Abstract
Hypoxia is common to several inflammatory diseases, where multiple cell types release adenine-nucleotides (particularly adenosine triphosphate/adenosine diphosphate). Adenosine triphosphate/adenosine diphosphate is metabolized to adenosine through a 2-step enzymatic reaction initiated by CD39 (ectonucleoside-triphosphate-diphosphohydrolase-1). Thus, extracellular adenosine becomes available to regulate multiple inflammatory endpoints. Here, we hypothesized that hypoxia transcriptionally up-regulates CD39 expression. Initial studies revealed hypoxia-dependent increases in CD39 mRNA and immunoreactivity on endothelia. Examination of the human CD39 gene promoter identified a region important in hypoxia inducibility. Multiple levels of analysis, including site-directed mutagenesis, chromatin immunoprecipitation, and inhibition by antisense, revealed a critical role for transcription-factor Sp1 in hypoxia-induction of CD39. Using a combination of cd39−/− mice and Sp1 small interfering RNA in in vivo cardiac ischemia models revealed Sp1-mediated induction of cardiac CD39 during myocardial ischemia. In summary, these results identify a novel Sp1-dependent regulatory pathway for CD39 and indicate the likelihood that CD39 is central to protective responses to hypoxia/ischemia.
Introduction
Circulating or locally released nucleotides are rapidly metabolized by vascular cell surface ectoenzymes.1,2 It is now accepted that the major pathway for extracellular hydrolysis of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) is the ectonucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1),3 previously identified as ecto-ATPase, ecto-ATPDase, or CD39.4,5 CD39 is expressed on the endothelium and inflammatory cells6 and modulates vascular cell and platelet purinoreceptor activity by the sequential hydrolysis of extracellular ATP or ADP to adenosine monophosphate (AMP).5,7 The thromboregulatory and anti-inflammatory role of CD39 has been recently demonstrated by the generation of mutant mice with disruption of the CD39 gene,8,9 and by a series of experiments where high levels of ATPDase expression are attained by administration of adenoviral vectors at injured tissue sites.10-12 These and other studies indicate that ATP hydrolysis by CD39 provides a readily available extracellular source for adenine nucleotides during hypoxia/inflammation.13,14
Surface-bound CD39 converts ATP/ADP to AMP, which in turn is enzymatically hydrolyzed by ecto-5′-nucleotidase (CD73) to adenosine (Ado). When generated extracellularly by this cascade, Ado can activate each of 4 7-transmembrane-spanning adenosine receptors or can be internalized through dipyridamole-sensitive carriers.2 These pathways have been shown to regulate diverse physiologic and pathophysiologic outcomes.1,15-17
At present, only very little is known about the regulation of CD39 and whether regulated expression provides a physiologic role.18-20 Several studies have suggested that CD39 contributes to the protective aspects of adenine nucleotide metabolism during hypoxia and ischemia.13 For example, CD39 has been strongly implicated in thromboregulatory roles during stroke,9,21 intestinal ischemia reperfusion,22 and in protection from vascular leak during hypoxia.6 These pathways appear to function primarily through adenosine, where activation of either the A2A23-25 or A2B26-32 receptors serves as tissue-protective mechanisms after insult.1,2,15,33
Our previous studies revealed that CD39 is induced by hypoxia at the mRNA, protein, and functional levels in vitro.6 Based largely on these previous studies, we hypothesized that CD39-regulatory mechanisms control tissue damage in response to ischemia. Initial studies with the cloned CD39 promoter revealed a prominent role for the transcription factor Sp1 in hypoxia inducibility. Examination of this same regulatory region of the mouse gene identified identical sequence homology, and studies using a cardiac ischemia model identified a central role for Sp1-regulated CD39 in tissue protection during ischemia.
Methods
Endothelial cell culture
5′-RACE and cloning
RNA was isolated from HMEC-1 using TRIzol (Invitrogen, Carlsbad, CA) for RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE). 5′–RLM-RACE cDNA was prepared using the FirstChoice Kit (Ambion, Austin, TX) as per the manufacturer's protocol. 5′-RACE experiments were carried out using FirstChoice 5′ forward primers and CD39-specific reverse primers with SuperTaq recombinant DNA polymerase (Ambion). The resulting band obtained was cloned into a TA cloning vector (pCR 2.1; Invitrogen) and sequenced using T7 primer (Biopolymers Facility, Harvard Medical School, Boston, MA). Cloning of the 5′-untranslated region (UTR) was performed using genomic DNA extracted from HeLa cells using a commercially available method (QIAGEN, Valencia, CA). A polymerase chain reaction (PCR) fragment was generated extending 1417 base pairs (bp) upstream of the transcription start site (TSS). The gel-purified band was then cloned into a pGL3 luciferase reporter vector (Promega, Madison, WI). Transcription factor analysis of this sequence was performed using online software (MatInspector; Genomatix, Ann Arbor, MI).
CD39 reporter assays
HMEC-1 cells were used here to assess CD39 inducibility by hypoxia. Plasmids expressing sequence corresponding to full-length CD39 (−1417 to +83 bp) or the following 5′ truncations: CD39-898 (−815 to +83 bp), CD39-300 (−217 to +83 bp), CD39-150 (−67 to +83 bp), and CD39-122 (−39 to +83 bp) upstream from the luc reporter gene. All constructs were cotransfected with Renilla vector (Invitrogen) using standard methods of overnight transfection using Geneporter transfection reagent (Gene Therapy Systems, San Diego, CA). In subsets of experiments, cells were transfected with a promoterless vector (pGL3-Basic; Promega) to control for background luciferase activity. After transfection, cells were subjected to hypoxia or normoxia for 48 hours. Luciferase activity was assessed (Turner Designs, Sunnyvale, CA) using a luciferase assay kit (Stratagene, La Jolla, CA). All luciferase activity was normalized with respect to a constitutively expressed β-galactosidase reporter gene.
In subsets of experiments, Sp1 depletion was accomplished using antisense oligonucleotide loading as described previously35 using phosphorothioate derivatives of antisense (5′-ATA TTA GGC ATC ACT CCA GG-3′) or control sense (5′-CCT GGA GTG ATG CCT AAT AT-3′) oligonucleotides.36 Endothelial cells were washed in serum-free medium containing 20 μg/mL Geneporter transfection reagent (Gene Therapy Systems) with 2 μg/mL Sp1 antisense or sense oligonucleotide. Cells were incubated for 4 hours at 37°C and then replaced with serum-containing growth media. Treated cells were then subjected to hypoxia or normoxia for indicated periods of time.
In subsets of experiments, Sp1-binding site mutations or GATA-3–binding site mutations were introduced in the CD39-300 construct using the GeneEditor in vitro site-directed mutagenesis system (Promega). Briefly, a mutation encoding a 7-nucleotide mutation in the CD39 Sp1-binding site (consensus motif 5′-AGCCCCTCCCAC-3′ mutated to 5′-AGCAATTATA-CAC-3′ within the putative SP1 site) by PCR. A 4-nucleotide mutation in the CD39 GATA-3–binding site (consensus 5′-TGTTATATCTCTG-3′ mutated to 5′-TGTTATAGAGATG-3′) by PCR. (Bold letters represent mutated sequence.) All mutations were confirmed by sequencing using pGL3-basic primers. Hypoxia inducibility in transient transfectants using such mutated luciferase constructs was exactly as described in this section.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described previously.37,38 The sequences of the CD39 promoter-specific primers spanning the putative Sp1-binding region were as follows: sense, 5′-ACC TGG AAA AGG CTTACGA GT-3′; and antisense, 5′-CCG TCT CCG TGA GAG AGG T-3′. The size of the amplified product resulting from the use of this primer pair was 192 bp.
In vivo cardiac ischemia/reperfusion model
In pharmacologic studies, age-, sex-, and weight-matched C57BL/6J mice were used. In studies of gene-targeted mice, homozygous mice for CD39 were compared with littermate controls and matched in age, sex, and weight as described previously.39,40 In subsets of experiments, mice were treated with the prolyl-hydroxylase inhibitor dimethyloxalylglycine (1 mg/animal).27 All animal protocols were in accordance with the German guidelines for use of living animals and were approved by the Institutional Animal Care and Use Committee of the Tübingen University Hospital and the Regierungspräsidium Tübingen (Tübingen, Germany) or the Animal Care and Use Committee at the University of Colorado Health Science Center (Denver).
In vivo measurement of cardiac nucleotide levels
Myocardial nucleotide levels (ATP, AMP, and adenosine) after ischemia were determined as described previously.28,31,40 In short, myocardial ischemia (60 minutes) or sham operations were performed in cd39−/− mice or littermate controls. Ischemic cardiac tissue was snap-frozen with clamps precooled to the temperature of liquid nitrogen. The cardiac tissues were pulverized under liquid nitrogen, and tissue protein was precipitated with 0.6 N ice-cold perchloric acid. Tissue adenosine and AMP levels were determined via high performance liquid chromatography (HPLC) as described previously.41
In vivo siRNA repression
To achieve in vivo repression of Sp1 within cardiac tissues, we developed a system to apply small interfering RNA (siRNA)/transfection agent via continuous infusion into the left ventricle, to achieve sufficient intracardial siRNA concentrations for successful siRNA repression. For this purpose, we advanced a carotid artery catheter into the left ventricle and confirmed its correct position by pressure monitoring. Once the appearance of a diastolic left ventricular pressure reading confirmed the position of the catheter in the left ventricle (diastolic pressure values of 5-10 mm Hg), the infusion of siRNA together with transfection agent (siPORT Amine; Ambion) was started (1.5 μg siRNA/g body weight over indicated time periods). SMARTpool siRNA targeting Sp1 was synthesized by Dharmacon RNA Technologies (Lafayette, CO). SMARTpool reagents combine 4 SMARTselection-designed siRNAs into a single pool, resulting in even greater probability that the SMARTpool reagent will reduce target mRNA to low levels. As control, an siRNA (Dharmacon RNA Technologies) with at least 4 mismatches to any human, mouse, or rat gene was used.27
Analysis of messenger RNA levels by real-time PCR
Western blot analysis for CD39 and immunohistochemistry
Data analysis
CD39 bioactivity and paracellular permeability data were compared by 2-factor analysis of variance (ANOVA) or by the Student t test, where appropriate. Values are expressed as the mean plus or minus SEM from at least 3 separate experiments.
Results
Characterization of the human CD39 promoter
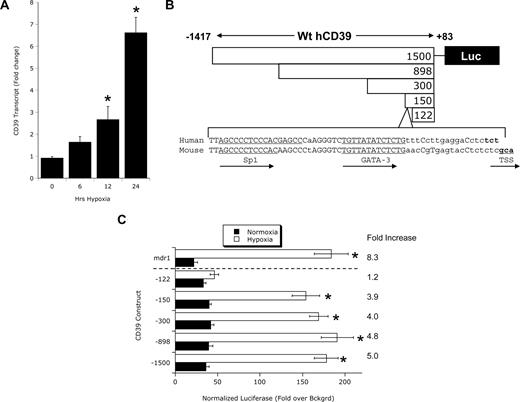
We have previously demonstrated that CD39 is transcriptionally induced on endothelial cells in response to hypoxia.6 Consistent with these studies, real-time reverse-transcribed PCR (RT-PCR) studies confirm CD39 induction on vascular endothelia (HMEC-1) by hypoxia (Figure 1A). To define these molecular principles, we initially determined whether the human CD39 (hCD39) promoter was hypoxia responsive. In view of the likelihood of a transcription-mediated induction of CD39 during hypoxia, attention was directed at the 5′-UTR for potential hypoxia regulated transcription factor sequences. Our 5′-RACE results confirmed the findings of Maliszewski et al identifying the TSS 81 bp upstream of the start codon.43 Based on this sequence, we cloned approximately 1.5 kb upstream of the major TSS in hCD39 (from chromosome 10, NW_001838005.2) into a pGL3 luciferase reporter vector and generated various length truncations of this promoter construct (Figure 1A) to address functionality. As shown in Figure 1B, endothelial cells (HMEC-1) transiently transfected with the full-length hCD39 promoter (nucleotides −1417 to +83) and exposed to hypoxia (24 hours) showed a 5.0- plus or minus 0.4-fold increase in luciferase activity over normoxia controls (P < .01). A promoter-expressing sequence for the MDR1 gene promoter, which we have shown is hypoxia-inducible,35 served as a positive control for these experiments (P < .01). Similar inducibility by hypoxia was observed with sequential 5′ truncations of the hCD39 promoter to −815, −217, and −67 (Figure 1B, all P < .01). An additional truncation of this promoter sequence to bp −39 to +83 resulted in a nearly complete loss of hypoxia inducibility compared with normoxia controls (P < .01), delineating a regulatory sequence in the −67 to −39 region relative to the major TSS.
CD39 is induced by hypoxia. (A) Vascular endothelia (HMEC-1) were exposed to hypoxia (2% oxygen) over indicated time periods, and CD39 transcript levels were determined by real-time RT-PCR. β-actin was used to control for starting template (n = 3, *P < .05). CD39 luciferase reporter assays. (B) Orientation of the human CD39 promoter, the location of Sp1 and GATA-3–binding sites in the human and mouse CD39 promoter, the location of the TSS, and the location of truncations used for transient transfections (see “CD39 promoter assays” for additional details). (C) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to full-length CD39 (−1417 to +83 bp) or the following 5′ truncations: CD39-898 (−815 to +83 bp), CD39-300 (−217 to +83 bp), CD39-150 (−67 to +83 bp), and CD39-122 (−39 to +83 bp) upstream from the luc reporter gene. Also shown is full-length mdr-1 promoter as a positive control. Twelve hours later, cells were exposed to hypoxia or normoxia for 48 hours and assessed for luciferase activity. All transfections were normalized to cotransfected Renilla promoter. Data are mean plus or minus SEM from 3 separate experiments.
CD39 is induced by hypoxia. (A) Vascular endothelia (HMEC-1) were exposed to hypoxia (2% oxygen) over indicated time periods, and CD39 transcript levels were determined by real-time RT-PCR. β-actin was used to control for starting template (n = 3, *P < .05). CD39 luciferase reporter assays. (B) Orientation of the human CD39 promoter, the location of Sp1 and GATA-3–binding sites in the human and mouse CD39 promoter, the location of the TSS, and the location of truncations used for transient transfections (see “CD39 promoter assays” for additional details). (C) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to full-length CD39 (−1417 to +83 bp) or the following 5′ truncations: CD39-898 (−815 to +83 bp), CD39-300 (−217 to +83 bp), CD39-150 (−67 to +83 bp), and CD39-122 (−39 to +83 bp) upstream from the luc reporter gene. Also shown is full-length mdr-1 promoter as a positive control. Twelve hours later, cells were exposed to hypoxia or normoxia for 48 hours and assessed for luciferase activity. All transfections were normalized to cotransfected Renilla promoter. Data are mean plus or minus SEM from 3 separate experiments.
Sequence analysis of the proximal CD39 promoter revealed binding sites for the transcription factors Sp1 (Figure 1A positions −42 to −54 relative to the TSS) and GATA-3 (Figure 1A positions −21 to −30 relative to the TSS), both of which have been identified as hypoxia-inducible transcription factors.44 Importantly, sequence comparison of this region in the human (from chromosome 10, NW_001838005.2) and mouse (from chromosome 19, NW_001030643.1) CD39 promoter regions revealed only a single base pair difference and an identical expression profile for both Sp1 and GATA-3–binding sites (Figure 1A), suggesting that this region is highly conserved.
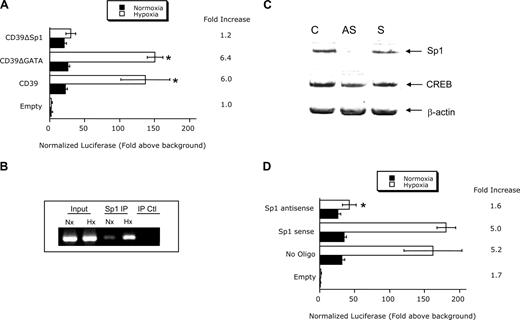
Site-directed mutagenesis of the central transcription factor binding site in Sp1 or GATA-3 in the hD39 promoter and analysis of hypoxia inducibility in HMEC revealed that only Sp1 contributed to this response (Figure 2A). Based on these findings, we determined whether Sp1 would bind the CD39 promoter. For these purposes, we used ChIP analysis to examine binding of Sp1 to the CD39 promoter spanning the putative Sp1 binding site in intact cells. As shown in Figure 2B, this analysis revealed a prominent band of 192 bp in nuclei derived from hypoxic and, to a lesser extent, in normoxic cells. No bands were evident in the beads only control, and pre-immunoprecipitation samples revealed equivalent DNA input.
Role of Sp1 in CD39 hypoxia inducibility. (A) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to truncations at the 5′ end (CD39-300, −217 to +83 bp) or plasmids encoding Sp1 or GATA3 mutations, as indicated. Twelve hours later, cells were exposed to hypoxia or normoxia for 48 hours and assessed for luciferase activity. All transfections were normalized to cotransfected Renilla plasmid. Data are mean plus or minus SEM from 3 separate experiments (*P < .025 compared with the corresponding nonmutated control). (B) ChIP assay was used to examine Sp1 binding to the CD39 promoter in normoxic and hypoxic HMEC-1 cells. Reaction controls included immunoprecipitations using a nonspecific IgG monoclonal antibody and PCR performed using HMEC-1 DNA (Input). A representative experiment of 3 is shown. (C) Confluent HMEC-1 monolayers were exposed to mock treatment (C), Sp1 sense oligonucleotides (S), or Sp1 antisense (AS) oligonucleotides for 48 hours. Total protein was solubilized, and Sp1 expression was examined by Western blot. As additional control, the transcription factor cAMP-response element-binding protein was probed. (D) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to truncations at the 5′ end (CD39-300, −217 to +83 bp) in the presence or absence of SP1 sense or antisense oligonucleotides. Twelve hours later, cells were exposed to hypoxia for 48 hours and assessed for luciferase activity. Data are mean plus or minus SEM from 3 separate experiments (*P < .01).
Role of Sp1 in CD39 hypoxia inducibility. (A) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to truncations at the 5′ end (CD39-300, −217 to +83 bp) or plasmids encoding Sp1 or GATA3 mutations, as indicated. Twelve hours later, cells were exposed to hypoxia or normoxia for 48 hours and assessed for luciferase activity. All transfections were normalized to cotransfected Renilla plasmid. Data are mean plus or minus SEM from 3 separate experiments (*P < .025 compared with the corresponding nonmutated control). (B) ChIP assay was used to examine Sp1 binding to the CD39 promoter in normoxic and hypoxic HMEC-1 cells. Reaction controls included immunoprecipitations using a nonspecific IgG monoclonal antibody and PCR performed using HMEC-1 DNA (Input). A representative experiment of 3 is shown. (C) Confluent HMEC-1 monolayers were exposed to mock treatment (C), Sp1 sense oligonucleotides (S), or Sp1 antisense (AS) oligonucleotides for 48 hours. Total protein was solubilized, and Sp1 expression was examined by Western blot. As additional control, the transcription factor cAMP-response element-binding protein was probed. (D) Confluent HMEC-1 monolayers were transiently transfected with plasmids expressing sequence corresponding to truncations at the 5′ end (CD39-300, −217 to +83 bp) in the presence or absence of SP1 sense or antisense oligonucleotides. Twelve hours later, cells were exposed to hypoxia for 48 hours and assessed for luciferase activity. Data are mean plus or minus SEM from 3 separate experiments (*P < .01).
In extensions of these studies, we depleted Sp1 in HMEC through the use of antisense oligonucleotides.35 As shown in Figure 2C, Western blotting of nuclear lysates derived from control, sense, or antisense loaded cells revealed a prominent reduction in Sp1 expression in antisense loaded cells. In contrast, protein levels of the transcription factor cAMP-response element-binding protein were unaltered. Analysis of luciferase activity in cells subjected to hypoxia was diminished compared with the wild-type promoter (Figure 2D, P < .025), providing additional evidence for Sp1 in hypoxia-induced expression of CD39. These findings were not a result of differences in background luciferase because parallel transfections with promoterless luciferase vectors showed no differences in activity between sense and antisense oligonucleotides directed against Sp1 (1.1- ± 0.3- and 1.3- ± 0.3-fold increase over mock-transfected for sense and antisense, respectively, P = not significant). Such findings strongly implicate Sp1 in the hypoxia inducibility of hCD39.
Induction and function of CD39 in myocardial ischemia/reperfusion
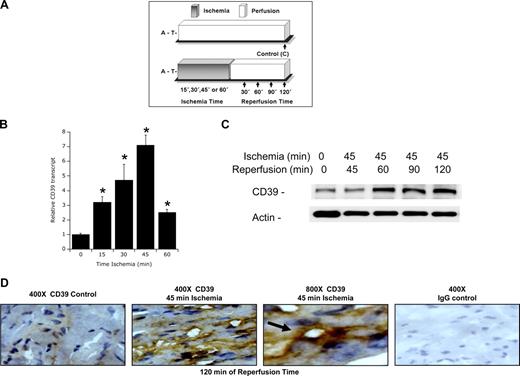
Given the strong similarity between regulatory regions of the human and murine CD39 gene (Figure 1A), we extended these findings from human endothelial cells to a murine disease model. As shown in Figure 3A, we used a previously described model of murine myocardial ischemia/reperfusion (I/R) facilitated by a hanging-weight system for intermittent coronary occlusion in ventilated animals.39,40 Analysis of ischemic tissues derived from this model revealed a time-dependent induction of cardiac CD39 mRNA (Figure 3B), with maximal induction at 45 minutes of ischemia (P < .01). Western blot analysis of reperfused (45 min-utes of ischemia) cardiac tissue identified a prominent, time-dependent induction of CD39 protein through the 120-minute period of reperfusion examined here. Similarly, immunohistochemical staining of CD39 confirmed cardiac induction of CD39 in vivo after 45/120 minutes I/R, respectively (Figure 3D). Similar to myocardial ischemia, intraperitoneal treatment with the prolyl-hydroxylase inhibitor dimethyloxalylglycine resulted in enhanced CD39 transcript and protein levels (as assessed by real-time RT-PCR and immunohistochemistry, Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
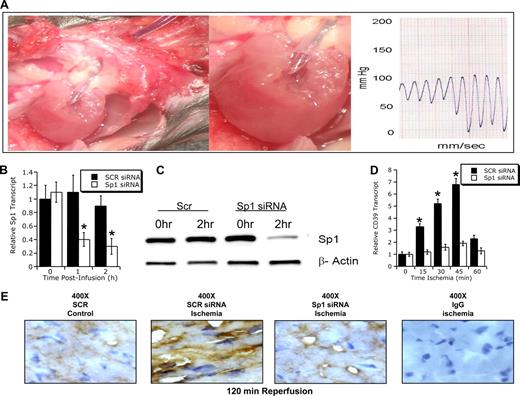
Induction of cardiac CD39 during murine myocardial ischemia in vivo. (A) Murine model of cardiac ischemia. To study in vivo expression of cardiac CD39 during cardiac ischemia, we exposed mice to in vivo coronary artery ligation (B) over indicated time periods (0-60 minutes) followed by 2 hours of reperfusion or (C) exposed mice to 45 minutes of coronary ischemia followed by indicated time periods of reperfusion. A indicates anesthesia induction; T, thoracotomy. (B) CD39 transcript from myocardial biopsies of the area at risk. C57BL/6 mice were subjected to myocardial ischemia over 0 to 60 minutes of ischemia followed by 2 hours of reperfusion, and cardiac tissue from the area at risk was excised, flash frozen, and CD39 transcript levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 minutes of myocardial ischemia ± SEM at each indicated time, n = 6; *P < .01). (C) C57BL/6 mice were subjected to 45 minutes of in situ ligation of the left coronary artery, and myocardial tissue from the area at risk was excised after indicated reperfusion times (0-120 minutes), lysed, proteins resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose. Membranes were probed with CD39 antibody. The same blot was probed for β-actin expression as a control for protein loading. One representative experiment of 3 is shown. (D) C57BL/6 mice were subjected to 45 minutes of myocardial ischemia, and cardiac tissue from the area at risk was harvested after 120 minutes of reperfusion, sectioned, and stained using CD39 antibody. Cardiac tissue from sham-operated mice served as control (original magnification ×400 or ×800, as indicated). To control for nonspecific staining, IgG was used at identical concentrations and staining conditions as the target primary antibodies for CD39.
Induction of cardiac CD39 during murine myocardial ischemia in vivo. (A) Murine model of cardiac ischemia. To study in vivo expression of cardiac CD39 during cardiac ischemia, we exposed mice to in vivo coronary artery ligation (B) over indicated time periods (0-60 minutes) followed by 2 hours of reperfusion or (C) exposed mice to 45 minutes of coronary ischemia followed by indicated time periods of reperfusion. A indicates anesthesia induction; T, thoracotomy. (B) CD39 transcript from myocardial biopsies of the area at risk. C57BL/6 mice were subjected to myocardial ischemia over 0 to 60 minutes of ischemia followed by 2 hours of reperfusion, and cardiac tissue from the area at risk was excised, flash frozen, and CD39 transcript levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 minutes of myocardial ischemia ± SEM at each indicated time, n = 6; *P < .01). (C) C57BL/6 mice were subjected to 45 minutes of in situ ligation of the left coronary artery, and myocardial tissue from the area at risk was excised after indicated reperfusion times (0-120 minutes), lysed, proteins resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose. Membranes were probed with CD39 antibody. The same blot was probed for β-actin expression as a control for protein loading. One representative experiment of 3 is shown. (D) C57BL/6 mice were subjected to 45 minutes of myocardial ischemia, and cardiac tissue from the area at risk was harvested after 120 minutes of reperfusion, sectioned, and stained using CD39 antibody. Cardiac tissue from sham-operated mice served as control (original magnification ×400 or ×800, as indicated). To control for nonspecific staining, IgG was used at identical concentrations and staining conditions as the target primary antibodies for CD39.
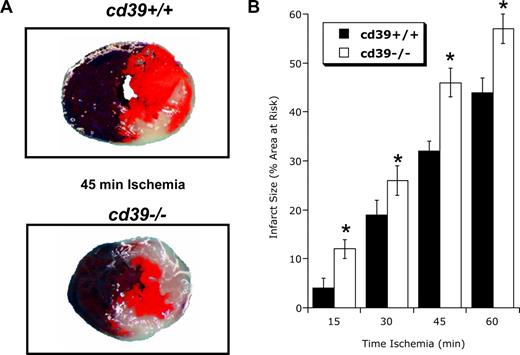
We next defined the functional role of cardiac CD39 during ischemia. For these purposes, we compared myocardial infarct sizes between cd39+/+ and cd39−/− mice after increasing periods of ischemia. As shown in Figure 4A, representative images of infarct staining (double label with Evan blue and triphenyltetrazolium chloride) revealed a significant increase in myocardial infarct size (expressed as percentage of the area at risk) in cd39−/− animals relative to their cd39+/+ counterparts, where increased susceptibility to ischemia was also demonstrable from gross morphology of cardiac tissue. Pooled results these studies revealed that infarct size increased with each 15-minute period of ischemia (P < .025 by ANOVA) in cd39+/+ mice. Moreover, these studies revealed that cd39−/− animals were more susceptible than their cd39+/+ counterparts to cardiac damage at each period of ischemia (P < .01 for each time point examined). Such differences in myocardial infarct size were accompanied by corresponding changes in cardiac nucleotide levels, with enhanced ATP, and attenuated AMP and adenosine levels in cd39−/− mice (Figure S2). These studies implicate a protective role for the induction of CD39 during cardiac hypoxia/ischemia.
Influence of CD39 on cardiac ischemia-reperfusion injury. CD39−/− or littermate controls matched by age and sex (CD39+/+) were exposed to 15 to 60 minutes of coronary ischemia via in situ ligation of the left coronary artery and were killed after 2 hours of reperfusion. Infarct sizes were measured by double staining with Evan blue and 2,3,5-triphenyltetrazolium chloride (TTC). (A) Representative images of infarcts from the experiment are displayed (blue represents retrograde Evan blue staining; red and white, area at risk; white, infarcted tissue). (B) Infarct sizes expressed as the percentage of the area at risk that underwent infarction (mean ± SD, n = 6). *P < .05 compared to wild-type mice (cd39+/+).
Influence of CD39 on cardiac ischemia-reperfusion injury. CD39−/− or littermate controls matched by age and sex (CD39+/+) were exposed to 15 to 60 minutes of coronary ischemia via in situ ligation of the left coronary artery and were killed after 2 hours of reperfusion. Infarct sizes were measured by double staining with Evan blue and 2,3,5-triphenyltetrazolium chloride (TTC). (A) Representative images of infarcts from the experiment are displayed (blue represents retrograde Evan blue staining; red and white, area at risk; white, infarcted tissue). (B) Infarct sizes expressed as the percentage of the area at risk that underwent infarction (mean ± SD, n = 6). *P < .05 compared to wild-type mice (cd39+/+).
Role of Sp1-regulated CD39 in myocardial protection
To further elucidate the functional contribution of Sp1 to CD39-mediated cardioprotection, an in vivo siRNA approach was used to repress cardiac Sp1. For these purposes, we advanced a carotid artery catheter into the left ventricle and confirmed its correct position by pressure monitoring (Figure 5A). At the appearance of a diastolic left ventricular pressure confirming the position of the catheter in the left ventricle (diastolic pressure values of 5-10 mm Hg), we infused pooled siRNAs in combination with transfection agent (1.5 μg Sp1 or scrambled siRNA/g body weight over 2 hours, Figure 5A). We then determined Sp1 transcript levels at indicated time points (0-2 hours) after the start of the siRNA infusion. These studies revealed maximal repression of Sp1 transcript at 2 hours of infusion (76% ± 6% reduction, P < .025), whereas transcript levels of Sp1 were unchanged with infusion of the control siRNA (Figure 5B). These changes of transcript levels were accompanied by corresponding changes of Sp1 protein, as determined by Western blot analysis (Figure 5C). In strong support of our hypothesis, Sp1 siRNA significantly attenuated ischemia-induced cardiac CD39 mRNA (Figure 5D, P < .01 by ANOVA) and protein (Figure 5E) compared with scramble siRNA.
Influence of Sp1 siRNA siRNA in vivo on cardiac ischemia. (A) Murine model of in situ siRNA repression. A surgically inserted carotid catheter was advanced into the left ventricle. Blood pressure recording confirmed correct placement of the catheter tip within the left ventricle. After placement of the catheter's infusion port within the left ventricle, an infusion of target (Sp1 siRNA) or control siRNA (SCR) together with transfection agent (siPORT Amine; Ambion) was given over indicated time periods (1.5 μg siRNA/g body weight over indicated time periods). Changes in cardiac Sp1 transcript (B) or protein (C) after in vivo siRNA repression. C57BL/6 mice were treated with a left ventricular infusion (as described for panel A) of siRNA specific for Sp1 or SCR siRNR. After indicated time periods, hearts were excised, total RNA isolated, and Sp1 transcript levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 hour of siRNA infusion ± SEM at each indicated time, n = 6). (C) Western blot. (D) CD39 transcript of myocardial biopsies from the area at risk after Sp1 repression. C57BL/6 mice were treated with scrambled control siRNA (SCR siRNA) or Sp1-specific siRNA (Sp1 siRNA) via intraventricular infusion over 2 hours before exposure to in situ myocardial ischemia (0-60 minutes). Cardiac tissue from the area at risk was excised at indicated time points, flash-frozen, and CD39 transcript was determined by real-time RT-PCR. Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 minutes of myocardial ± SEM at each indicated time, n = 6). *P < .05 compared to 0 hours (B,D). (E) C57BL/6 mice were treated with control (SCR siRNA) or Sp1-specific siRNA (Sp1 siRNA) via intraventricular infusion over 2 hours before exposure to in situ myocardial ischemia (45 minutes). Cardiac tissue from the area at risk was harvested after 120 minutes of reperfusion, sectioned, and stained using CD39 antibody. Cardiac tissue from sham-operated mice served as control (original magnification ×400). To control for nonspecific staining, IgG was used at identical concentrations and staining conditions as the target primary antibodies for CD39.
Influence of Sp1 siRNA siRNA in vivo on cardiac ischemia. (A) Murine model of in situ siRNA repression. A surgically inserted carotid catheter was advanced into the left ventricle. Blood pressure recording confirmed correct placement of the catheter tip within the left ventricle. After placement of the catheter's infusion port within the left ventricle, an infusion of target (Sp1 siRNA) or control siRNA (SCR) together with transfection agent (siPORT Amine; Ambion) was given over indicated time periods (1.5 μg siRNA/g body weight over indicated time periods). Changes in cardiac Sp1 transcript (B) or protein (C) after in vivo siRNA repression. C57BL/6 mice were treated with a left ventricular infusion (as described for panel A) of siRNA specific for Sp1 or SCR siRNR. After indicated time periods, hearts were excised, total RNA isolated, and Sp1 transcript levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 hour of siRNA infusion ± SEM at each indicated time, n = 6). (C) Western blot. (D) CD39 transcript of myocardial biopsies from the area at risk after Sp1 repression. C57BL/6 mice were treated with scrambled control siRNA (SCR siRNA) or Sp1-specific siRNA (Sp1 siRNA) via intraventricular infusion over 2 hours before exposure to in situ myocardial ischemia (0-60 minutes). Cardiac tissue from the area at risk was excised at indicated time points, flash-frozen, and CD39 transcript was determined by real-time RT-PCR. Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold change compared with control (0 minutes of myocardial ± SEM at each indicated time, n = 6). *P < .05 compared to 0 hours (B,D). (E) C57BL/6 mice were treated with control (SCR siRNA) or Sp1-specific siRNA (Sp1 siRNA) via intraventricular infusion over 2 hours before exposure to in situ myocardial ischemia (45 minutes). Cardiac tissue from the area at risk was harvested after 120 minutes of reperfusion, sectioned, and stained using CD39 antibody. Cardiac tissue from sham-operated mice served as control (original magnification ×400). To control for nonspecific staining, IgG was used at identical concentrations and staining conditions as the target primary antibodies for CD39.
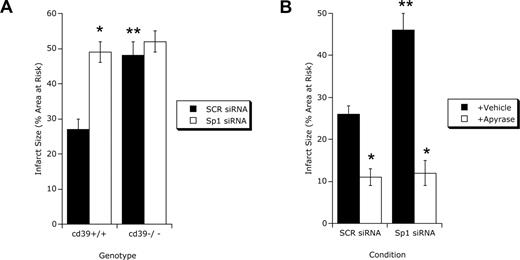
Quantification of infarct size (Evan blue and triphenyltetrazolium chloride) after ischemia in Sp1 siRNA-repressed hearts revealed a prominent increase in infarct size compared with control siRNA (Figure 6A). Ischemia in cd39−/− animals served as a control for these studies (Figure 6A) and revealed that repression of Sp1 in cd39−/− did not increase infarct size compared with control siRNA, collectively implicating both CD39 and Sp1 in cardioprotection. Previous studies have used soluble apyrase (rapidly converts ATP/ADP to AMP) to reconstitute gene-targeted mice for cd39.40,45 To determine whether the Sp1-repressed hearts could be reconstituted, animals were exogenously administered soluble potato apyrase (5 U intraperitoneally) before I/R (45/120 minutes) and examined for infarct size. As shown in Figure 6B, infarct sizes in animals administered apyrase were not different between Sp1-repressed and scrambled control siRNA myocardium, indicating a central role for apyrase activity in cardioprotection. Such studies strongly implicate Sp1-mediated induction of CD39 in cardioprotection during hypoxia/ischemia.
Myocardial infarct sizes after Sp1 repression. (A) CD39−/− mice or littermate controls matched in age, sex, and gender (CD39+/+ mice) were exposed to 45 minutes of myocardial ischemia after 2 hours of infusion of control siRNA (SCR siRNA) or siRNA specific for Sp1 (Sp1 siRNA) via a catheter placed with its infusion port into the left ventricle. Myocardial infarct sizes were measured by double staining of cardiac tissue with Evan blue and TTC. Infarct sizes are expressed as the percentage of the area at risk that underwent infarction (mean ± SD, n = 6). *P < .05 compared to control siRNA–treated mice (SCR); **P < .05 compared to wild-type mice (cd39+/+). (B) Reconstitution with soluble apyrase. C57BL/6J mice underwent treatment with control siRNA (SCR siRNA) or siRNA specific for Sp1 (Sp1 siRNA, see description for panel A). Thirty minutes before coronary ischemia, they were treated intraperitoneally with soluble apyrase (+Apyrase, 80 U/kg) or vehicle. After 45 minutes of myocardial ischemia and 2 hours of reperfusion, infarct sizes were determined by Evan blue and TCC double staining (mean ± SEM, n = 6). *P < .05 compared to vehicle-treated mice (Vehicle); **P < .05 compared to control siRNA–treated mice (SCR).
Myocardial infarct sizes after Sp1 repression. (A) CD39−/− mice or littermate controls matched in age, sex, and gender (CD39+/+ mice) were exposed to 45 minutes of myocardial ischemia after 2 hours of infusion of control siRNA (SCR siRNA) or siRNA specific for Sp1 (Sp1 siRNA) via a catheter placed with its infusion port into the left ventricle. Myocardial infarct sizes were measured by double staining of cardiac tissue with Evan blue and TTC. Infarct sizes are expressed as the percentage of the area at risk that underwent infarction (mean ± SD, n = 6). *P < .05 compared to control siRNA–treated mice (SCR); **P < .05 compared to wild-type mice (cd39+/+). (B) Reconstitution with soluble apyrase. C57BL/6J mice underwent treatment with control siRNA (SCR siRNA) or siRNA specific for Sp1 (Sp1 siRNA, see description for panel A). Thirty minutes before coronary ischemia, they were treated intraperitoneally with soluble apyrase (+Apyrase, 80 U/kg) or vehicle. After 45 minutes of myocardial ischemia and 2 hours of reperfusion, infarct sizes were determined by Evan blue and TCC double staining (mean ± SEM, n = 6). *P < .05 compared to vehicle-treated mice (Vehicle); **P < .05 compared to control siRNA–treated mice (SCR).
Discussion
Pathophysiologic conditions of hypoxia/ischemia result in numerous adenine nucleotide metabolic changes, and adenosine has a demonstrated role in organ function under such conditions. In the present studies, we explored the molecular mechanisms and impact of CD39 induction by hypoxia, which along with CD73 provides the primary determinants for localized production of adenosine at tissue interfaces.6 These studies extend our previous work implicating hypoxia in the transcriptional induction of CD396 and identify precise molecular mechanisms of CD39 regulation under such conditions. Additional insights are gained using a relevant animal model (cardiac I/R) and provide important insight for the novel role of Sp1 in CD39 regulation.
Multiple levels of evidence implicate adenosine in tissue responses to ischemia and hypoxia.40 Although the source of interstitial adenosine in hypoxia has been the basis of much debate, it is generally accepted that enzymatic processing of phosphorylated nucleotide substrates represents the major pathway of adenosine formation during oxygen supply imbalances.23 Adenosine production in the ischemic myocardium, for example, is attributable to activity of CD39,40 and both CD39 activity and adenosine metabolism have been demonstrated in cardiac preconditioning by brief periods of ischemia.28,40 Increased CD39 activity in ischemic preconditioning has been attributed to a variety of acute activation pathways, and another study provides direct evidence that CD39 is transcriptionally regulated by hypoxia in pheochromocytoma cells in vitro.46 Once liberated in the extracellular space, adenosine either interacts with cell surface adenosine receptors or is taken up into the cell through dipyridamole-sensitive carriers.2,16,17 Presently, 4 subtypes of G protein–coupled adenosine receptors exist, designated A1, A2A, A2B, and A3. These receptors are classified according to the use of pertussis toxin–sensitive pathways (A1AR and A3AR) or adenylate cyclase activation pathways (A2AAR and A2BAR).47 Endothelial cells of many origins express adenosine receptors constitutively, primarily of the A2A and A2B subtypes.26,47
Initial studies with promoter constructs confirmed previous work indicating hypoxia inducibility of human CD39.6 A truncation approach narrowed the search for potential transcriptional regulators to a small region within approximately 50 bp of the TSS. A search of putative binding sites revealed a classic Sp1 and GATA-3–binding site. Sp1 and, to a lesser extent, GATA-3 have been implicated in hypoxia-induced transcriptional regulation.44 To better define the specific role in CD39, 3 approaches were used. First, site-directed mutagenesis of the putative Sp1 and GATA-3 sites revealed a nearly complete loss of hypoxia inducibility with the Sp1, but not GATA-3, site mutant. Second, ChIP assays revealed increased binding of Sp1 to this site with exposure to hypoxia. Third, the use of previously published Sp1 antisense oligonucleotides,35 but not sense controls, resulted in a nearly complete blockade of CD39 induction by hypoxia. Thus, although we have not exhaustively examined other transcriptional regulators of this pathway, it is quite convincing that Sp1 contributes significantly to hypoxia-inducible CD39.
Sp1 is a member of a ubiquitously expressed family of Sp/XKLF transcription factors.48 These transcription factors are best known for maintenance and transcriptional housekeeping responsibilities. However, Sp1 has been strongly implicated in hypoxic gene transcription. A prime example is the endothelial mitogen vascular endothelial growth factor (VEGF). VEGF is one of the quintessential hypoxia-responsive genes important in normal tissue vascularization and in pathogenic tumor development.49 Whereas VEGF expression is regulated by hypoxia-inducible factor, Sp1 has been strongly implicated in the integrated regulation of VEGF during hypoxia and in inflammation.49 In this regard, Sp1 may act directly and indirectly on VEGF regulation. Although Sp1 directly binds DNA and has transcriptional regulatory functions,48 this factor can also associate with nontranscriptional regulatory proteins (eg, the tumor suppressor pVHL critical for hypoxia-inducible factor induction)50 as well as other transcription factors (eg, p53)51 to enhance hypoxia-inducible VEGF. In the case of CD39, we have no evidence for Sp1 interaction with other regulatory factors. Rather, several levels of evidence implicate Sp1 as a sole functioning factor in CD39 regulation. First, truncation of the CD39 promoter beyond the Sp1 site resulted in a nearly complete loss of hypoxia inducibility. Second, hypoxia-induced DNA binding to the promoter was provided through ChIP analysis, probably ruling out indirect actions of Sp1. Third, site-directed mutagenesis of only the Sp1 site resulted in a loss of hypoxia inducibility. Fourth, directed loss of Sp1 through antisense oligonucleotides attenuated hypoxia-driven CD39 promoter activity. Such findings strongly implicate Sp1-regulated transcription as the primary means of CD39 regulation in the vasculature.
In the course of these studies, we compared the human promoter region with that of the mouse and rat genes and revealed a particularly high homology near the TSS. Notably, both the Sp1 and GATA-3 binding sites were preserved in the TSS proximal region of the mouse and rat (NM_022587, data not shown) CD39 5′-UTR, suggesting an additional level of conservation between species. We provide evidence here that Sp1-regulated CD39 functions as an overall protective element in response to hypoxia/ischemia. In cd39−/− mice subjected to ischemia, significantly increased cardiac damage was observed, suggesting that CD39 provides a physiologic function in this regard. The directed loss of Sp1 via siRNA provided an additional level of evidence in this regard and revealed both a loss of CD39 induction as well as a functional deficit observed through cardiac damage to ischemia.
Although the present studies are the first to define a physiologic role for CD39 in cardiac damage in vivo, significant work has implicated CD39 in endothelial barrier protection in vitro. During modeled inflammation, neutrophils release several biologically active mediators, including ATP and 5′-AMP.6,52,53 Barrier-protective actions are mediated by adenosine, and metabolism of ATP to adenosine requires CD39 as a first enzymatic step.6 Inhibitor-based studies as well as murine models in vivo have implicated CD39 in regulation of endothelial permeability during polymorphonuclear leukocyte (PMN) transmigration and suggested that the conversion of ATP to adenosine by CD39 and CD73 may be rate limiting (ie, increased CD39 and CD73 results in parallel increases in adenosine-mediated bioactivity).6,54 The competitive inhibitor polyoxometalate 1, a potent E-NTPDase inhibitor, abolishes the influence of ATP.40
Although we do not know the exact mechanism(s) of cardiac protection by adenosine, recent studies suggest that, during myocardial ischemia, extracellular levels of adenosine are significantly elevated.28,40 For example, during ischemic preconditioning, in which treatment with short and repeated episodes of nonlethal ischemia causes significant cardioprotection from subsequent myocardial infarction, cardiac adenosine levels are approximately 5-fold elevated. Treatment with an ectonucleotidase inhibitor polyoxometalate 1 or the use of gene-targeted mice for Cd39 completely abolishes elevations of cardiac adenosine levels above baseline, thereby confirming the extracellular nature of these findings.28,40 Although the contribution of individual adenosine receptors to cardioprotection has been the basis of much debate, a recent study compared cardiac preconditioning phenomena in gene-targeted mice for each individual adenosine receptor.28 This study found a selective role for the A2BAR in mediating cardioprotection during IP,28 converging on protein kinase C–dependent signaling pathways.
In contrast, recent elegant studies found a critical role of signaling through myeloid A2AARs in attenuating myocardial ischemia and reperfusion injury.55 These studies indicate that A2AAR activation on bone marrow–derived cells, specifically T or B lymphocytes, is responsible for the infarct-sparing and anti-inflammatory effect of ATL146e-a–specific adenosine A2AAR agonist when administered at the time of reperfusion after coronary occlusion. This is consistent with other studies of mice deficient in the A2AAR showing increased inflammation-associated tissue damage, thereby providing evidence for A2AAR signaling as a mechanism for regulating acute inflammatory responses in vivo.24 In this study, a subthreshold dose of an inflammatory stimulus was associated with minimal tissue damage in control animals. A similar stimulus was sufficient to induce extensive tissue damage, more prolonged and higher levels of proinflammatory cytokines, and death in A2AR−/− mice. Similar observations were made using other models of inflammation, liver damage, or bacterial endotoxin-induced septic shock, suggesting A2AR in the limitation and termination of tissue-specific and systemic inflammatory responses.
In conclusion, these results define hypoxia-regulated CD39 expression in both endothelial cells and cardiac tissue and identify a previously unappreciated Sp1 regulatory binding site in the CD39 promoter. This regulatory pathway extends to the transcriptional level in vitro and in vivo and identifies an important role for CD39 in the regulation of tissue protection during hypoxia and ischemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (grants DK50189, HL60569, and HL076540), a Foundation for Anesthesia Education and Research grant (H.K.E.), and German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]) (grant El274/4-1) (H.K.E.).
National Institutes of Health
Authorship
Contribution: H.K.E. designed the research, performed experiments, and wrote part of the manuscript; D.K., T.E., and T.K. performed experiments and analyzed data; S.C.R. designed the research, reviewed the manuscript, and provided gene-targeted mice; and S.P.C. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger K. Eltzschig, Mucosal Inflammation Program, 12700 E 19th Avenue, Mailstop B112, Research Complex 2, Denver, CO 80220; e-mail: holger.eltzschig@uchsc.edu; and Sean P. Colgan, Mucosal Inflammation Program, 4200 E 9th Avenue, Denver, CO 80220; e-mail: sean.colgan@uchsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal