Abstract
Hypoxia is a well-documented inflammatory stimulus and results in tissue polymorphonuclear leukocyte (PMN) accumulation. Likewise, increased tissue adenosine levels are commonly associated with hypoxia, and given the anti-inflammatory properties of adenosine, we hypothesized that adenosine production via adenine nucleotide metabolism at the vascular surface triggers an endogenous anti-inflammatory response during hypoxia. Initial in vitro studies indicated that endogenously generated adenosine, through activation of PMN adenosine A2A and A2B receptors, functions as an antiadhesive signal for PMN binding to microvascular endothelia. Intravascular nucleotides released by inflammatory cells undergo phosphohydrolysis via hypoxia-induced CD39 ectoapyrase (CD39 converts adenosine triphosphate/adenosine diphosphate [ATP/ADP] to adenosine monophosphate [AMP]) and CD73 ecto-5′-nucleotidase (CD73 converts AMP to adenosine). Extensions of our in vitro findings using cd39- and cd73-null animals revealed that extracellular adenosine produced through adenine nucleotide metabolism during hypoxia is a potent anti-inflammatory signal for PMNs in vivo. These findings identify CD39 and CD73 as critical control points for endogenous adenosine generation and implicate this pathway as an innate mechanism to attenuate excessive tissue PMN accumulation. (Blood. 2004;104:3986-3992)
Introduction
Tissue hypoxia has been implicated as a contributing factor to inflammatory diseases initiated at the vascular surface. For example, ongoing inflammatory responses are characterized by dramatic shifts in tissue metabolism including lactate accumulation, increased nucleotide metabolism, and diminished availability of oxygen (hypoxia).1 Such shifts in tissue metabolism result, at least in part, from extensive recruitment of inflammatory cells, particularly myeloid cells such as polymorphonuclear leukocytes (PMNs) and monocytes. Moreover, it has recently been appreciated that hypoxia may also contribute to productive inflammatory responses. For example, studies in myeloid cells of mice conditionally deficient in the hypoxia-responsive transcription factor hypoxiainducible factor-1α (HIF-1α) revealed that activation of HIF-1α is essential for myeloid cell infiltration and activation,2 implicating hypoxia as an important endogenous mediator of inflammation.
In spite of the severe course in which inflammatory diseases can proceed, most inflammation is self-limiting. One important factor may be increased production of endogenous adenosine, a naturally occurring anti-inflammatory agent.3 Several lines of evidence support this assertion. First, adenosine receptors are widely expressed on target cell types as diverse as leukocytes, vascular endothelia, and mucosal epithelia and have been studied for their capacity to modulate inflammation.4 Second, murine models of inflammation provide evidence for adenosine receptor signaling as a mechanism for regulating inflammatory responses in vivo. For example, mice deficient in the A2A-adenosine receptor (AdoRA2A) show increased inflammation-associated tissue damage.5 Third, hypoxia is a common feature of inflamed tissues1 and is accompanied by significantly increased levels of adenosine.6-8 At present, the exact source of adenosine is not well defined but likely results from a combination of increased intracellular metabolism and amplified extracellular phosphohydrolysis of adenine nucleotides via surface ecto-nucleotidases. With regard to this latter point, it was recently shown that hypoxia coordinates both transcriptional and metabolic control of the surface ecto-nucleotidases CD39 and CD739-11 and, as such, significantly amplifies the extracellular production of adenosine from adenine nucleotide precursors.
In the present studies, we defined the role of extracellular adenine nucleotide phosphohydrolysis in attenuating adhesive interactions between PMNs and vascular endothelia. Using a combination of in vitro and in vivo hypoxia models, we identify the control points for adenosine-mediated attenuation of PMN accumulation. Through the use of 2 genetically deficient murine models, namely cd39- and newly generated cd73-null mice,12 evidence is provided that endogenous production of adenosine at vascular surfaces significantly attenuates tissue PMN accumulation. Such findings provide new insight into endogenous pathways to regulate leukocyte trafficking at inflammatory sites.
Materials and methods
Endothelial cell isolation and culture
Isolation of human neutrophils
PMNs were freshly isolated from whole blood obtained by venipuncture from human volunteers and anticoagulated with acid citrate/dextrose.14 Resulting cell population was greater than 97% PMNs as assessed by microscopic evaluation. PMNs were studied within 2 hours of their isolation. Approval for these studies was obtained from the Brigham and Women's Hospital's institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Preparation of activated PMN supernatants
To measure the time course of adenosine triphosphate (ATP) released from PMNs, freshly isolated PMNs (107 cells/mL in Hanks balanced salt solution [HBSS]) were incubated end-over-end at 37°C following 10-6 M FMLP (formyl-methionyl-leucyl-phenylalanine) activation for indicated periods of time, supernatants were collected, and ATP content was quantified using CHRONO-LUME reagent (Chrono-log, Haverton, PA). Luciferase activity was assessed on a luminometer (Turner Designs, Sunnyvale, CA) and compared with internal ATP standards.
PMN adhesion assay
Freshly isolated PMNs were isolated as described in “Isolation of human neutrophils” and labeled for 30 minutes at 37°C with 5 μM BCECF-am (2′,7′-bis(carboxyethyl)-56-carboxyfluorescein-acetoxymethyl ester; 5 μM final concentration; Calbiochem, San Diego, CA) and used to assess adhesion to activated endothelial cells as described previously.15
In experiments where adenosine receptor antagonists were used, both PMN and HMEC-1 monolayers were preincubated prior to adhesion assays with the nonspecific adenosine receptor antagonist 8-phenyl-theophylline (8-PT; Sigma Chemical, St Louis, MO); the specific AdoRA1 antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine; Sigma Chemical); the AdoRA2A antagonist 8 (3-chlorostyryl) caffeine (CSC; Sigma Chemical); the AdoRA2A antagonist ZM 241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol; Tocris Cookson, Ellisville, MO); the AdoRA2B antagonist MRS 1754 (N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide; Molecular Recognition Section, National Institutes of Health [NIH], Bethesda, MD); and the specific AdoRA3 antagonist MRS 1334 (1,4-dihydro-2-methyl-6-phenyl-4-(phenylethynyl)-3,5-pyridinedicarboxylic acid 3-ethyl-5-[(3-nitrophenyl)methyl] ester; Tocris Cookson).
CD39 suppression with RNA interference
SiRNA-directed suppression of CD39 in HMEC-1s was accomplished using the following ribonucleotides, sense strand (5′-GAA UAU CCU AGC CAU CCU UdTdT-3′) and antisense strand (5′-dTdT CUU AUA GGA UCG GUA GGA A-3′), as described previously.11 A nonspecific control ribonucleotide sense strand (5′-ACU CUA UCU GCA CGC UGA CdTdT-3′) and antisense strand (5′-dTdT UGA GAU AGA CGU GCG ACU G-3′), as well as specific control siRNA-directed against lamin A/C (Qiagen, Valencia, CA), were used under identical conditions. Protein levels were detected by Western blot using antibodies directed against CD39 (Research Diagnostics, Flanders, NJ; 5 μg/mL) or lamin C (ImmunQuest, Cleveland, United Kingdom; 5 μg/mL).
Immunoprecipitation
Confluent normoxic or hypoxic (48 hours hypoxia exposure, pO2 20 mm Hg) HMEC-1s were surface labeled with biotin, and CD39 was immunoprecipitated and probed as described previously.11
Immunofluorescence
Confluent normoxic or hypoxic (48 hours hypoxia exposure, pO2 20 mm Hg) HMEC-1s on coverslips were washed in phosphate-buffered saline (PBS) and preincubated with HBSS+ with or without a combination of the selective AdoRA2A antagonist 8 (3-chlorostyryl) caffeine (CSC) and the selective AdoRA2B antagonist MRS 175416 or the CD73 inhibitor APCP (αβ-methylene-adenosine 5′-diphosphate) for 5 minutes. To each coverslip, 1 × 106 freshly isolated and FMLP-activated PMNs were added following preincubation with or without adenosine receptor antagonists or APCP in HBSS, as described above. The coverslips were centrifuged at 150g for 2 minutes to settle PMNs uniformly, and adhesion was allowed for 10 minutes at 37°C. Following washing, the coverslips were fixed in 1% paraformaldehyde and 100 mM cacodylate buffer for 10 minutes at room temperature and washed again. The coverslips were incubated with rhodamineconjugated phalloidin (Molecular Probes, Eugene, OR), washed, mounted with antifade mounting media (Molecular Probes), and analyzed with a Nikon E-6000 (Melville, NY) microscope using a 40 × objective/400 × NA and Spot 3.0 software (Sterling Heights, MI) as previously described.17
In vivo hypoxia model
Mice deficient in cd39 on the C57BL/6/129 SVJ strain (12th generation backcross to C57BL/6) were generated, validated, and characterized as described previously.18 Mice deficient in cd73 on the C57BL/6/129 svj strain (seventh generation backcross to C57BL/6) were generated, validated, and characterized as described elsewhere.12 Control mice were matched according to sex, age, and weight. For the purpose of quantifying PMN tissue concentrations, the animals were exposed to normobaric hypoxia (8% O2, 92% N2) or room air for 4 hours (n = 4-6 animals per condition). Following hypoxia/normoxia exposure, the animals were killed and the liver, brain, skeletal muscle, kidney, colon, and lungs were harvested. The PMN marker myeloperoxidase (MPO) was quantified as previously described.19 In subsets of experiments, mice were reconstituted with 5′-nucleotidase (5′-NT; purified from Crotalus atrox venom; Sigma Chemical). Pilot dosing experiments revealed that formulation of 5′-NT could be used at concentrations as high as 500 U/kg intraperitoneally. This protocol was in accordance with NIH guidelines for use of live animals and was approved by the Institutional Animal Care and Use Committee at Brigham and Women's Hospital.
Data analysis
Data were compared by 2-factor analysis of variance (ANOVA) or by Student t test, where appropriate. Values are expressed as the mean ± SD from at least 3 separate experiments.
Results
PMN adenosine receptor signaling regulates adhesion to the posthypoxic endothelium
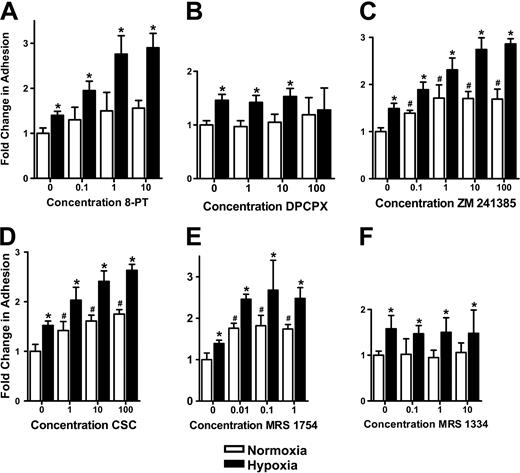
We recently demonstrated that metabolism of extracellular nucleotides by surface ecto-nucleotidases coordinates posthypoxic endothelial adenosine responses.11 Given the potent anti-inflammatory actions of adenosine on PMN function,3 we hypothesized that endogenous adenosine generated at the endothelial surface could influence PMN-endothelial adhesion. To test this hypothesis, endothelial cells were pre-exposed to conditions of hypoxia, which induce ecto-nucleotidase (CD39 and CD73) activity (20 mm Hg, 48 hours),11 and assessed for adenosine-dependent PMN adhesion using the nonselective adenosine receptor antagonist 8-PT. As shown in Figure 1A, 8-PT did not significantly influence FMLP-stimulated PMN adhesion to normoxic endothelia. However, with posthypoxic endothelia (3- to 5-fold increase in both CD39 and CD73 expression; data not shown), 8-PT increased FMLP-stimulated PMN adhesion in a concentration-dependent manner (P < .05 by ANOVA), with maximal increases of 2.8 ± 0.8-fold at 10 μM 8-PT (no additional increases at higher concentrations; data not shown). These data indicate that endogenous adenosine liberated at the surface of posthypoxic endothelia attenuates PMN-endothelial adhesion.
Influence of adenosine receptor inhibition on PMN adhesion to posthypoxic endothelia. HMEC-1s were subjected to normoxia (pO2 147 mm Hg for 48 h, □) or hypoxia (pO2 20 mm Hg for 48 h, ▪) followed by determination of FMLP-stimulated PMN adhesion in the presence or absence of indicated concentrations of adenosine receptor antagonists. PMN adhesion was determined by assessment of BCECF-labeled PMNs binding to normoxic or posthypoxic HMEC-1s. Results are presented as the fold change (± SD) in BCECF fluorescence in the presence of (A) the nonselective antagonist 8-phenyl-theophylline (8-PT); (B) the AdoRA1 antagonist DPCPX; (C) the AdoRA2A antagonist ZM 241385; (D) the AdoRA2A antagonist CSC; (E) the AdoRA2B antagonist MRS 1754; (F) or the AdoRA3 antagonist MRS 1334. Results are presented as the fold change (± SD) in BCECF fluorescence (μM concentrations; *P < .05 compared with normoxia; #P < .025 compared with no antagonist control).
Influence of adenosine receptor inhibition on PMN adhesion to posthypoxic endothelia. HMEC-1s were subjected to normoxia (pO2 147 mm Hg for 48 h, □) or hypoxia (pO2 20 mm Hg for 48 h, ▪) followed by determination of FMLP-stimulated PMN adhesion in the presence or absence of indicated concentrations of adenosine receptor antagonists. PMN adhesion was determined by assessment of BCECF-labeled PMNs binding to normoxic or posthypoxic HMEC-1s. Results are presented as the fold change (± SD) in BCECF fluorescence in the presence of (A) the nonselective antagonist 8-phenyl-theophylline (8-PT); (B) the AdoRA1 antagonist DPCPX; (C) the AdoRA2A antagonist ZM 241385; (D) the AdoRA2A antagonist CSC; (E) the AdoRA2B antagonist MRS 1754; (F) or the AdoRA3 antagonist MRS 1334. Results are presented as the fold change (± SD) in BCECF fluorescence (μM concentrations; *P < .05 compared with normoxia; #P < .025 compared with no antagonist control).
We next determined whether PMN or endothelial adenosine receptors accounted for the differences observed in PMN-endothelial interactions. To do this, we desensitized PMN or endothelial adenosine receptors through incubation with N-ethylcarboxamido-adenosine (NECA), a nonmetabolized adenosine analog. Such pretreatment has been shown to effectively desensitize adenosine receptor signal transduction.20 Effective desensitization of PMNs was demonstrated by the loss of adenosine-mediated inhibition of FMLP-stimulated MPO release (data not shown) and by the loss of adenosine-mediated increases in endothelial barrier function (data not shown). We then compared endothelial adhesion of NECA-desensitized PMNs with that of untreated PMNs under normoxic or posthypoxic conditions (HMEC-1s, 48 h normoxia or 48 h hypoxia, 20 mm Hg O2). When measuring adhesion of BCECF-labeled PMNs to normoxic endothelial cells, NECA pretreatment was not associated with a significant change in adhesion. In contrast, increases in PMN adhesion in the presence of 10-μM concentrations of the nonspecific adenosine receptor antagonist 8-PT were obviated in NECA-desensitized PMNs (P < .01). As a control for these responses, NECA pretreatment did not influence inhibition of PMN adhesion associated with functional blockade of CD11b (clone 44a, 10 μg/mL) by monoclonal antibody (mAb) treatment (71% ± 7% and 76% ± 9% decrease in PMN adhesion with and without NECA desensitization, respectively; both P < .025). No significant differences in adhesion were observed with NECA-desensitized endothelial cells under any of these conditions. Taken together, these results indicate that PMN adenosine receptors, as opposed to endothelial adenosine receptors, mediate diminished PMN adhesion to the posthypoxic endothelium.
PMN-AdoRA2A and -AdoRA2B are responsible for limiting hypoxia-induced adhesion to the endothelium
Since PMNs are known to express all 4 subtypes of adenosine receptors,21-24 we used selective antagonists of the different adenosine receptor subtypes to further identify the functional receptor responsible for limiting PMN adhesion to posthypoxic endothelia. As shown in Figure 1B, incubation with the AdoRA1 antagonist DPCPX did not change PMN adhesion. As with 8-PT, the AdoRA2A antagonists ZM 241385 (Figure 1C) and CSC (Figure 1D), as well as the AdoRA2B antagonist MRS 1754 (Figure 1E), were associated with amplified hypoxia-induced increases of PMN adhesion. The AdoRA3 receptor antagonist MRS 1334 (Figure 1F) was not associated with changes in hypoxia-induced adhesion of PMNs to the endothelium. Imaging of this interaction (data not shown) revealed an almost complete loss of BCECF-labeled PMN adhesion with antagonists of the AdoRA2A and AdoRA2B. Taken together, these results implicate PMN AdoRA2A and AdoRA2B in limiting increased PMN adhesion to posthypoxic endothelia.
PMNs release ATP in an activation-dependent fashion
We next determined the potential source(s) of extracellular nucleotide(s) during PMN-endothelial interactions. Initial studies with endothelial cells revealed that levels of adenosine in supernatants derived from either hypoxic or normoxic endothelia were below the level of high-performance liquid chromatography (HPLC) resolution (< 100 nM; data not shown), most probably due to rapid uptake via membrane nucleoside transporters.25 Similarly, extracellular ATP levels (measured using standard luminometric ATP-detection assays) in both hypoxic and normoxic endothelia were at or below the level of detection (< 300 fM; data not shown). Such data suggest that endothelial cells are not a major source of extracellular nucleotides.
We then determined whether activated PMNs are a source of extracellular nucleotide(s), particularly since it has been shown that PMNs release both adenosine monophosphate (AMP)26,27 and ATP11 under some conditions. Only small amounts of ATP accumulated in the supernatants of resting PMNs (eg, without activation at 4°C in Ca2+-free HBSS, maximal levels 45 ± 7 nmol/107 PMNs). Higher ATP levels were observed at 37°C in Ca2+ containing buffer (maximal levels 123 ± 27 nmol/107 PMNs; P < .01 by ANOVA). However, following FMLP activation, ATP concentrations in the supernatant were profoundly increased, with a rapid ATP peak at 1 minute after FMLP activation (at 1 minute following activation: 315 ± 44 nmol/107 PMNs; P < .01 by ANOVA). Measurable levels of ATP rapidly dissipated after activation and approached levels observed without FMLP activation. These results indicate that PMNs rapidly release ATP in an activation-dependent fashion, thus providing a readily available source for adenosine generation at the endothelial surface.
In vitro knockdown of endothelial CD39 is associated with increased PMN adhesion to posthypoxic endothelia
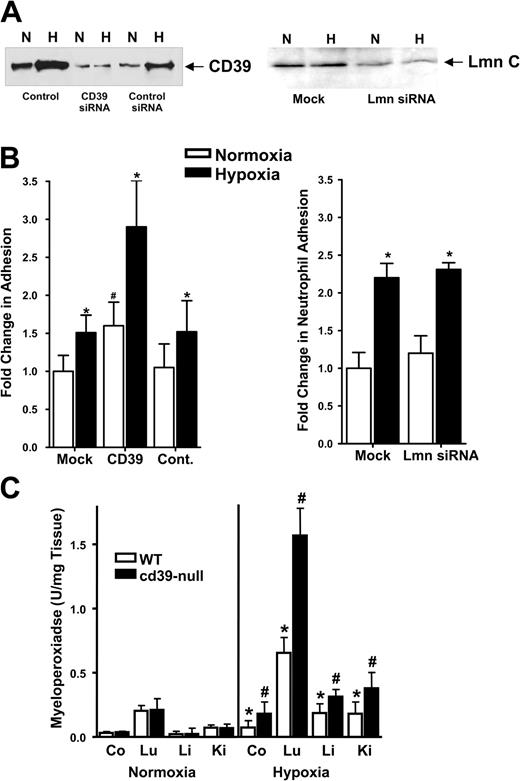
Having demonstrated that PMN-derived ATP is a potential source for extracellular adenosine in the setting of inflammation, we used our in vitro model to further investigate the role of ectonucleotidase activity on PMN-endothelial interactions during hypoxia. We reasoned that inhibition of hypoxia-induced CD39 should reveal the contribution of ATP/adenosine diphosphate (ADP) metabolism to adenosine-mediated attenuation of PMN adhesion (Figure 1). To accomplish this, we used siRNA-mediated suppression of CD39 expression, as previously described.11 As expected, siRNA directed against CD39 significantly decreased surface-expressed CD39 under both normoxic and posthypoxic conditions, as determined by immunoprecipitation of biotinylated surface CD39 protein (Figure 2A). Mock-transfected cells, control ribonucleotide-loaded cells, as well as siRNA directed against lamin A/C, were used to demonstrate specificity (Figure 2A-B).
Decreased CD39 expression is associated with increased PMN adhesion to posthypoxic endothelia in vitro and in vivo. (A) HMEC-1 cells were loaded with CD39-specific siRNA, control ribonucleotide, mock-treated (control) or lamin A/C (Lmn C) siRNA controls and exposed to normoxia (N) or hypoxia (H; 2% normobaric oxygen for 48 h). Monolayers were washed, surface proteins were biotinylated, and cells were lysed. CD39 was immunoprecipitated and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and resultant Western blots were probed with avidin-peroxidase. (B) HMEC-1s were loaded with specific CD39 siRNA, a nonspecific control siRNA, mock-treated (control), or lamin A/C siRNA and exposed to hypoxia or normoxia (48 h). BCECF-labeled FMLP-activated PMNs were added (105 PMNs per condition) and PMN adhesion was quantified by measuring fluorescence (*P < .05 compared with normoxic control and to normoxia; #P < .05 compared with normoxia). (C) cd39-null (▪) or wild-type littermate (□) mice were subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by measuring myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). Data are presented as mean ± SEM.
Decreased CD39 expression is associated with increased PMN adhesion to posthypoxic endothelia in vitro and in vivo. (A) HMEC-1 cells were loaded with CD39-specific siRNA, control ribonucleotide, mock-treated (control) or lamin A/C (Lmn C) siRNA controls and exposed to normoxia (N) or hypoxia (H; 2% normobaric oxygen for 48 h). Monolayers were washed, surface proteins were biotinylated, and cells were lysed. CD39 was immunoprecipitated and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and resultant Western blots were probed with avidin-peroxidase. (B) HMEC-1s were loaded with specific CD39 siRNA, a nonspecific control siRNA, mock-treated (control), or lamin A/C siRNA and exposed to hypoxia or normoxia (48 h). BCECF-labeled FMLP-activated PMNs were added (105 PMNs per condition) and PMN adhesion was quantified by measuring fluorescence (*P < .05 compared with normoxic control and to normoxia; #P < .05 compared with normoxia). (C) cd39-null (▪) or wild-type littermate (□) mice were subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by measuring myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). Data are presented as mean ± SEM.
Having shown that siRNA suppression of CD39 expression is efficient under our conditions, we loaded cultured endothelial cells with specific CD39 siRNA and measured adhesion of BCECF-labeled FMLP-activated PMNs (HMEC-1s, 48 h normoxia or 48 h hypoxia, 20 mm Hg O2). In untreated controls, hypoxia exposure was associated with a significant increase of PMN adhesion to posthypoxic endothelia (1.41 ± 0.12-fold increase in BCECF fluorescence above normoxia; P < .05). Following siRNA knockdown of CD39, a significant increase of adhesion was observed in normoxic endothelia (1.6 ± 0.19-fold increase above normoxia controls; P < .05; Figure 2B). Moreover, adhesion was even more dramatically increased after hypoxia exposure (1.8-fold increase above normoxia and 2.68 ± 0.69-fold above untreated controls; P < .01; Figure 2B). No difference was observed between the controls and endothelial cells loaded with control siRNA. Similar results were obtained by using MPO activity as a measurement of PMN adhesion to the endothelium (data not shown). These results suggest that hypoxia-mediated induction of CD39 may contribute to attenuated PMN accumulation to the endothelial surface following hypoxia exposure.
Hypoxia exposure of cd39-null mice promotes increased concentrations of PMNs in multiple organs
We and others have shown that hypoxia is a potent stimulus for tissue PMN accumulation in vivo.28-30 Based on the above findings that adenine nucleotide metabolism at the endothelial surface may attenuate PMN-endothelial adhesion (Figures 1 and 2), we hypothesized that targeted disruption of CD39 would result in more pronounced PMN tissue accumulation.
To test this hypothesis, we used a previously described in vivo hypoxia model.10,11,31 cd39-null mice18 were subjected to either normoxia or normobaric hypoxia (8% O2 and 92% N2) for 4 hours and multiple mucosal organs were screened for PMN accumulation by measuring MPO activity (Figure 2C). There was no difference in organ MPO activity of normoxic wild-type and cd39-null mice. Consistent with previous studies,30 MPO concentrations were significantly increased after hypoxia exposure of the wild-type animals (colon, liver, kidney, and lung; Figure 2C). In support of our hypothesis, MPO activity was increased by 2- to 4-fold in all cd39-null organs examined following hypoxia (colon, liver, kidney, lung; P < .025 by ANOVA; Figure 2C). These in vivo findings suggest that nucleotide phosphohydrolysis via CD39 is a critical control point in the regulation of PMN accumulation within hypoxic tissues.
Inhibition of CD73 increases PMN adhesion to the posthypoxic endothelium in vitro
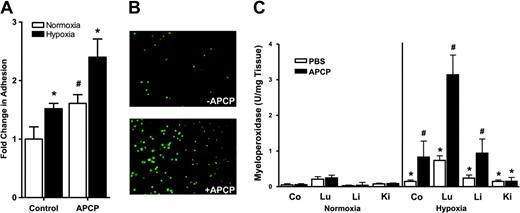
The final metabolic step for generation of extracellular adenosine from AMP requires CD73. Therefore, we extended the above observations regarding CD39 (Figure 2) to examine whether inhibition of CD73 might also influence PMN-endothelial interactions. CD73 is effectively blocked by the stable ADP analog α,β-methylene ADP (APCP).10 Thus, we investigated PMN adhesion to normoxic or posthypoxic endothelial cells (HMEC-1s, 48 h normoxia or 48 h hypoxia, 20 mm Hg O2) in the presence of 10-μM concentrations of APCP.26 Similar to the above results regarding CD39-siRNA depletion, we observed that under normoxic conditions, APCP inhibition of CD73 was associated with increased PMN adhesion to endothelial monolayers compared with untreated, normoxic controls (1.43 ± 0.11-fold increase above untreated controls; P < .05; Figure 3A). Moreover, the observed increase of PMN adhesion to the posthypoxic endothelium (1.46 ± 0.13-fold increase above normoxia; P < .05; Figure 3A) was further amplified in the posthypoxic endothelium following the addition of APCP (2.37 ± 0.34-fold increase above normoxia; P < .01; Figure 3A). Imaging of this interaction revealed a large increase in BCECF-labeled PMN adhesion to posthypoxic endothelia in the presence of APCP (10 μM; Figure 3B). These results support our hypothesis that PMN adhesion to the posthypoxic endothelium is increased by blockade of CD73 in vitro.
Influence of CD73 inhibition on PMN adhesion to the posthypoxic endothelia in vitro and in vivo. HMEC-1s were subjected to normoxia (pO2 147 mm Hg for 48 h, □) or hypoxia (pO2 20 mm Hg for 48 h, ▪) followed by measurement of FMLP-stimulated PMN adhesion in the presence and absence of the CD73 inhibitor αβmethylene-ADP (APCP, 10 μM). (A) PMN adhesion was quantified by assessment of adherent BCECF-labeled PMNs to normoxic or posthypoxic HMEC-1s. Results are presented as the fold change (± SD) in BCECF fluorescence (*P < .05 compared with normoxia; #P < .025 compared with PBS control). Panel B represents immunofluorescence imaging of BCECF-labeled PMNs adherent to posthypoxic endothelia in the absence (top) and presence of APCP (10 μM; bottom). In panel C, wild-type mice were administered APCP (20 mg/kg ntraperitoneally) or PBS and subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours and indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). Data are presented as mean ± SEM.
Influence of CD73 inhibition on PMN adhesion to the posthypoxic endothelia in vitro and in vivo. HMEC-1s were subjected to normoxia (pO2 147 mm Hg for 48 h, □) or hypoxia (pO2 20 mm Hg for 48 h, ▪) followed by measurement of FMLP-stimulated PMN adhesion in the presence and absence of the CD73 inhibitor αβmethylene-ADP (APCP, 10 μM). (A) PMN adhesion was quantified by assessment of adherent BCECF-labeled PMNs to normoxic or posthypoxic HMEC-1s. Results are presented as the fold change (± SD) in BCECF fluorescence (*P < .05 compared with normoxia; #P < .025 compared with PBS control). Panel B represents immunofluorescence imaging of BCECF-labeled PMNs adherent to posthypoxic endothelia in the absence (top) and presence of APCP (10 μM; bottom). In panel C, wild-type mice were administered APCP (20 mg/kg ntraperitoneally) or PBS and subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours and indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). Data are presented as mean ± SEM.
CD73 and PMN trafficking in vivo
The studies described above implicate both CD39 and CD73 in PMN accumulation within hypoxic tissues. To further define the role of CD73 in vivo, we determined the influence of APCP (APCP, 20 mg/kg intraperitoneally, a concentration previously shown to effectively block CD73 in vivo)10 on hypoxia-induced PMN trafficking. As shown in Figure 3C, administration of APCP to control normoxic animals did not change PMN accumulation in any organ studied. However, under hypoxic conditions, inhibition of CD73 with APCP increased MPO activity above that of hypoxia alone in all organs examined (P < .025 by ANOVA) except kidney, thus supporting our hypothesis that extracellular adenosine functions to attenuate PMN accumulation.
We next used newly generated cd73-null mice12 to more clearly define the role of ecto-nucleotidases to PMN trafficking in vivo. As shown in Figure 4A, cd73-/- mice subjected to our above-described hypoxia protocol revealed a large increase in tissue-associated PMNs. Indeed, while hypoxia maximally increased tissue PMNs by 7 ± 0.4-fold in wild-type animals (Figure 4A colon), similar analysis in cd73-/- animals resulted in a 19 ± 3.1-fold increase over normoxic controls (Figure 4A colon). This increase in tissue-associated PMNs was apparent in all organs examined (P < .025 by ANOVA). Histologic examination of lungs derived from hypoxic cd73-/- mice (Figure 4B) confirmed previous observations of perivascular edema and circumferential cuffing with aggregates of inflammatory cells surrounding large pulmonary vessels.12
Influence of CD73 on PMN accumulation during hypoxia in vivo: partial rescue with exogenous 5′-nucleotidase. (A) cd73-null (▪) or wild-type littermate (□) mice were subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). (B) Representative low-power (top, × 100 total magnification) and high-power (bottom, × 400 total magnification) lung section from cd73-null animal subjected to hypoxia. Note inflammatory infiltration in the perivascular region of large vessels. (C) cd73-null (▪) or wild-type littermate (□) mice were administered purified 5′-nucleotidase (500 U/kg intraperitoneally [ip]), subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia). Data are presented as mean ± SEM.
Influence of CD73 on PMN accumulation during hypoxia in vivo: partial rescue with exogenous 5′-nucleotidase. (A) cd73-null (▪) or wild-type littermate (□) mice were subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). (B) Representative low-power (top, × 100 total magnification) and high-power (bottom, × 400 total magnification) lung section from cd73-null animal subjected to hypoxia. Note inflammatory infiltration in the perivascular region of large vessels. (C) cd73-null (▪) or wild-type littermate (□) mice were administered purified 5′-nucleotidase (500 U/kg intraperitoneally [ip]), subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia). Data are presented as mean ± SEM.
In additional series of experiments, we determined whether reconstitution of cd73-/- mice with 5′-nucleotidase purified from Crotalus atrox venom (used at 500 U/kg intraperitoneally) would normalize PMN accumulation in response to in vivo hypoxia. This treatment protocol has been shown to effectively elevate circulating nucleotidase activity.12 As depicted in Figure 4C, exogenous administration 5′-nucleotidase to cd73-/- mice rescued, at least in part, the cd73-null phenotype of increased tissue-associated PMNs in hypoxia. While hypoxia-associated increases in PMNs were evident in both wild-type and cd73-null mice (P < .025 by ANOVA), no significant differences were observed between wild-type and cd73-null mice. These results suggest that the terminal enzymatic step for adenosine production (ie, 5′AMP to adenosine) attenuates, at least in part, the accumulation of PMNs within posthypoxic tissues. Taken together, these results implicate both CD39 and CD73 as critical control points to dampen inflammatory cell accumulation associated with tissue hypoxia in vivo.
Discussion
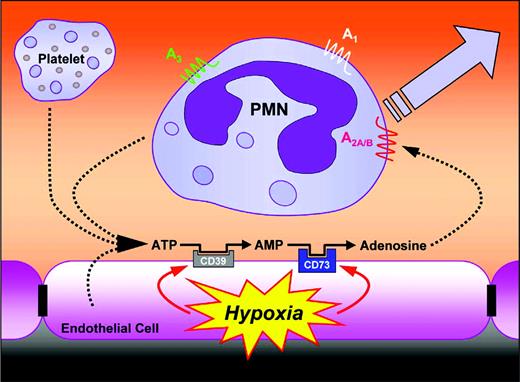
The vascular endothelium is the primary interface between a hypoxic insult and the surrounding tissues. At the same time, the endothelium is central to the orchestration of leukocyte trafficking in response to chemotactic stimuli. This critical anatomic location places vascular endothelial cells in an ideal position to coordinate extracellular metabolic events important to endogenous anti-inflammatory responses. We report here the existence of extracellular metabolic neutrophil-endothelial cell cross-talk coordinated by hypoxia. This pathway uses extracellular nucleotide substrates, likely liberated from a number of sources, to generate adenosine at the vascular surface. Such adenosine binds to surface-expressed PMN adenosine receptors to limit excessive accumulation of PMNs within tissues and, as such, functions as a feedback loop to attenuate potential tissue damage (Figure 5).
Proposed model of leukocyte-endothelial cross-talk at sites of inflammation. In areas of ongoing inflammation, diminished oxygen supply coordinates the induction of CD39 and CD73. At such sites, activated PMNs provide a readily available extracellular source of ATP, which through 2 enzymatic steps is metabolized to adenosine. Adenosine generated in this fashion is available for activation of PMN adenosine receptors, particularly the AdoRA2A and AdoRA2B, leading to decreased in PMN adhesion and transmigration. As such, this form of leukocyte-endothelial cell cross-talk may provide an innate mechanism to dampen ongoing inflammatory responses.
Proposed model of leukocyte-endothelial cross-talk at sites of inflammation. In areas of ongoing inflammation, diminished oxygen supply coordinates the induction of CD39 and CD73. At such sites, activated PMNs provide a readily available extracellular source of ATP, which through 2 enzymatic steps is metabolized to adenosine. Adenosine generated in this fashion is available for activation of PMN adenosine receptors, particularly the AdoRA2A and AdoRA2B, leading to decreased in PMN adhesion and transmigration. As such, this form of leukocyte-endothelial cell cross-talk may provide an innate mechanism to dampen ongoing inflammatory responses.
For more than 2 decades, it has been appreciated that adenosine attenuates potentially harmful aspects of PMN activation.32 More recent studies have focused on targeting adenosine receptors to limit tissue injury in a variety of diseases4,33,34 using either native adenosine or pharmacologic agonism/antagonism with receptor-selective analogs. Less is known about the dynamic influences of endogenously generated adenosine on PMN-endothelial interactions. New information regarding metabolic pathways to generate extracellular adenosine (ie, CD39 and CD73) provides an opportunity to understand these pathways in more detail. Here, we sought to better define whether endogenous adenosine might functionally influence leukocyte accumulation. Studies using selective AdoR antagonists in vitro identified the PMN AdoRA2 as critical to attenuation of initial PMN-endothelial adhesive responses. Others have demonstrated that PMNs express all 4 AdoR subtypes,22,23,35 and as some cross-reactivity may occur between the different antagonists used here, we could not distinguish between AdoRA2A and AdoRA2B. In general, these observations are consistent with previous work.21,22,36 With the availability of specific agonists/antagonists of adenosine receptors and the use of targeted gene disruption, the role of PMN adenosine receptor activation could be further clarified. As such, more recent studies show that transendothelial migration of PMNs can be attenuated by adenosine activation of AdoRA2B.37 Similarly, targeted disruption of the AdoRA2A receptor is associated with dramatically increased inflammatory reactions to subthreshold stimuli in an animal model.5 Therefore, these results that PMN AdoRA2A/2B activation are responsible for dampening hypoxia-elicited increases in PMN adhesion to the vascular endothelium are not surprising. However, the mechanism by which adenosine activation of AdoRA2A/2B receptors on PMNs contributes to the inhibition and termination of inflammation remains to be further elucidated.
We show here, that PMNs release ATP to the extracellular milieu in an activation-dependent fashion. At present, we do not know the mechanism of ATP release. Several mechanisms have been proposed, including direct transport through ATP-binding cassette (ABC) proteins, transport through connexin hemichannels, as well as vesicular release.38,39 As part of the present study, some experiments were done in an attempt to identify the intracellular ATP compartment (data not shown). For example, we compared the kinetics of ATP and granule release from activated PMNs. These studies indicated that while ATP levels were maximal within 1 minute, activated degranulation contents such as MPO were maximal at time points more than 5 minutes, suggesting that ATP is not granule bound. Moreover, isolated granules from unactivated PMNs contained greater than 95% of MPO activity but less than 5% of ATP, suggesting that ATP is unlikely to be granule-bound in PMNs. At present, the mechanism by which PMNs release ATP remains unclear.
Results from these studies implicate the rapid release and metabolism of ATP to adenosine at the endothelial surface as a mechanism to attenuate PMN adhesion and transmigration. It remains to be seen whether such rapid kinetics of PMN nucleotide release and metabolism contribute to the sustained process of adhesion and transendothelial migration. Of note, previous work using real-time flow fluorescence microscopy has indicated that following PMN adhesion to the vascular endothelium, the process of transmigration can occur in a relatively short period of time (approximately 1 minute).40 These same studies indicated that gaps in endothelial tight junction formed by transmigrating PMNs and monocytes rapidly reseal.40 Important in this regard, our previous work has implicated extracellular nucleosides (eg, adenosine) generated during PMN-endothelial interactions as a mechanism to dampen permeability increases associated with PMN transmigration.11,26,41 Thus, it would seem reasonable to propose that adenosine generated at the vascular cell surface assumes multiple protective roles at inflammatory foci.
Activated platelets comprise an additional source of extracellular adenine nucleotides.42,43 Endothelial CD39 has been viewed as playing a protective, thromboregulatory role in restricting the size of the hemostatic plug by limiting excessive platelet aggregation.43-46 Indeed, excessive platelet accumulation and recruitment can be treated with soluble forms of CD39.47,48 Moreover, a thromboregulatory role could be demonstrated in a model of stroke or ischemia-reperfusion injury, where damage in cd39-null mice was readily treated with soluble forms of CD39/apyrase.49,50 However, a role of CD39 in regulating neutrophil adhesion and transmigration has not been demonstrated. The present results suggest that similarly to CD73, CD39 is responsible for increases in adenine nucleotide metabolism, thereby promoting increases in intravascular adenosine concentrations. Similar to its role in limiting excessive activation of the prothrombotic system via decreasing intravascular ADP concentrations, CD39 also appears to limit excessive PMN infiltration by providing increased adenosine concentrations at sites of hypoxia and inflammation.
Taken together, we describe here a metabolic pathway that results in increased intravascular adenosine concentrations. In this form of neutrophil-endothelial cell cross-talk, metabolism of ATP derived from neutrophils is increased due to the induction of the ecto-nucleotidases CD39 and CD73 on endothelial cells by hypoxia. Similar anti-inflammatory pathways resulting in increased adenosine concentrations have been described previously. For example, it is well documented that the anti-inflammatory influence of methotrexate and sulfasalazine is mediated by adenosine.51,52 However, it was recently reported that both drugs take effect by increasing the production of adenosine by CD73.53 In this study, the anti-inflammatory effects of methotrexate and sulfasalazine can be obviated by the CD73 inhibitor APCP. Similarly to the present investigation, this study suggests that methotrexate/sulfasalazine-induced increases in adenosine concentrations are related to increased phosphohydrolysis of adenine nucleotides that are released by cells. However, this study could not further identify the source of adenine nucleotides.
In summary, the present investigations reveal a novel form of neutrophil-endothelial cell cross-talk to dampen neutrophil adhesion to the endothelium and transmigration into hypoxic tissues, thereby limiting hypoxia-associated inflammatory responses. On the part of the circulating PMNs, activation results in the release of ATP that serves as a substrate for the ecto-enzymes CD39 and CD73. The increased availability of adenosine in hypoxic tissues then decreases PMN adhesion and transmigration through the endothelial barrier along a gradient of inflammatory chemoattractants. On the part of the endothelium, hypoxia coordinately induces functional ecto-nucleotidase activity (via CD39 and CD73),10,11 thereby resulting in increased phosphohydrolysis of adenine nucleotide substrates. We propose that this biochemical cross-talk contributes to an endogenous, anti-inflammatory mechanism for highly vascular tissues during adaptation to hypoxia.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-06-2066.
Supported by National Institutes of Health (NIH) grants AI18220 (L.F.T.), HL60569 (S.P.C), DK50189 (S.P.C.), DE13499 (S.P.C.), and by fellowship awards from the Foundation for Anesthesia Education and Research (J.C.I.) and the Crohn's and Colitis Foundation of America (J.K.). L.F.T. holds the Putman City Schools Chair in Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 4. Influence of CD73 on PMN accumulation during hypoxia in vivo: partial rescue with exogenous 5′-nucleotidase. (A) cd73-null (▪) or wild-type littermate (□) mice were subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia; #P < .025 compared with wild-type). (B) Representative low-power (top, × 100 total magnification) and high-power (bottom, × 400 total magnification) lung section from cd73-null animal subjected to hypoxia. Note inflammatory infiltration in the perivascular region of large vessels. (C) cd73-null (▪) or wild-type littermate (□) mice were administered purified 5′-nucleotidase (500 U/kg intraperitoneally [ip]), subjected to either normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours, and the indicated organs (colon, Co; lung, Lu; liver, Li; or kidney, Ki) were assessed for PMN accumulation by myeloperoxidase activity (*P < .025 compared with normoxia). Data are presented as mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2066/6/m_zh80240470940004.jpeg?Expires=1769123535&Signature=4NlJ-DppFadUTgBYNusfr1BjbB-w6SFuirXBtahJPnutTxAh2VJDHwJPAMnQV~TM0OwvoHmhPqrxtk8mrBQmvCYF2tllxhzWOGMAI5MAtQSO8mqzeiZqD4SypCTRI71VtOoPUgZyxycCzuDm86OTP-c4stMQqaGY-9OZc2Di3TdXcvBhhIy3eqQdJXUCVscQUtj1GMOOJD9tksyzkDhSAn0upVpkXOYjf8e~F-fMR8j3Y1h2wzflcHBjjmEzWF5gZh9GfcfAZlS1Sna62Tf7uMW23m3YReulkudFLD3P-42C0V6buFf9uQo~uPA~2L4ubdkWmVnu-s8RFwA4NE53PA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal