Extracellular adenosine has been implicated in adaptation to hypoxia and previous studies demonstrated a central role in vascular responses. Here, we examined the contribution of individual adenosine receptors (ARs: A1AR/A2AAR/A2BAR/A3AR) to vascular leak induced by hypoxia. Initial profiling studies revealed that siRNA-mediated repression of the A2BAR selectively increased endothelial leak in response to hypoxia in vitro. In parallel, vascular permeability was significantly increased in vascular organs of A2BAR−/−-mice subjected to ambient hypoxia (8% oxygen, 4 hours; eg, lung: 2.1 ± 0.12-fold increase). By contrast, hypoxia-induced vascular leak was not accentuated in A1AR−/−-, A2AAR−/−-, or A3AR−/−-deficient mice, suggesting a degree of specificity for the A2BAR. Further studies in wild type mice revealed that the selective A2BAR antagonist PSB1115 resulted in profound increases in hypoxia-associated vascular leakage while A2BAR agonist (BAY60-6583 [2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)-. phenyl]pyridin-2-ylsulfanyl]acetamide]) treatment was associated with almost complete reversal of hypoxia-induced vascular leakage (eg, lung: 2.0 ± 0.21-fold reduction). Studies in bone marrow chimeric A2BAR mice suggested a predominant role of vascular A2BARs in this response, while hypoxia-associated increases in tissue neutrophils were, at least in part, mediated by A2BAR expressing hematopoietic cells. Taken together, these studies provide pharmacologic and genetic evidence for vascular A2BAR signaling as central control point of hypoxia-associated vascular leak.
Introduction
Previous studies have implicated extracellular nucleotide metabolites, predominantly adenosine, as triggers of endogenous protective mechanisms in a number of acute injury models.1,,,,,–7 Extracellular adenosine is derived primarily through phosphohydrolysis of adenosine 5′-monophosphate (AMP). Ecto-5′-nucleotidase (CD73), a ubiquitously expressed ectoenzyme, is the pacemaker of this reaction.8 Studies on the role of CD73 in tissue-injury showed that cd73−/− mice develop profound vascular leakage and pulmonary edema upon hypoxia exposure.8 Once generated into the extracellular space, adenosine can signal through any of 4 G-protein coupled adenosine-receptors (ARs: A1AR/A2AAR/A2BAR/A3AR). All of these receptors are expressed on vascular endothelia9 and have been implicated in tissue-protection in different models of injury.1,–3,7,10,,,,,,,–18
Changes in vascular barrier function closely coincide with tissue injury of many etiologies, and result in fluid loss, edema, and organ dysfunction.19,–21 The predominant barrier (∼90%) to movement of macromolecules across a blood vessel wall is presented by the vascular endothelium.20,22 Under physiologic conditions, macromolecules such as albumin (molecular weight ∼70 kD) can cross the endothelial monolayer via a paracellular route (eg, by passing between adjacent endothelia) with some contribution of transcellular passage.23,24 Endothelial barrier function correlates inversely with the size of molecules that can gain entry into tissues and differs between tissues of different origins. Endothelial permeability is highly regulated and may increase markedly upon exposure to inflammatory stimuli (eg, lipopolysaccharide, bacteria, bacterial compounds, prostaglandins, reactive oxygen species, leukotrienes) or adverse conditions such as ischemia or hypoxia.18,20,25,,,–29
Given that activation of ARs can lead to an elevation of intracellular cAMP, and that elevated cAMP in endothelia promotes barrier function,20,30 we considered the possibility of endothelial AR-signaling to regulate vascular permeability. In contrast to previous studies that found tissue protection during hypoxia or inflammation through signaling pathways involving the A2AAR,1,3,7,31,32 the present studies conclude that the A2BAR is central to the control of vascular leak in hypoxia.
Methods
Cell culture
Human microvascular endothelial cells (HMEC)-1 were cultured as described previously.9,18 For preparation of experimental HMEC-1 monolayers, confluent endothelial cells were seeded at approximately less than 105 cells/cm2 onto either permeable polycarbonate inserts or 100-mm Petri dishes. Endothelial cell purity was assessed by phase microscopic “cobblestone” appearance and uptake of fluorescent acetylated low-density lipoprotein.
Stable repression of AR expression by siRNA
With the help of the siRNA Wizard (www.sirnawizard.com; InvivoGen, San Diego, CA) the following primer sequences were chosen within the coding region of the gene of interest. A1AR: 5′-ACC TCG AAG AGA GGC CTG ATG ACT AGT CAA GAG CTA GTC ATC AGC CCT CTC TTC TT-3′and 5′-CAA AAA GAA GAG AGC CCT GAT GAC TAG CTC TTG ACT AGT CAT CAG GCC TCT CTT CG-3′; A2AAR: 5′-ACC TCG GAT CAA GGA TAG GGA GTT GTT CAA GAG ACA ACT CCC TAT CCT TGA TCC TT-3′and 5′-CAA AAA GGA TCA AGG ATA GGG AGT TGT CTC TTG AAC AAC TCC CTA TCC TTG ATC CG-3′; A2BAR: 5′-ACC TCA ACC GAG ACT TCC GCT ACA CTT CAA GAG AGT GTA GCG GAA GTC TCG GTT TT-3′and 5′-CAA AAA AAC CGA GAC TTC CGC TAC ACT CTC TTG AAG TGT AGC GGA AGT CTC GGT TG-3′; A3AR: 5′-ACC TCG CGT CAA CTC GTG CAA GAA CTT CAA GAG AGT TCT TGC ACG AGT TGA CGC TT-3′ and 5′-CAA AAA GCG TCA ACT CGT GCA AGA ACT CTC TTG AAG TTC TTG CAC GAG TTG ACG CG-3′. Primers were annealed for 2 minutes at 80°C to create the hairpin structure and ligated into the Bbs1/Bbs1 digested psiRNA-hH1neo G2 vector. After transformation using the Lyocomp GT116 Escherichia coli strain, cells were spread on a KanXgal agar plate with the advantage of white/blue selection. A recombinant white clone was grown, DNA was extracted and HMEC-1 cells were transfected using an electroporation procedure. Two days after transfection, cells were selected with G418 (1 mg/mL) and stable transfectants were individualized after 2 to 3 weeks. The control cell line was transfected with an empty psiRNA-hH1 neoscr plasmid. Successful siRNA repression was confirmed by realtime reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis as described previously.9,33
Macromolecule paracellular permeability assay
Using a modification of methods previously described,9,18,34 HMEC-1 were grown on polycarbonate permeable supports (0.4-μm pore, 6.5-mm diam; Corning Life Sciences, Acton, MA) and studied 7 to 10 days after seeding (2–5 days after confluence). Inserts were placed in Hank basic salt solution (HBSS)-containing wells (0.9 mL), and HBSS (alone or with indicated concentrations of adenosine, with and without 1 μM PSB1115 [Tocris Cookson, Bristol, United Kingdom] or indicated concentrations of BAY 60-6583 [Bayer HealthCare, Leverkuden, Germany] was added to inserts (100 μL). At the start of the assay (t = 0), 3.5 μM fluorescein isothiocyanate (FITC)-labeled 70 kD dextran was added to fluid within the insert. The size of FITC-dextran, 70 kD, approximates that of human albumin, both of which have been used in similar endothelial paracellular permeability models. Fluid from the reservoir was sampled (50 μL) over 60 minutes (t = 20, 40, and 60 minutes). Fluorescence intensity of each sample was measured (excitation, 485 nm; emission, 530 nm; Cytofluor 2300; Millipore, Billerica, MA); and FITC-dextran concentrations were determined from standard curves generated by serial dilution of FITC-dextran. Paracellular flux was calculated by linear regression of sample fluorescence.
In vivo hypoxia model
Previously characterized mice gene-targeted for the A1AR,35 A2AAR,36 or A3AR,13 and corresponding littermate controls were matched according to sex, age, and weight. A2BAR-deficient mice were generated by Deltagen (San Carlos, CA) by replacing a 112-bp fragment (from base 156 to base 267) from the 1076-bp protein-coding region of the A2BAR gene with a 6.9-kb IRES-lacZ reporter and neomycin resistance cassette. The targeted A2BAR mutation was generated in strain 129/OlaHsd-derived embryonic stem cells. The chimeric mice were bred to C57BL/6N mice to generate F1 heterozygous animals. These mice were backcrossed to C57BL/6N (The Jackson Laboratory, Bar Harbor, ME) for more than 12 generations to generate congenic C57BL/6N strain A2BAR mutant mice. Heterozygotes were then intercrossed to generate wildtype, heterozygous, and homozygous mutant progeny. Characterization and validation was performed by Deltagen and in our laboratory in Tübingen as described previously.16 In pharmacologic studies, age-, weight- and sex-matched C57BL/6 mice were used. Total organ vascular permeability was quantified by intravascular administration of Evan blue as described previously.8,9,18 In additional studies, mice were injected with PSB1115 (10 mg/kg intraperitoneally) or BAY 60-6583 (80 mg/kg intraperitoneally) or vehicle. For the purpose of quantifying vascular permeability, 0.2 mL of Evan blue (0.5% in phosphate-buffered saline [PBS]) were injected intravenously. Animals were exposed to normobaric hypoxia (8% O2, 92% N2) or room temperature air for 4 hours (n = 6 animals per condition). After hypoxia/normoxia exposure, the animals were killed and the heart, colon, kidney, lung, spleen, brain, muscle, and liver were harvested. Organ Evan blue concentrations were quantified after formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Pulmonary edema was assessed in additional experiments. For this purpose, lungs of the animals (n = 6) were collected, weighed, and dried by speed-vac (Eppendorf Vacufuge). Weight differences before and after drying were used to calculate lung water content. For the purpose of quantifying prime neutrophil (PMN) tissue concentrations, the animals were exposed to normobaric hypoxia (8% O2, 92% N2) or room temperature air for 4 hours (n = 6 animals per condition). After hypoxia/normoxia exposure, the animals were killed, organs were harvested, and the PMN marker myeloperoxidase (MPO) was quantified as previously described.18,37 This protocol was in accordance with National Institutes of Health guidelines for use of live animals and was approved by the Institutional Animal Care and Use Committee at University of Colorado, Health Science Center in Denver.
Immunohistochemistry
To examine the influence of hypoxia on vascular A2BAR expression in vivo, immunohistochemistry was performed on lungs from C57BL/6J mice subjected to normoxia or hypoxia as detailed above. Mice were killed, and whole lungs were fixed with 10% formalin at total lung capacity and subsequently stained with A2BAR rabbit polyclonal antibody (epitope corresponding to amino acids 293–332 mapping at the C-terminus, Santa Cruz Biotechnology, Santa Cruz, California) as described previously.16,17,38 In controls, normal rabbit IgG was used at identical concentrations and staining conditions as the target primary antibody (Ab).
Isolation of hematopoietic cells and RT-PCR to assess A2BAR mRNA expression
Bone marrow was flushed from the tibia and femurs of wild-type mice and sorted into lymphocytes, macrophages, and neutrophils based on the typical light scattering properties of these 3 populations using a FACSAria (Becton Dickinson, Mountain View, CA). Each sorted population was more than 95% free of the other 2 populations of interest. RNA was isolated from the 3 populations using TriReagent (Molecular Research Center, Cincinnati, OH) and cDNA was synthesized using SuperScript III First-Strand (Invitrogen, Carlsbad, CA). Semiquantitative RT-PCR was performed using 3 serial 5-fold dilutions of cDNA and previously described primers specific for the murine A2BAR and β-actin.39 The results are expressed as the means plus or minus the SEM of the ratio of A2BAR mRNA to β-actin mRNA for 3 determinations. The PCR cycling conditions were 94°C for 5 minutes, followed by 40 cycles of 94°C for 45 seconds, 52°C (A2BAR) or 56°C (β-actin) for 45 seconds, and 72°C for 90 seconds.
Generation of A2BAR bone marrow chimeric mice
This animal protocol was in accordance with the German guidelines for use of living animals and were approved by the Institutional Animal Care and Use Committee of the Tübingen University Hospital and the Regierungspräsidium Tübingen. To define the contribution of vascular or hematopoietic cell A2BAR to vascular permeability during hypoxia, we generated bone marrow chimeric mice in which bone marrow was ablated by radiation in WT mice (C57BL/6) followed by reconstitution with bone marrow derived from previously characterized mice gene-targeted for the A2BAR16 and vice versa (A2BAR−/− → A2BAR+/+, and A2BAR+/+ → A2BAR−/−). Experiments with A2BAR+/+→ A2BAR+/+ and A2BAR−/− → A2BAR−/− mice served as controls. In short, male donor mice (8–10 weeks old, 20–25 g) were killed and the marrow from the tibia and femur were harvested by flushing the marrow cavity with sterile isotonic sodium chloride solution. The bone marrow cells were then centrifuged at 400g for 5 minutes, and resuspended and counted. Recipient mice (8–10 weeks of age, 20–25 g) were irradiated with a total dose of 12 Gy from a 137Cs source. Immediately after irradiation, 107 BM cells/recipient were injected in0.3 mL 0.9% sodium chloride into the jugular vein. The resulting chimeric mice were housed in microisolators for at least 8 weeks before experimentation and fed with water containing tetracycline (100 mg/L) in the first 2 weeks after BM transplantation. Preliminary experiments using the same conditioning regimen and transplanting CD45.1+ bone marrow into irradiated CD45.1− mice resulted in more than 95% chimerism in B cells, neutrophils, and monocytic cells, and approximately 85% chimerism in CD4+ and CD8+ T cells of recipient mice (data not shown).
Data analysis
Data were compared by 2-factor ANOVA, or by Student t test where appropriate. Values were expressed as the means plus or minus the SD from 6 animals per condition.
Results
A2BAR attenuates hypoxia-associated endothelial leakage in vitro
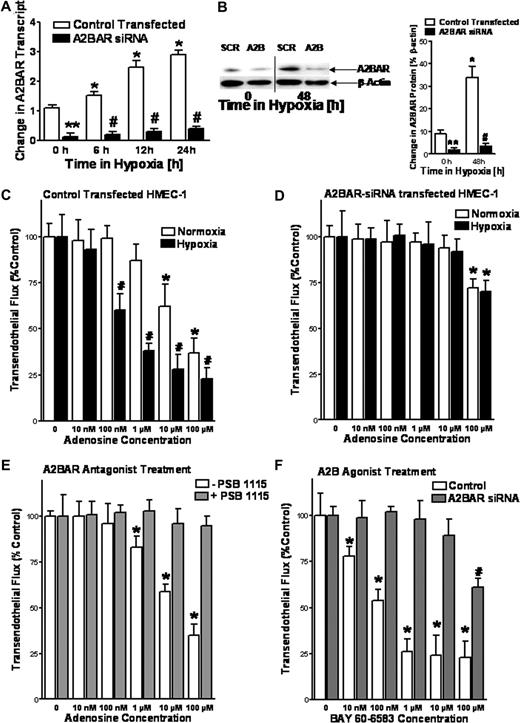
Previous studies have shown a central role of extracellular adenosine in vascular responses.3,8,9,12,15,18,33,37 Based on these studies, we hypothesized a contribution of extracellular adenosine signaling for the preservation of endothelial barrier during hypoxia in vitro. To identify which adenosine receptor is responsible for regulating vascular permeability, we used a previously characterized in vitro model of endothelial barrier function.9,18,34 Here, we performed siRNA-mediated stable repression of individual ARs and studied endothelial barrier function in normoxic or posthypoxic endothelia (HMEC-1 cells, 48 hours at 2% oxygen concentration). As shown for the A2BAR, transcript and protein levels were less than 20% of control-transfected cells and the induction of A2BR expression that normally occurs as a result of hypoxia9,33 was largely abolished (Figure 1A,B). Consistent with previous in vitro studies of endothelial barrier function,9 the addition of adenosine to control-transfected HMEC-1 resulted in a dose-dependent reduction of paracellular flux rates (Figure 1C). These responses were enhanced in posthypoxic endothelia (Figure 1C). In contrast, adenosine effects on endothelial barrier function were almost completely abolished after siRNA-mediated repression of the A2BAR (Figure 1D). Moreover, siRNA repression of the A1AR, A2AAR, or A3AR did not alter endothelial barrier responses to adenosine (data not shown). Similar to siRNA repression, pretreatment of endothelia with the selective A2BAR antagonist PSB1115 (1 μM) effectively blocked adenosine-elicited barrier responses (Figure 1E). Moreover, treatment of control-transfected HMEC-1 with a specific A2BAR agonist (BAY 60-6583)16 was associated with dose-dependent reduction in paracellular flux rates, while BAY 60-6583 effects on endothelial barrier function were almost completely abolished after siRNA-repression of the A2BAR (Figure 1F). Taken together, these data suggest the A2BAR is responsible for adenosine-mediated changes in endothelial barrier responses during hypoxia in vitro.
A2B adenosine receptor (A2BAR) on HMEC-1 cells promotes endothelial barrier function in vitro. Inhibition of A2BAR mRNA expression by stable expression of A2BAR siRNA. HMEC-1 cells were stably transfected with a plasmid expressing A2BAR siRNA or a nonspecific siRNA (Control Transfected, SCR). Cells were exposed to hypoxia (pO2 = 20 torr) and real-time RT-PCR was used to confirm repression of the A2BAR (A) Data were calculated relative to β-actin and are expressed as fold change over control plus or minus SD after indicated time periods of hypoxia exposure. Results are derived from 3 experiments (*P < .05, different from normoxia; #P < .01, compared with normoxia and control transected; **P < .01 different from control transfected). Western blot analysis was used to confirm siRNA repression of the A2BAR protein (B). A representative blot of 3 is shown (a vertical line has been inserted to indicate a repositioned gel lane.), in addition to densitometric analysis of A2BAR protein levels relative to β-actin (*P < .01, different from normoxia; #P < .05, compared with normoxia and control transected; **P < .05 different from control transfected, n = 3). Repression of A2BAR expression inhibits adenosine-mediated enhancement of endothelial barrier function. Indicated concentrations of adenosine in HBSS were added to the apical surface of confluent normoxic (48-hour exposure to pO2 = 147 torr) or hypoxic (48-hour exposure to pO2 = 20 torr) HMEC-1 that were stably transfected with A2BAR (D) or control (C) siRNA. Permeability to FITC-dextran (70 kDa) was quantified by measuring transendothelial flux (3 samples over 60 minutes) and normalized as a percentage of control (HBSS). Baseline flux rates for normoxic conditions where approximately 200 to 250 pM/min per cm2 and 800 to 1000 pM/min per cm2 after hypoxia exposure (less than 5% of tracer). Data are derived from 6 monolayers in each condition (*P < .05, compared with baseline; #P < .05, compared with baseline and normoxia). (E) The A2BAR antagonist PSB1115 inhibits adenosine-mediated enhancement of endothelial barrier function. Adenosine elicited barrier responses were measured in normoxic endothelia (HMEC-1) with or without the addition of 1 μM PSB1115 (*P < .05, compared with baseline). (F) The A2BAR agonist BAY 60-6583 promotes barrier responses. Hypoxic endothelia (48-hour exposure to pO2 = 20 torr) that were stably transfected with A2BAR or control siRNA were assessed for BAY 60-6583–elicited barrier responses. Data are expressed as means plus or minus SD (*P < .05, compared with baseline. #P < .05, compared with baseline and untreated controls, n = 6).
A2B adenosine receptor (A2BAR) on HMEC-1 cells promotes endothelial barrier function in vitro. Inhibition of A2BAR mRNA expression by stable expression of A2BAR siRNA. HMEC-1 cells were stably transfected with a plasmid expressing A2BAR siRNA or a nonspecific siRNA (Control Transfected, SCR). Cells were exposed to hypoxia (pO2 = 20 torr) and real-time RT-PCR was used to confirm repression of the A2BAR (A) Data were calculated relative to β-actin and are expressed as fold change over control plus or minus SD after indicated time periods of hypoxia exposure. Results are derived from 3 experiments (*P < .05, different from normoxia; #P < .01, compared with normoxia and control transected; **P < .01 different from control transfected). Western blot analysis was used to confirm siRNA repression of the A2BAR protein (B). A representative blot of 3 is shown (a vertical line has been inserted to indicate a repositioned gel lane.), in addition to densitometric analysis of A2BAR protein levels relative to β-actin (*P < .01, different from normoxia; #P < .05, compared with normoxia and control transected; **P < .05 different from control transfected, n = 3). Repression of A2BAR expression inhibits adenosine-mediated enhancement of endothelial barrier function. Indicated concentrations of adenosine in HBSS were added to the apical surface of confluent normoxic (48-hour exposure to pO2 = 147 torr) or hypoxic (48-hour exposure to pO2 = 20 torr) HMEC-1 that were stably transfected with A2BAR (D) or control (C) siRNA. Permeability to FITC-dextran (70 kDa) was quantified by measuring transendothelial flux (3 samples over 60 minutes) and normalized as a percentage of control (HBSS). Baseline flux rates for normoxic conditions where approximately 200 to 250 pM/min per cm2 and 800 to 1000 pM/min per cm2 after hypoxia exposure (less than 5% of tracer). Data are derived from 6 monolayers in each condition (*P < .05, compared with baseline; #P < .05, compared with baseline and normoxia). (E) The A2BAR antagonist PSB1115 inhibits adenosine-mediated enhancement of endothelial barrier function. Adenosine elicited barrier responses were measured in normoxic endothelia (HMEC-1) with or without the addition of 1 μM PSB1115 (*P < .05, compared with baseline). (F) The A2BAR agonist BAY 60-6583 promotes barrier responses. Hypoxic endothelia (48-hour exposure to pO2 = 20 torr) that were stably transfected with A2BAR or control siRNA were assessed for BAY 60-6583–elicited barrier responses. Data are expressed as means plus or minus SD (*P < .05, compared with baseline. #P < .05, compared with baseline and untreated controls, n = 6).
Vascular leakage of AR−/− mice during hypoxia
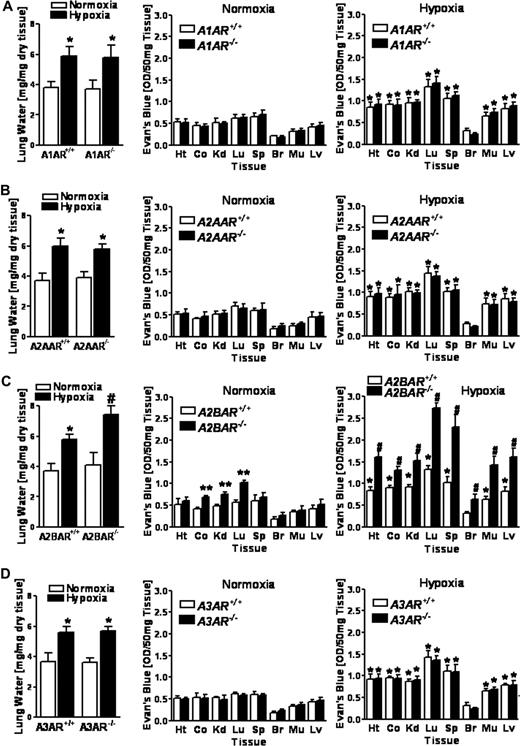
Based on the above in vitro studies indicating that extracellular adenosine is critical for control of hypoxia-induced endothelial leak, we hypothesized that AR deficiency would significantly influence vascular leakage in vivo. To this end, we exposed previously characterized mice gene-targeted for the A1AR,35 A2AAR,36 A2BAR,16 or A3AR13 to normobaric hypoxia as a stimulus for increases in lung water and vascular leakage of multiple organs (4 hours of 8% oxygen).8,9,18 During the initial period of hypoxia (∼30 minutes), A2BAR−/− mice were more lethargic and less compliant than their wild-type littermates or mice gene-targeted for the A1AR, A2AAR, or A3AR. Nonetheless, all AR−/− and corresponding littermate controls recovered and survived the 4-hour period of hypoxia. While hypoxia was associated with a similar degree of lung water increases in gene-targeted mice for A1AR, A2AAR, or A3AR or their corresponding littermates (Figure 2A,B,D), this increase was dramatically enhanced in A2BAR−/− mice (Figure 2C). Moreover, comparative analysis of basal permeability (ie, in normoxia) as assessed by Evan blue dye extravasation revealed that in 3 of 8 organs studied (colon, kidney, and lung), vascular leakage was significantly increased in A2BAR−/− mice (Figure 2C, P < .05) compared with wild-type littermate controls. Normobaric hypoxia increased leak in highly vascular organs of A1AR−/−, A2AAR−/−, or A3AR−/− mice to a similar degree as in corresponding littermate controls (Figure 2A,B,D, P < .05 by ANOVA). In contrast, hypoxia-induced vascular leak was significantly enhanced in all examined organs of A2BAR−/− mice compared with that in wild-type littermate controls (Figure 2C, P < .05 by ANOVA). Changes in overall vascular leakage (Evan blue dye extravasation) between A2BAR+/+ and A2BAR−/− mice under both normoxic and hypoxic conditions were also evident in open abdominal images taken at necropsy (Figure 3A). These findings identify signaling through the A2BAR as a critical control point for permeability changes associated with adenine nucleotide metabolism, particularly in the posthypoxic vasculature.
Vascular permeability in A1, A2A, A2B and A3 adenosine receptor (AR)-deficient mice during hypoxia in vivo. (A) A1AR−/−, (B) A2AAR−/−, (C) A2BAR−/−, or (D) A3AR−/− mice and age-, weight-, and sex-matched littermate controls were exposed to room air (▭) or normobaric hypoxia ( , 8% O2, 92% N2) for 4 hours and lung water content was measured. In additional studies, mice were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) prior to hypoxia or normoxia exposure. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with littermate controls; and #P < .05, compared with littermate controls and normoxia).
, 8% O2, 92% N2) for 4 hours and lung water content was measured. In additional studies, mice were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) prior to hypoxia or normoxia exposure. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with littermate controls; and #P < .05, compared with littermate controls and normoxia).
Vascular permeability in A1, A2A, A2B and A3 adenosine receptor (AR)-deficient mice during hypoxia in vivo. (A) A1AR−/−, (B) A2AAR−/−, (C) A2BAR−/−, or (D) A3AR−/− mice and age-, weight-, and sex-matched littermate controls were exposed to room air (▭) or normobaric hypoxia ( , 8% O2, 92% N2) for 4 hours and lung water content was measured. In additional studies, mice were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) prior to hypoxia or normoxia exposure. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with littermate controls; and #P < .05, compared with littermate controls and normoxia).
, 8% O2, 92% N2) for 4 hours and lung water content was measured. In additional studies, mice were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) prior to hypoxia or normoxia exposure. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with littermate controls; and #P < .05, compared with littermate controls and normoxia).
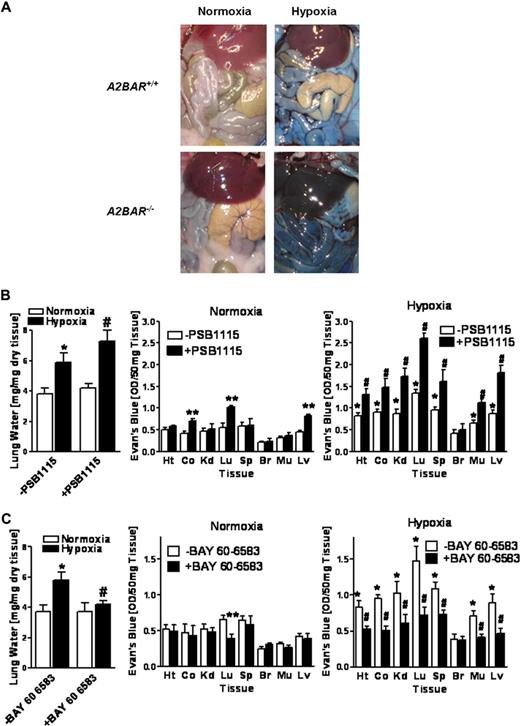
A2BAR and vascular permeability during hypoxia in vivo. (A) A2BAR−/− mice or age-, weight-, and sex-matched littermate controls were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) and exposed to room temperature air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Representative images of abdominal dissections are shown. Images were obtained using a Canon Power Shot G9 digital camera (Canon, Krefeld, Germany). (B) Age-, weight-, and sex-matched wild type mice were administered a selective A2BAR antagonist (PSB1115, 20 mg/kg intraperitoneally) or an equal volume of PBS and lung water content was measured. In other experiments, mice were injected with intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) after PBS1115 or vehicle treatment and exposed to room air or to normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with normoxic −PSB1115 controls; and #P < .05, compared with normoxia and −PSB115 controls). (C) Age-, weight-, and sex-matched wild type mice were administered a selective A2BAR agonist (BAY 60-6583, 80 μg/kg.) or an equal volume of PBS and lung water content was measured. In other experiments, mice were injected with intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) after BAY 60-6583 or vehicle treatment and exposed to room air or to normobaric hypoxia (8% O2, 92% N2) for 4 hours. Data are expressed as means plus or minus SD of Evans blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with −BAY 60-6583 normoxic controls and #P < .01, compared with −BAY 60-6583 hypoxic controls).
A2BAR and vascular permeability during hypoxia in vivo. (A) A2BAR−/− mice or age-, weight-, and sex-matched littermate controls were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) and exposed to room temperature air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Representative images of abdominal dissections are shown. Images were obtained using a Canon Power Shot G9 digital camera (Canon, Krefeld, Germany). (B) Age-, weight-, and sex-matched wild type mice were administered a selective A2BAR antagonist (PSB1115, 20 mg/kg intraperitoneally) or an equal volume of PBS and lung water content was measured. In other experiments, mice were injected with intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) after PBS1115 or vehicle treatment and exposed to room air or to normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue dye concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evan blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with normoxic −PSB1115 controls; and #P < .05, compared with normoxia and −PSB115 controls). (C) Age-, weight-, and sex-matched wild type mice were administered a selective A2BAR agonist (BAY 60-6583, 80 μg/kg.) or an equal volume of PBS and lung water content was measured. In other experiments, mice were injected with intravenous Evan blue dye (0.2 mL of 0.5% in PBS per mouse) after BAY 60-6583 or vehicle treatment and exposed to room air or to normobaric hypoxia (8% O2, 92% N2) for 4 hours. Data are expressed as means plus or minus SD of Evans blue OD/50 mg wet tissue; n = 6 animals/condition (*P < .05, compared with normoxia; **P < .05, compared with −BAY 60-6583 normoxic controls and #P < .01, compared with −BAY 60-6583 hypoxic controls).
Influence of A2BAR antagonist (PSB1115) and agonist (BAY 60-6583) on vascular leak during hypoxia
After having shown that A2BAR−/− mice exhibit profound vascular leakage upon exposure to ambient hypoxia, we next sought to confirm these findings using pharmacologic approaches. Here, we first tested the highly selective A2BAR inhibitor PSB1115 (10 mg/kg intraperitoneally)16 on pulmonary edema and Evan blue accumulation in vivo in multiple organs from wild-type mice subjected to normoxia or normobaric hypoxia (8% O2, 92% N2) for 4 hours. As shown in Figure 3B, lung water content was significantly increased in hypoxic mice treated with PSB1115 compared with vehicle control (P < .05). Moreover, permeability of the lung, liver, and colonic vasculature to Evan blue dye was increased in normoxic mice pretreated with PSB1115 (P < .05 compared with vehicle controls), suggesting that signaling through the A2BAR contributes to basal barrier function in these organs. In addition, PSB1115 also increased vascular leakage in all examined organs of mice subjected to normobaric hypoxia (Figure 3B, P < .05 by ANOVA compared with vehicle controls) with the notable exception of the brain. Next, we treated mice with a previously described highly selective agonist of the A2BAR (BAY 60-6583, 80 μg/kg intraperitoneally).16 As shown in Figure 3C, pretreatment with BAY 60-6583 significantly attenuated hypoxia-induced pulmonary edema (P < .05). Moreover, pulmonary permeability was decreased in normoxic mice and hypoxia-induced increases in vascular leakage of all examined organs (except the brain) were almost completely reversed to normoxic levels (Figure 3C, P < .05). The fact that neither A2BAR antagonist PSB1115 nor A2BAR agonist BAY 60-6583 had any effects on vascular permeability in the brain may be related to the special anatomic structure and regulation of the blood brain barrier.40 Taken together, these data suggest the A2BAR as therapeutic target in hypoxia-associated vascular leakage.
The vascular A2BAR is induced by ambient hypoxia in vivo
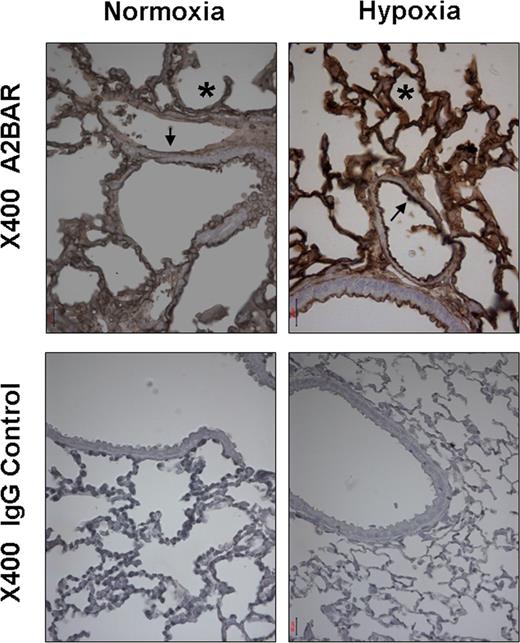
Previous studies have shown endothelial or vascular expression of the A2BAR. For example, a study by Yang et al using an A2BAR reporter mouse model found highest levels of expression of the A2BAR in the vasculature of multiple organs.12 To confirm previous in vitro studies showing induction of the vascular A2BAR in vitro,9,33 we next examined expression of the pulmonary A2BAR during normoxia or hypoxia in vivo. Immunohistochemistry revealed A2BAR on murine pulmonary vasculature. In addition, hypoxia exposure was associated with a robust induction of the vascular pulmonary A2BAR (Figure 4), consistent with results obtained with HMEC-1 cells (Figure 1A,B).
Pulmonary A2BAR expression increases after exposure to hypoxia in vivo. Wild-type mice were subjected to normoxia or 4 hours of ambient hypoxia (8% oxygen). Whole lungs were fixed with 10% formalin at total lung capacity. Lungs were stained with antibody for the A2BAR. Normal goat IgG was used in controls at identical concentration and staining conditions as the target primary Ab (arrow marks vascular, asterisk alveolar structures). Evaluation of the histologic and immunohistochemical stainings and photographic documentation was performed using an Olympus Vanox AH-3 light microscope (Hamburg, Germany), original magnification, ×400.
Pulmonary A2BAR expression increases after exposure to hypoxia in vivo. Wild-type mice were subjected to normoxia or 4 hours of ambient hypoxia (8% oxygen). Whole lungs were fixed with 10% formalin at total lung capacity. Lungs were stained with antibody for the A2BAR. Normal goat IgG was used in controls at identical concentration and staining conditions as the target primary Ab (arrow marks vascular, asterisk alveolar structures). Evaluation of the histologic and immunohistochemical stainings and photographic documentation was performed using an Olympus Vanox AH-3 light microscope (Hamburg, Germany), original magnification, ×400.
A2BAR expression on hematopoietic cells
Based on previous studies showing A2BAR expression on hematopoietic cells,12 we next screened the 3 major populations of hematopoietic cells in murine bone marrow, lymphocytes, macrophages, and neutrophils, for A2BAR expression by semiquantitative RT-PCR. As shown in Table 1, all 3 populations were positive for A2BAR mRNA expression, with macrophages having the highest level of expression. These results are consistent with recent studies reported by Yang et al12 using a reporter mouse model for the A2BAR.
A2BAR mRNA expression in hematopoietic cell populations
| Population . | A2BAR mRNA/β-actin mRNA . |
|---|---|
| Macrophages | 0.099 ± 0.017 |
| Neutrophils | 0.023 ± 0.008 |
| Lymphocytes | 0.024 ± 0.007 |
| Population . | A2BAR mRNA/β-actin mRNA . |
|---|---|
| Macrophages | 0.099 ± 0.017 |
| Neutrophils | 0.023 ± 0.008 |
| Lymphocytes | 0.024 ± 0.007 |
mRNA was isolated from sorted populations of bone marrow macrophages, neutrophils, and lymphocytes and converted into cDNA. Semiquantitative RT-PCR was performed with primers specific for the A2BAR and β-actin using three 5-fold serial dilutions of cDNA. The data are expressed as the mean plus or minus SEM of the ratio of A2BAR mRNA/β-actin mRNA for three determinations.
Vascular permeability in A2BAR bone marrow chimeric mice
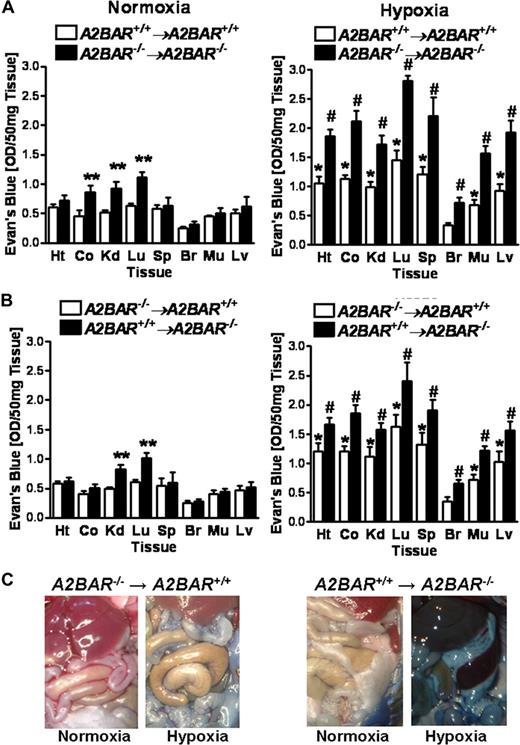
Based on our above studies showing vascular and hematopoietic cell A2BAR expression, we next pursued functional studies of ambient hypoxia in A2BAR bone marrow chimeric mice. As expected, A2BAR+/+→A2BAR+/+ chimeric mice showed a similar degree of hypoxia-associated increases in vascular leakage as wild type mice. Similarly, vascular leak was significantly enhanced at baseline in the colon, kidney, and lungs (Figure 5A, P < .05) and in all examined organs upon hypoxia exposure (Figure 5A, P < .05) in A2BAR−/−→A2BAR−/− mice. While baseline permeability in the kidneys and lungs was increased in A2BAR+/+→A2BAR−/− chimeric mice, colon permeability was not increased, suggesting a component of myeloid A2BAR signaling in baseline barrier regulation of the colon. Interestingly, A2BAR−/−→A2BAR+/+ chimeric mice showed a similar degree of hypoxia-associated vascular leakage as A2BAR+/+ mice (Figure 5B). In contrast, A2BAR+/+→A2BAR−/− chimeric mice showed increased hypoxia-induced vascular leakage, very similar to A2BAR−/− mice (Figure 5B). This was also evident in open abdominal images taken at necropsy (Figure 5C). Taken together, these studies suggest a critical role of vascular (ie, nonhematopoietic) A2BARs in attenuating hypoxia-induced vascular leakage.
Vascular permeability in A2BAR bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR+/+→A2BAR+/+ and A2BAR−/−→A2BAR−/−; B: A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/−) were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) and exposed to room air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evan blue OD/50 mg wet tissue (n = 6 animals/condition). (A: *P < .05, compared with normoxia; **P < .05, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice; and #P < .05, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice and normoxia. B: *P < .05, compared with normoxia; **P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice; and #P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice and normoxia). (C) Representative images of abdominal dissections are shown from A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/− bone marrow chimeric mice. Images were obtained using a Canon Power Shot G9 digital camera (Canon).
Vascular permeability in A2BAR bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR+/+→A2BAR+/+ and A2BAR−/−→A2BAR−/−; B: A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/−) were administered intravenous Evan blue dye (0.2 mL of 0.5% in PBS) and exposed to room air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver were harvested. Organ Evan blue concentrations were quantified as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evan blue OD/50 mg wet tissue (n = 6 animals/condition). (A: *P < .05, compared with normoxia; **P < .05, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice; and #P < .05, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice and normoxia. B: *P < .05, compared with normoxia; **P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice; and #P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice and normoxia). (C) Representative images of abdominal dissections are shown from A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/− bone marrow chimeric mice. Images were obtained using a Canon Power Shot G9 digital camera (Canon).
Barrier protection of the A2BAR agonist BAY 60-6853 requires vascular A2BARs
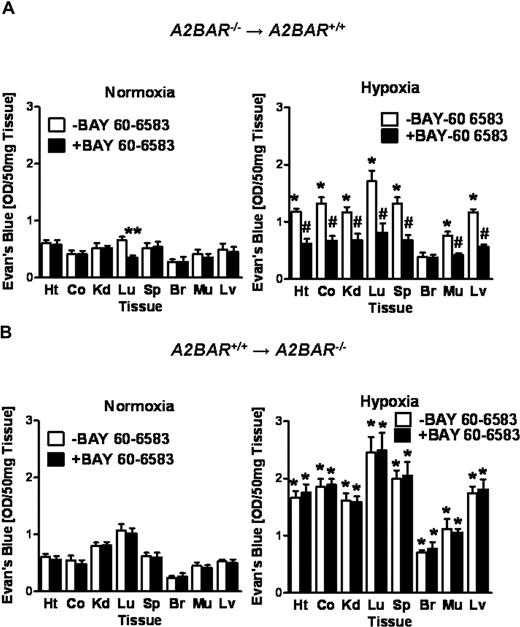
To further understand the contribution of vascular or hematopoietic A2BARs to barrier protection during hypoxia-induced vascular leak, we next performed ambient hypoxia studies in A2BAR bone-marrow chimeric mice pretreated with the selective A2BAR agonist BAY 60-6853. Bone marrow chimeric A2BAR mice expressing the A2BAR on the tissues but not on their hematopoeitic cells (A2BAR−/−→ A2BAR+/+ mice) showed reduced tissue Evan blue dye accumulation at baseline in the lungs after BAY 60-6583 pretreatment (80 μg/kg intraperitoneally). Moreover, and similar to A2BAR+/+ mice, BAY 60-6583 pretreatment resulted in a profound reduction of hypoxia-associated vascular leakage in all examined organs except the brain (Figure 6A, P < .05). In contrast, BAY 60-6583 pretreatment of A2BAR bone marrow chimeric mice with the A2BAR expressed exclusively on hematopoietic cells (A2BAR+/+→ A2BAR−/− mice) showed no reduction in Evan blue dye tissue accumulation (Figure 6B). Taken together, these data suggest that signaling through the vascular A2BAR is critical for attenuating hypoxia associated vascular leakage in vivo.
Influence of the A2BAR agonist (BAY 60-6583) on vascular permeability in bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR−/−→A2BAR+/+; B: A2BAR+/+→A2BAR−/−) were treated with the selective A2BAR agonist (BAY 60-6583, 80 μg/kg intraperitoneally) or an equal volume of PBS 30 minutes before intravenous Evan blue dye (0.2 mL of 0.5% in PBS) and exposed to room air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver (Lv) were harvested. Organ Evan blue dye concentrations were quantified after formamide extraction (55°C for 2 hours) as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evans blue OD/50 mg wet tissue (n = 6 animals/condition). (A: *P < .05, compared with normoxia; **P < .05, compared with −BAY 60-6583 control; and #P < .05, compared with −BAY 60-6583 control and normoxia; B: *P < .05, compared with normoxia).
Influence of the A2BAR agonist (BAY 60-6583) on vascular permeability in bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR−/−→A2BAR+/+; B: A2BAR+/+→A2BAR−/−) were treated with the selective A2BAR agonist (BAY 60-6583, 80 μg/kg intraperitoneally) or an equal volume of PBS 30 minutes before intravenous Evan blue dye (0.2 mL of 0.5% in PBS) and exposed to room air or normobaric hypoxia (8% O2, 92% N2) for 4 hours. Animals were killed and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver (Lv) were harvested. Organ Evan blue dye concentrations were quantified after formamide extraction (55°C for 2 hours) as described in “In vivo hypoxia model.” Data are expressed as means plus or minus SD of Evans blue OD/50 mg wet tissue (n = 6 animals/condition). (A: *P < .05, compared with normoxia; **P < .05, compared with −BAY 60-6583 control; and #P < .05, compared with −BAY 60-6583 control and normoxia; B: *P < .05, compared with normoxia).
Hematopoeitic cell A2BAR signaling attenuates hypoxia-induced increases of neutrophil tissue accumulation in multiple organs
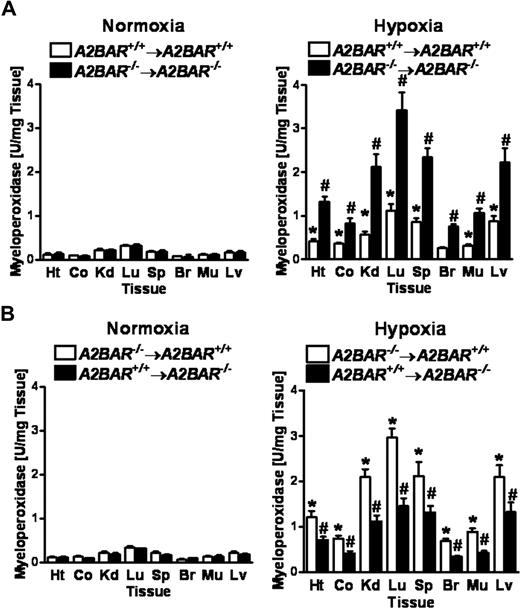
We and others have shown that hypoxia is a potent stimulus for tissue PMN accumulation in vivo.41,–43 Based on the above findings that signaling through vascular A2BARs attenuates hypoxia-induced vascular leak, we next studied the effects of A2BAR signaling on hypoxia-induced PMN tissue accumulation. Here, we again exposed bone marrow chimeric A2BAR mice to ambient hypoxia (4 hours of 8% oxygen) and multiple mucosal organs were screened for PMN accumulation by measuring the PMN marker myeloperoxidase (MPO) activity (Figure 7). As expected, hypoxia-associated PMN tissue numbers were increased in bone marrow chimeric A2BAR−/−→A2BAR−/− mice compared with A2BAR+/+→A2BAR+/+ mice (Figure 7A, P < .01), similar to A2BAR−/− mice (data not shown). Both bone marrow chimeric A2BAR−/−→A2BAR+/+ mice and A2BAR+/+→A2BAR−/− mice showed higher PMN numbers after hypoxia exposure. However, this increase was significantly more dramatic in A2BAR−/−→A2BAR+/+ mice (Figure 7B, P < .05). Taken together, these studies suggest that hypoxia-associated increases in PMN tissue accumulation are attenuated by A2BAR signaling events on both hematopoietic and nonhematopoeitic cells, with those on hematopoietic cells playing the dominant role, while hypoxia associated fluid leakage is dampened by vascular A2BARs.
Hypoxia-induced increases in PMN-tissue accumulation in A2BAR bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR+/+→A2BAR+/+ and A2BAR−/−→A2BAR−/−; B: A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/−) were subjected to either normoxia (room air) or normobaric hypoxia (8% oxygen) for 4 hours, and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver (Lv) were assessed for PMN accumulation by myeloperoxidase activity (A: *P < .01, compared with normoxia and #P < .01, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice and normoxia; B: *P < .01, compared with normoxia and #P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice and normoxia). Error bars represent SD.
Hypoxia-induced increases in PMN-tissue accumulation in A2BAR bone marrow chimeric mice.A2BAR bone marrow chimeric mice (A: A2BAR+/+→A2BAR+/+ and A2BAR−/−→A2BAR−/−; B: A2BAR−/−→A2BAR+/+ and A2BAR+/+→A2BAR−/−) were subjected to either normoxia (room air) or normobaric hypoxia (8% oxygen) for 4 hours, and the heart (Ht), colon (Co), kidney (Kd), lung (Lg), spleen (Sp), brain (Br), muscle (Mu) and liver (Lv) were assessed for PMN accumulation by myeloperoxidase activity (A: *P < .01, compared with normoxia and #P < .01, compared with A2BAR+/+→A2BAR+/+ bone marrow chimeric mice and normoxia; B: *P < .01, compared with normoxia and #P < .05, compared with A2BAR−/−→A2BAR+/+ bone marrow chimeric mice and normoxia). Error bars represent SD.
Discussion
Adenosine exerts autocrine and paracrine actions on most cell types. Adverse conditions like hypoxia or ischemia result in the elevation of extracellular adenosine,16,17,38,44,45 which has beneficial consequences including ischemic preconditioning16,38,44,,–47 and promotion of endothelial barrier function.8,9,18 In this study, our goal was to identify the specific adenosine receptor responsible for the maintenance of vascular barrier function during normoxia or hypoxia. Our results point toward a pivotal role of the A2BAR in attenuating hypoxia-induced increases in vascular leak. In contrast, mice gene-targeted for other ARs failed to show an influence on vascular barrier function during hypoxia, providing some degree of specificity for the A2BAR. Moreover, in vivo studies of A2BAR bone marrow chimeric mice suggest an important contribution of vascular A2BARs in attenuating vascular leakage during hypoxia. In contrast, attenuation of hypoxia-associated increases in tissue neutrophil numbers appeared to depend largely on hematopoietic cell A2BAR signaling.
Previous studies have shown that A2BARs are highly expressed in the vasculature.9,12,33,48 In fact, in vitro studies of posthypoxic endothelia revealed a selective induction of the A2BAR transcript, protein and function in human vascular endothelia (HMEC-1) upon hypoxia exposure.9 Other studies identified a previously unrecognized hypoxia-inducible-factor (HIF)-1 binding site in the promoter of the A2BAR.33 In fact, HIF-1α loss and gain of function studies suggested a critical role of HIF-1α in the transcriptional induction of the A2BAR during hypoxia. This is consistent with the present studies showing in vivo induction of the A2BAR during hypoxia. In addition, a very elegant study by Yang et al used an “A2BAR-knockout/reporter gene-knock-in” approach to study the role of the A2BAR in inflammatory models.12 The authors found highest levels of expression of the A2BAR in the vasculature and on macrophages.12 In addition, genetic deletion of the A2BAR was associated with low-grade vascular inflammation, augmentation of proinflammatory cytokines, such as TNFα, and a consequent down-regulation of IκBα in addition to up-regulation of adhesion molecules of the vasculature. Consistent with our studies of PMN accumulation in posthypoxic organs, leukocyte adhesion to the vasculature was significantly increased in mice gene-targeted for the A2BAR.12 Moreover, the authors showed that exposure to endotoxin resulted in augmented proinflammatory cytokine levels in gene-targeted mice. However, and in contrast to our observations of the importance of vascular A2BAR in attenuating hypoxia-induced increases in vascular leak, Yang et al concluded that bone marrow (and to a lesser extent vascular) A2BARs regulated the low-grade inflammation observed in their studies.12
The present study is consistent with previous work suggesting a role of extracellular adenosine in vascular barrier function. For example, studies of PMN-endothelial crosstalk revealed that PMN release adenosine-precursor molecules (particularly ATP),9,49 which are readily converted to adenosine on the endothelial surface and contribute to barrier protection in vitro.50 Moreover, murine studies using gene-targeted deletion of enzymes involved in extracellular adenosine generation (CD39, CD73) showed profound increases in vascular leakage upon hypoxia exposure.8,9 Moreover, pharmacologic approaches resulting in a prolonged action of extracellular adenosine, either by inhibition of adenosine uptake via nucleoside transporters18 or metabolism by adenosine deaminase,51 found enhanced extracellular adenosine signaling effects and protection of the vascular barrier function from hypoxia-induced vascular leakage in vivo.
Adenosine has been shown to have anti-inflammatory actions,7,11,15,32,52 to attenuate diabetes mellitus,53 to promote wound healing,54 and to participate in tissue protection, particularly during conditions of limited oxygen availability.3,11 An excellent study by Khoury et al used a microarray approach to investigate mechanisms of adenosine-mediated tissue protection in a model of pulmonary hypoxic preconditioning.55 They found that expression of a NF-κB–regulated gene cluster was attenuated by preconditioning.55 Further studies using an NF-κB reporter confirmed suppression of NF-κB activation by hypoxic preconditioning and identified the eliciting activity as adenosine. Guided by recent studies demonstrating bacterial inhibition of NF-κB activity through cullin-1 (Cul-1) deneddylation, the authors found a dose-dependent deneddylation of Cul-1 by A2BAR stimulation, thereby providing mechanistic insight into how A2BAR signaling attenuates inflammation and provides tissue protection during hypoxia.55
In contrast to the present studies, there is previous evidence pointing to a role for A2AAR in tissue protection from hypoxia.1,3,7,31,56 For example, mice deficient in the A2AAR showed increased inflammation-associated tissue damage, providing evidence for A2AAR signaling in the regulation of acute inflammatory responses in vivo.7 Moreover, the same group has provided convincing evidence that oxygenation inhibits the physiologic tissue-protecting mechanism via hypoxia-elicited A2AAR signaling, and thereby exacerbates acute inflammatory lung injury.32 Why these results differ from the present studies showing A2BAR signaling in hypoxia-associated vascular leakage are not clearly understood. However, some factors may be contributing. At first, hypoxia causes a very robust induction of the endothelial A2BAR via HIF-1 dependent pathways.9,33 This may lead to an endothelial phenotype where the A2BAR becomes the predominant adenosine receptor of the vasculature during hypoxia.48 Secondly, other studies on myocardial,11 renal57,58 or hepatic14 ischemia/hypoxia demonstrated tissue protection through the A2AAR on T lymphocytes. In conjunction with the present studies showing that vascular barrier resuscitation during hypoxia mainly involves vascular A2BARs, it is tempting to speculate that tissue protection during hypoxia may require a combination of myeloid A2AARs together with vascular A2BARs.
In summary, the present study reveals signaling through the vascular A2BAR as a critical pathway for attenuating hypoxia-associated decreases in vascular barrier function and suggests that selective A2BAR agonist treatment may be beneficial during acute pathologic conditions involving vascular leakage as a consequence of limited oxygen availability. Future challenges include the development of approaches to deal with A2BAR desensitization, the delivery of AR agonists to specific anatomic sites, and the definition of pharmacologic strategies to safely use A2BAR agonists in patients suffering from conditions involving vascular leakage in the setting of limited oxygen availability such as may occur during sepsis or acute lung injury.59
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Cathrine Ledent, Marlene Jacobson, and Jürgen Schnermann for kindly providing us with gene-targeted mice and David Koehler, Stephanie Zug, and Scott Hooker for excellent technical support. L.F.T. holds the Putman City Schools Distinguished Chair in Cancer Research.
This work was supported by grants from the German Research Foundation (DFG) (EL274/2-2 to H.K.E.), the Foundation for Anesthesia Research and Education (H.K.E.), Interdisciplinary Center for Clinical Research (IZKF) Nachwuchsgruppe (1605-0-0 to T.E.) and the Nationals Institutes of Health (AI18220 to L.F.T).
National Institutes of Health
Authorship
T.E. designed research, analyzed data, and helped with manuscript writing; M.F. performed histologic studies and helped with generation of bone marrow chimera; A.G. designed research, helped with manuscript writing, and generated bone marrow chimera; S.L. performed experiments; L.F.T. helped with manuscript writing and performed PCR studies on myeloid A2BAR expression; H.K.E. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger K. Eltzschig, MD, PhD, Associate Professor of Anesthesiology, Mucosal Inflammation Program, Department of Anesthesiology and Perioperative Medicine, Biochemistry Research Building (BRB), Room 852, 4200 E 9th Ave, Mailstop B112, Denver, CO 80262; e-mail: holger.eltzschig@uchsc.edu.
References
Author notes
T.E., M.F., and A.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal