenuated inflammatory response. Thus these studies indicate a protective role for P2 signaling during P aeruginosa infections of the lungs by enhancing a proinflammatory cytokine response.96 In contrast, pathways that promote ATP/ADP conversion to adenosine, and increase concomitant adenosine signaling events, are frequently associated with an attenuated inflammatory response during infections with pathogens. For example, a recent study induced polymicrobial sepsis in cd73−/− mice using cecal ligation and puncture. Interestingly, cd73−/− mice had elevated inflammatory cytokine and chemokine concentrations in the blood and peritoneum. This occurred in the context of increased lung injury, including elevated myeloperoxidase activity and neutrophil infiltration into the lungs.97 Consistent with these findings, subsequent studies to address the functional role of individual adenosine receptors suggest that genetic deficiency of the Adora2b is associated with increased mortality and increased levels of inflammatory cytokines and chemokines and augmented nuclear factor–κB activation in the spleen, heart, and plasma.63 Interestingly, a similar study that examined the role of Adora2b signaling during cecal ligation and puncture found that Adora2b−/− mice appear to be protected during polybacterial sepsis.98 Although both studies agree on the conceptual finding that Adora2b−/− mice show an enhanced inflammatory response, the outcome of increasing inflammatory responses during cecal ligation and puncture was protective in one study,98 whereas this appeared to be detrimental in the other study.63 Taken together, these findings indicate that P2 signaling is involved in promoting inflammatory responses during infections with pathogens, whereas adenosine-dependent activation of Adora2a or Adora2b receptors attenuates inflammatory activation. As such, P1 agonists are considered therapeutic in the context of excessive and harmful inflammation, whereas P2 agonists may be important in situations for which an appropriate inflammatory response is required to efficiently eliminate invading pathogens.
Platelet functions
Platelets are well known for their function as critical mediators of hemostasis including platelet adhesion, aggregation, and subsequent formation of a platelet thrombus.65 Because nucleotides are continuously released from many different cells within the blood stream (eg, platelets, inflammatory cells, erythrocytes), it is not surprising that signaling events through purinergic receptors (including P1 and P2 receptors) play very central roles in controlling platelet activation and hemostasis.11 Interestingly, and beyond their function in mediating thrombus formation, platelets are also known for the important role as major sources of inflammatory mediators (eg, serotonin, IL-1β, CD40-ligand, platelet-derived growth factor, platelet-activating factor). As such, it has become clear that platelets resemble an important link between coagulation and inflammation.11,65 Platelets are known to express P2X1R, P2Y1R, and P2Y12R, where predominantly ADP signaling via activation of the P2Y1 and P2Y12R is critical for initiating platelet aggregation. Activation of P2Y1R causes activation of phospholipase C and triggers shape changes, whereas P2Y12 couples to Gi to reduce adenylyl cyclase activity and inactivation of the fibrinogen receptor glycoprotein GP IIb/IIIa receptor, which is critically involved in platelet aggregation.11 The platelet P2Y12R has gained considerable recognition, because inhibitors of the P2Y12R such as clopidogrel are used clinically as antithrombotic therapy (eg, as treatment for patients with coronary artery disease or recurrent stroke). In addition, P2Y12R signaling has also been implicated in triggering mediator-release from platelets, thereby promoting platelet-elicited inflammation. Consistent with this concept, several clinical studies have been able to demonstrate reduced inflammatory markers in patients who are receiving treatment (eg, for coronary syndrome or for stroke) with the P2Y12R antagonist clopidogrel.99,100 Moreover, preclinical studies demonstrate a P2Y12R-driven pro-inflammatory role of platelets in models of vascular inflammation and asthma. For instance, P2y12−/− mice experience a milder phenotypic disease manifestation in experimentally induced atherosclerosis, which is related to a marked attenuation of platelet-mediated inflammation.101 Other studies suggest that a platelet-dependent cross-talk involving the leukotriene LTE4 and the P2Y12R plays a critical role in the pathogenesis of allergic airway inflammation.102
Several lines of experimental studies provide evidence that the phosphohydrolysis of ATP/ADP and the concomitant increases in adenosine signaling can function to attenuate platelet activation. Because of the central role of CD39 in the extracellular breakdown of ATP and ADP, it is not surprising that cd39−/− mice exhibited increased cerebral infarct volumes and reduced postischemic perfusion, whereas treatment with soluble CD39 reconstituted these mice.103 CD39 is also important in the maintenance of platelet functionality because it prevents platelet P2Y1R desensitization. As such, mouse genes targeted for cd39 show prolonged bleeding time with minimally perturbed coagulation parameters.104 Treatment with soluble nucleotidase, and concomitant increases in AMP conversion to adenosine, functions to inhibit platelet aggregation.105 Adenosine-dependent inhibition of platelet function has been shown to involve predominantly signaling events through the ADORA2A or the ADORA2B.106,107 Taken together, these findings indicate opposing functions in ATP/ADP vs adenosine in platelet functions. On one hand, activation of platelet-expressed P2 receptors causes platelet activation and release of inflammatory mediators. Conversely, activation of adenosine receptors on platelets—particularly the ADORA2A or the ADORA2B—leads to inhibition of platelet function. These studies also highlight the central role for extracellular nucleotide phosphohydrolysis via CD39 and CD73 in controlling the balance between P2 and P1 signaling on platelets.
Conclusions
Research over the past decade has implicated purinergic signaling as an important regulatory mechanism in a wide range of diseases and biological functions, with important implications for blood and vascular disorders. The implications of purinergic signaling extend far beyond the present review. For example, important findings on the role of purinergic signaling in disease mechanisms of the central nervous system—such as Parkinson disease108,109 or cancer110 —have not been addressed here. There are many instances in which signaling events through adenosine P1 vs nucleotide P2 receptors mediate effects that go in opposing directions and are often associated with completely opposite outcomes in biological systems. Important in this context is the observation that pharmacologic approaches to shift the balance between purinergic P2 and P1 signaling resemble an important therapeutic concept in modulating inflammation, platelet activation, or vascular barrier function. Although experimental studies in models of vascular inflammation, ischemia and reperfusion, or infectious disease models, or during platelet activation, implicate purinergic signaling in the treatment of these orders, few examples for the clinical use of purinergic signaling have been established. For example, established treatment modalities that target purinergic receptors in patients include P2Y12R inhibitors (such as clopidogrel) as an inhibitor of platelet function, or the intravenous treatment with adenosine for the pharmacologic termination of supraventricular tachycardia. It will be a challenge for this field to extend the use of pharmacologic approaches that target purinergic signaling beyond the indications that have been established, which will require additional clinical studies in patients. However, we are highly optimistic that these developments will result in novel treatment modalities for diseases of the blood and the vascular system.
Acknowledgment
The authors acknowledge Shelley A. Eltzschig for artwork during manuscript preparation.
The work was supported by the National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases grants R01 DK097075 and R01-DK083385 and National Heart, Lung, and Blood Institute grants R01-HL0921, R01- HL098294, and POIHL114457-01, and by a grant from the Crohn’s and Colitis Foundation of America (CCFA) (H.K.E.).
Authorship
Contribution: All authors wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger K. Eltzschig, Organ Protection Program, Department of Anesthesiology, University of Colorado School of Medicine, 12700 E 19th Ave, Mailstop B112, Research Complex 2, Room 7124, Aurora, CO 80045; e-mail: holger.eltzschig@ucdenver.edu.

![Figure 4. Purinergic signaling during sickle cell disease. In patients with sickle cell disease, extracellular adenosine levels are elevated. As an important antiinflammatory signal, Adora2a signaling on inflammatory cells (eg, on natural killer T cells [NKT-cells]) suppresses the innate immune response and limits inflammation and cellular injury. This endogenous antiinflammatory mechanism can be targeted for the treatment of ischemia and reperfusion injury such as occurs during vaso-occlusive syndrome in patients with sickle cell disease (eg, by using an Adora2a agonist therapeutically). However, noting a complication in the role of adenosine signaling during sickle cell disease, other studies that demonstrate that activation of the Adora2b by adenosine on erythrocytes increases their 2,3-DPG levels, thereby promoting red blood sickling.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/7/10.1182_blood-2013-09-402560/5/m_1029f4.jpeg?Expires=1767789168&Signature=n4WU3ctK-1qnyrSAg28rkUSYyYZMOXWzDIdCEwdNchV3alilPjyABmHYs-7aNqTqdpH6ZEy9dgY8vpKyuxF8E7ovFP9-mQ1OARYQuqdwMqIJedBf6AdMqKZ2~freHntauriUT5Zj~pcKtcdNhw4-SHJpeI3UxEnfRg0xaKFg3ml5hQVpmjd9JuatTca~eO0GUVDAr1FJUe~cZAhpKRHxwrgoz2wN9wCi3yYTuUCa4sQW41YftR7OfzO5tE4dDzWGVANG1WRqFU92M5YqsmxzWTxG2yhRL-wgDknPs5SGE0ya8ju1BeV3iWMnmpph5YVT8Jh2jvsLZ6DAIE53ElrVoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)