Key Points
In utero injection of an antibody against the c-Kit receptor can effectively deplete host HSCs in mice.
In utero depletion of host HSCs leads to significantly increased engraftment after neonatal congenic hematopoietic cell transplantation.
Abstract
Although in utero hematopoietic cell transplantation is a promising strategy to treat congenital hematopoietic disorders, levels of engraftment have not been therapeutic for diseases in which donor cells have no survival advantage. We used an antibody against the murine c-Kit receptor (ACK2) to deplete fetal host hematopoietic stem cells (HSCs) and increase space within the hematopoietic niche for donor cell engraftment. Fetal mice were injected with ACK2 on embryonic days 13.5 to 14.5 and surviving pups were transplanted with congenic hematopoietic cells on day of life 1. Low-dose ACK2 treatment effectively depleted HSCs within the bone marrow with minimal toxicity and the antibody was cleared from the serum before the neonatal transplantation. Chimerism levels were significantly higher in treated pups than in controls; both myeloid and lymphoid cell chimerism increased because of higher engraftment of HSCs in the bone marrow. To test the strategy of repeated HSC depletion and transplantation, some mice were treated with ACK2 postnatally, but the increase in engraftment was lower than that seen with prenatal treatment. We demonstrate a successful fetal conditioning strategy associated with minimal toxicity. Such strategies could be used to achieve clinically relevant levels of engraftment to treat congenital stem cell disorders.
Introduction
Hematopoietic stem cell (HSC) transplantation is a promising strategy to treat many nonmalignant genetic disorders such as hemoglobinopathies, immunodeficiencies, and inborn errors of metabolism,1 and may even provide tolerance for solid organ transplants.2 However, this approach often requires host myeloablation and immunosuppression, which carries significant morbidity.3,4 Transplantation into the immunologically naive fetal environment to circumvent the host immune response is an attractive alternative strategy to achieve sustained engraftment and donor-specific tolerance. This approach of in utero hematopoietic cell transplantation (IUHCTx) has been successful in many animal models, but clinical applications remain hampered by low levels of engraftment that are not sufficient to ameliorate symptoms or cure most diseases (reviewed in Nijagal et al5 ). Because the only clinical successes have been achieved in fetuses with severe combined immunodeficiency,6-8 it has been suggested that success is limited by barriers such as rejection of the stem cell graft and lack of space within the hematopoietic niche (reviewed in Flake and Zanjani9 ). We and others have previously explored the role of an immune response to donor cells and reported that the maternal immune system is a significant barrier to engraftment.10,11 The fetal host can become tolerant to transplanted cells through clonal deletion of alloreactive lymphocytes, allowing for donor-specific tolerance.12,13 However, it has been difficult to achieve therapeutic levels of donor cell engraftment, suggesting that lack of space in the hematopoietic niche is an additional barrier.
Given these observations, several approaches to increase engraftment after IUHCTx have been explored in animal models, such as improving the homing and competitive advantage of transplanted cells and increasing space in the host hematopoietic niche by conditioning. For example, inhibition of CD26 (a cell-surface peptidase that modulates chemokine-mediated HSC homing),14 can increase engraftment of transplanted allogeneic cells in mice after IUHCTx.15 The only reported in utero approach to increase space in the niche is maternal administration of busulfan, which has led to improved engraftment of transplanted cells in a fetal lamb model.16 However, the clinical use of this approach is limited by concerns for multiorgan toxicity,17 which can be considerable in the pediatric and fetal populations and requires careful experimental titration.18 Any strategy to deplete fetal host HSCs must have an excellent safety profile to be considered in clinical applications, because diseases considered for IUHCTx, except for α thalassemia, are typically nonfatal at birth and have some, although limited, postnatal treatment options.
The c-Kit receptor (CD117) is expressed on HSCs at all stages of development19 and interactions with its ligand, stem cell factor (SCF), are critical for HSC survival.20 The SCF-c-Kit signaling pathway plays an important role in the homing, adhesion, maintenance, and survival of HSCs in the hematopoietic niche (reviewed in Kent et al21 ). Thus, 1 strategy to deplete host HSCs selectively is to use an antibody against the c-Kit receptor to impede SCF-c-Kit signaling. The utility of this approach was initially suggested by experiments in c-Kit-defective wild-type/wild-type mutant mice, in which IUHCTx could cure the genetic anemia.22 In adult immunodeficient mice, an antibody against the c-Kit receptor, ACK2, has been used successfully to improve engraftment of transplanted cells.23 Although c-Kit is constitutively expressed on HSCs, its expression is downregulated during differentiation24 such that ACK2 treatment depletes true HSCs without depletion of mature progeny and avoids the toxicity of true myeloablation.23 This therapy has also been used in wild-type mice and led to increased levels of congenic chimerism when given concurrently with low-dose irradiation,25 but has not led to improved allogeneic chimerism. Because ACK2 provides no advantage for allogeneic stem cell transplantation, this strategy provides a unique opportunity to evaluate the “space” barrier independent of the “immune” barrier to transplantation.
In this study, we explored the strategy of ACK2-mediated depletion of fetal host HSCs to improve engraftment of donor hematopoietic cells. We defined the therapeutic window for this strategy in fetal mice and achieved clinically significant levels of donor chimerism in the congenic setting that were sustained for at least 5 months. These experiments suggest that safe techniques to modify the hematopoietic niche in the fetal host should be considered to improve levels of engraftment after IUHCTx.
Materials and methods
Mice
Wild-type C57Bl/6J (C57; CD45.2) and B6.SJL-PtrcaPep3b/BoyJ (BoyJ; CD45.1) mice were obtained from either the National Cancer Institute or the Jackson Laboratory. All mice were bred and maintained in a specific pathogen-free facility at the University of California, San Francisco (UCSF). All mouse experiments were performed according to the UCSF Institutional Animal Care and Use Committee–approved protocol.
Antibodies and flow cytometry
The following antibodies for flow cytometry were purchased from Becton Dickinson: CD3 (145-2C11), Gr-1 (RB6-8C5), CD45 (30F-11), and CD19 (1D3); Ebioscience: Pur-B220 (RA3-6B2), Pur-CD8 (53-6.7), Pur-CD4 (GK1.5), Pur-Gr-1(RB6-8C5), Pur-CD5 (53-7.3), Pur-Ter-119 (Ter-119), Pur-CD3 (17A2), Pur-Mac-1 (M1/70), CD117 (2B8), CD45.1 (A20), and CD45.2 (104); BioLegend: Ly-6A/E (D7); and Invitrogen: F(ab′)2 fragment of goat anti-rat (immunoglobulin G). ACK2 and rat immunoglobulin G-2b isotype control were obtained from the UCSF Hybridoma and Monoclonal Antibody Core. Cell viability was determined using propidium iodide (Ebioscience) or 4′,6-diamidino-2-phenylindole dihydrochloride (Invitrogen). Flow cytometry was performed on an LSR II (BD) or Accuri (Accuri Cytometers Inc.) flow cytometer and analyzed using FACS Diva.
In utero ACK2 injections and neonatal transplantation
B6.CD45.2 fetal mice were injected with given concentrations of ACK2 (2.5-50 µg) or with isotype antibody or phosphate-buffered saline (PBS) as controls in a volume of 5 µL per fetus at embryonic day 13.5 (E13.5) to E14.5. These doses were chosen based on the adult dose of 25 µg/g,23 which is equivalent to 5 µg per fetus. Exposure and fetal intrahepatic injections were performed as previously described.26 Briefly, under isoflurane-induced general anesthesia, a midline laparotomy was performed on pregnant dams, the uterus was exposed, and 5 µL of ACK2 antibody at the indicated concentration was injected into the fetal liver of each pup using pulled glass micropipettes. The abdominal incision was closed in multiple layers. Postnatally, neonates were transplanted with congenic (B6.CD45.1/CD45.2) fetal liver mononuclear cells (FLMC) on postnatal day 1 (P1) to P2. FLMC were isolated from E13.5 to E14.5 donor fetuses by density gradient separation using Ficoll-Paque Plus (GE Healthcare) as previously described11,13 and 2.5 × 106 FLMC were resuspended in 10 µL PBS and injected into the neonatal liver using a 31G insulin syringe (BD).
Quantification of HSC Depletion
Bone marrow (BM), spleens, and livers were dissected from mice at P1 after in utero ACK2 treatment and a single-cell suspension was created. For livers, the mononuclear cells were isolated by density gradient separation, whereas BM mononuclear cells were harvested by dissection of neonatal or maternal femurs and tibias. The cells were then incubated with antibodies against lineage markers (B220, CD8a, CD4, Gr-1, CD5, Ter119, CD3e, Mac-1), c-Kit, and Sca-1 to quantify Lin−Sca-1+c-Kit+ (KLS cells27 ).
Analysis of engraftment
Peripheral blood chimerism levels were determined by flow cytometry after staining with antibodies against CD45.1 and CD45.2. Chimerism was expressed as the percentage of donor/(donor+host) cells. Mice with >1% circulating donor cells were considered chimeric. Lineage analysis of chimeric cells was performed by staining peripheral blood with antibodies against CD45.1, CD45.2, Gr-1, CD3, and CD19. Engraftment of transplanted KLS cells within the BM was analyzed 1 and 20 weeks after transplantation.
Complete blood count analysis
Whole blood was collected into EDTA capillary tubes (Fisher Scientific, Hampton, NH). Samples were analyzed using Hemavet 850 FF (Drew Scientific) by the Mouse Pathology Core at UCSF.
ACK2 clearance
Clearance of ACK2 from host serum was determined as previously described23 and is detailed in the supplemental Methods on the Blood Web site.
Comparison of in utero to postnatal ACK2 administration and transplantation
CD45.2 neonates born after in utero ACK2 treatment (5 µg per fetus) and untreated control neonates were transplanted with congenic CD45.1/CD45.2 FLMC at P1. The treated group subsequently received a second ACK2 dose at 2 weeks by retroorbital injection (25 µg/g, weight based equivalent to in utero dose), and both groups were transplanted with 2.5 × 106 congenic CD45.1+/+ FLMC 7 days later. Peripheral blood chimerism (CD45.1/CD45.2 [first transplant] and CD45.1+/+ [second transplant]) was determined starting at 5 weeks of age. A separate group of mice received retroorbital injections of ACK2 at 2 weeks of age and BM and serum was subsequently collected and analyzed to confirm KLS depletion and antibody clearance.
Statistics
Data comparing 2 groups were evaluated using either the χ-square test or Student t test. Multiple grouped data were evaluated using analysis of variance (ANOVA) with Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM). A P value of <.05 was considered statistically significant. Statistical analysis was performed using Prism Graphpad (GraphPad Software, Inc., La Jolla, CA).
Results
Survival after in utero ACK2 treatment
We first monitored survival after in utero ACK2 treatment at doses ranging from 2.5 to 50 µg per fetus. We found that survival to birth among fetuses injected with 2.5, 5, or 10 µg of ACK2 was similar to controls (Figure 1A). However, survival to birth was significantly lower in fetuses injected with 20 or 50 µg of ACK2 (Figure 1A).
Toxicity associated with in utero ACK2 administration in mice. (A) Survival to birth after in utero injection of ACK2 at various concentrations. Control (isotype control antibody or PBS, n = 115); 2.5 µg, n = 44; 5 µg, n = 123; 10 µg, n = 45; 20 µg, n = 134; 50 µg, n = 70. ****P ≤ .0001 by χ-square test). (B) Survival to 1 month after neonatal transplantation in mice treated in utero with ACK2 antibody. Control, n = 15; 2.5 µg, n = 8; 5 µg, n = 13; 10 µg, n = 9; 20 µg, n = 17; 50 µg, n = 6. *P < .05 in both groups by χ-square test. (C) Hypopigmentation after in utero ACK2 administration. (D) Frequency of coat discoloration in 2-week-old mice after in utero injection of ACK2. Normal coat, n = 59; ≤50% of total body surface area (TBSA), n = 13; and >50% TBSA coat discoloration, n = 8. (E) Weight of control and treated (5 µg per fetus) mice at various time points. N ≥ 5 per group. (F) RBC concentration (hematocrit) and (G) leukocyte count of peripheral blood collected 7 days after ACK2 administration in control and treated (5 µg per fetus) animals. Control, n = 7; 2.5 µg, n = 5; 5 µg, n = 17; *P < .05, **P < .01 by ANOVA with Tukey’s multiple comparison test. (H) Hematocrit over time in control and treated (5 µg per fetus) pups (n ≥ 4 for each time point per group). (I) Leukocyte count over time in control and treated (5 µg per fetus) pups (n ≥ 4 for each time point per group). Data presented as percentage or mean ± SEM. n.s., no significance.
Toxicity associated with in utero ACK2 administration in mice. (A) Survival to birth after in utero injection of ACK2 at various concentrations. Control (isotype control antibody or PBS, n = 115); 2.5 µg, n = 44; 5 µg, n = 123; 10 µg, n = 45; 20 µg, n = 134; 50 µg, n = 70. ****P ≤ .0001 by χ-square test). (B) Survival to 1 month after neonatal transplantation in mice treated in utero with ACK2 antibody. Control, n = 15; 2.5 µg, n = 8; 5 µg, n = 13; 10 µg, n = 9; 20 µg, n = 17; 50 µg, n = 6. *P < .05 in both groups by χ-square test. (C) Hypopigmentation after in utero ACK2 administration. (D) Frequency of coat discoloration in 2-week-old mice after in utero injection of ACK2. Normal coat, n = 59; ≤50% of total body surface area (TBSA), n = 13; and >50% TBSA coat discoloration, n = 8. (E) Weight of control and treated (5 µg per fetus) mice at various time points. N ≥ 5 per group. (F) RBC concentration (hematocrit) and (G) leukocyte count of peripheral blood collected 7 days after ACK2 administration in control and treated (5 µg per fetus) animals. Control, n = 7; 2.5 µg, n = 5; 5 µg, n = 17; *P < .05, **P < .01 by ANOVA with Tukey’s multiple comparison test. (H) Hematocrit over time in control and treated (5 µg per fetus) pups (n ≥ 4 for each time point per group). (I) Leukocyte count over time in control and treated (5 µg per fetus) pups (n ≥ 4 for each time point per group). Data presented as percentage or mean ± SEM. n.s., no significance.
We also analyzed survival after neonatal transplantation in these groups of mice. Survival was similar (between 80% and 85%) in control pups and in those previously treated with ACK2 at 2.5, 5, or 10 µg per fetus, but was significantly lower after high concentrations of ACK2 (52% survival for 20 µg and 0% survival for 50 µg ACK2, P < .05 for each group) (Figure 1B).
Toxicity of ACK2
It has been reported that c-Kit is functionally important for normal melanocyte development and that maternal administration of an antibody against the c-Kit receptor leads to altered coat color of offspring when injected early in gestation.28 In our model, in utero treatment with ACK2 periodically resulted in coat discoloration (Figure 1C). Coat discoloration affected 26% of all treated mice and most often affected <50% of the body surface area (Figure 1D). Interestingly, coat discoloration was almost exclusively seen in animals injected at E13.5, suggesting that c-Kit expression on melanocytes is temporally regulated.
The c-Kit receptor is also expressed on interstitial cells of Cajal,29 which regulate intestinal motility. We did not note any gastrointestinal toxicity; the weights of treated animals were the same as those of controls at birth, 4 weeks, and 20 weeks of age (Figure 1E). Finally, the c-Kit receptor is expressed on germ cells, but both male and female offspring injected with ACK2 were fertile and delivered viable pups, similar to what has been seen in adult mice exposed to ACK2.23
We also analyzed complete blood counts to determine any effects of ACK2 treatment on mature circulating cells. We detected no alterations in the hematologic profile in mice treated with the lowest dose of ACK2 (2.5 µg per fetus) (Figure 1F). However, we detected anemia and leukopenia (both neutropenia and lymphopenia) 7 days after in utero injection of 5 µg/fetus ACK2 (Figure 1F-G; supplemental Table 1). The effect was transient, with full reconstitution by 2 weeks (Figure 1H-I). There was no significant thrombocytopenia at this dose (supplemental Table 1). Importantly, we did not detect any adverse effects of in utero administration of ACK2 on maternal hematopoiesis. The maternal circulating erythrocyte and leukocyte counts were similar between control and treated (5 µg per fetus) mothers (supplemental Figure 1A-B), as were the number of KLS cells within the BM at P1 (supplemental Figure 1C).
Host HSC depletion
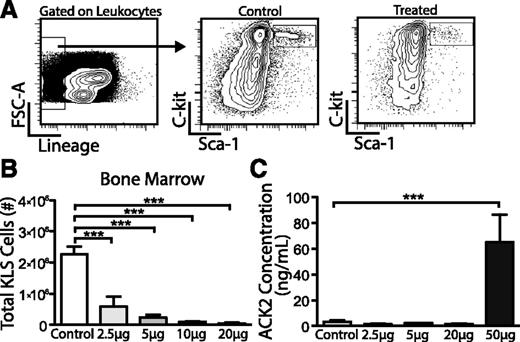
To quantify depletion of host HSCs after ACK2 treatment, we harvested BM and livers 7 days after in utero injection and stained for lineage markers, c-Kit, and Sca-1 to define KLS cells (Figure 2A). We found that ACK2 treatment led to a decrease in the absolute number of KLS cells in the BM at all concentrations tested, with a 74% to 98% depletion at doses of 2.5 to 20 µg per fetus (Figure 2B). Interestingly, KLS depletion was not seen in the liver on P1 (supplemental Figure 2). Analysis of neonatal splenocytes indicated no changes in the relative lineage distribution of granulocytes, B cells, and T cells between treated and control mice, suggesting ACK2 treatment depletes HSCs rather than committed progenitors (supplemental Figure 3).
Efficacy of HSC depletion and clearance of ACK2 antibody after in utero treatment in mice. Fetuses were treated with isotype control/PBS or varying doses of ACK2 at E13.5 to E14.5 and blood and BM were harvested 7 days later. (A) Gating strategy to detect KLS cells (Lin−c-Kit+Sca-1+) in neonatal pups following in utero ACK2 treatment after first gating on live leukocytes. (B) Total number of BM KLS cells within long bones 7 days after ACK2 treatment. Control, n = 14; 2.5 µg, n = 8; 5 µg, n = 17; 10 µg, n = 5; 20 µg, n = 4. (C) Serum concentrations of ACK2 7 days after in utero isotype control/PBS or ACK2 injections. N ≥ 3 in each group. Data presented as mean ± SEM. ***P < .001 by ANOVA with Tukey’s multiple comparison test.
Efficacy of HSC depletion and clearance of ACK2 antibody after in utero treatment in mice. Fetuses were treated with isotype control/PBS or varying doses of ACK2 at E13.5 to E14.5 and blood and BM were harvested 7 days later. (A) Gating strategy to detect KLS cells (Lin−c-Kit+Sca-1+) in neonatal pups following in utero ACK2 treatment after first gating on live leukocytes. (B) Total number of BM KLS cells within long bones 7 days after ACK2 treatment. Control, n = 14; 2.5 µg, n = 8; 5 µg, n = 17; 10 µg, n = 5; 20 µg, n = 4. (C) Serum concentrations of ACK2 7 days after in utero isotype control/PBS or ACK2 injections. N ≥ 3 in each group. Data presented as mean ± SEM. ***P < .001 by ANOVA with Tukey’s multiple comparison test.
ACK2 clearance
We next analyzed the timing of ACK2 clearance from the serum to ensure that transplanted HSCs would not be affected by this initial dose. We found that ACK2 was cleared from the serum 7 days after injection at all concentrations <50 µg per fetus (Figure 2C). At the 20 µg per fetus dose, ACK2 levels were detectable 5 days after treatment but undetectable at 7 days (data not shown). We therefore performed neonatal transplantations at doses 2.5 to 20 µg per fetus on P1-P2, 7 days after ACK2 injection.
Engraftment after in utero ACK2 treatment followed by neonatal HSC transplantation
We performed congenic fetal liver transplantation (CD45.1/CD45.2 into CD45.2) in mice treated in utero with various doses of ACK2 or controls and analyzed levels of peripheral blood chimerism starting 3 weeks after transplantation. We calculated rates of engraftment (number of surviving mice with >1% donor cells/total number of transplanted animals) as well as the levels of donor cell engraftment (donor CD45/(donor + host CD45) × 100%). Rates of engraftment were high both in control and treated animals at all concentrations (Figure 3A).
Increased engraftment after in utero ACK2 treatment followed by neonatal transplantation. B6.CD45.2 mice treated in utero with ACK2 or control antibody were transplanted on P1-2 with congenic B6.CD45.1/CD45.2 fetal liver mononuclear cells and levels of peripheral blood chimerism (donor CD45 cells/(donor+host CD45 cells) × 100) were determined by flow cytometry starting 3 weeks after transplantation. (A) Rate of engraftment (number of animals with >1% chimerism/surviving animals) at 4 weeks. Control n = 16; 2.5 µg, n = 8; 5 µg, n = 10; 10 µg, n = 9; 20 µg, n = 4. (B) Levels of donor CD45 chimerism at 4 weeks. N, number of chimeric animals reported in panel A, excluding 1 nonchimeric animal. *P < .05 and **P < .01 by ANOVA with Tukey’s multiple comparison test. There is a significant negative correlation between ACK2 dose and level of chimerism: Pearson R = −0.96, P = .04. (C) Levels of donor CD45 chimerism over time. N = 4-9 animals from ≥2 independent experiments for each group. Data presented as percentage or mean ± SEM. *P < .05 by ANOVA with Tukey’s comparison between doses of 2.5 to 10 µg and control.
Increased engraftment after in utero ACK2 treatment followed by neonatal transplantation. B6.CD45.2 mice treated in utero with ACK2 or control antibody were transplanted on P1-2 with congenic B6.CD45.1/CD45.2 fetal liver mononuclear cells and levels of peripheral blood chimerism (donor CD45 cells/(donor+host CD45 cells) × 100) were determined by flow cytometry starting 3 weeks after transplantation. (A) Rate of engraftment (number of animals with >1% chimerism/surviving animals) at 4 weeks. Control n = 16; 2.5 µg, n = 8; 5 µg, n = 10; 10 µg, n = 9; 20 µg, n = 4. (B) Levels of donor CD45 chimerism at 4 weeks. N, number of chimeric animals reported in panel A, excluding 1 nonchimeric animal. *P < .05 and **P < .01 by ANOVA with Tukey’s multiple comparison test. There is a significant negative correlation between ACK2 dose and level of chimerism: Pearson R = −0.96, P = .04. (C) Levels of donor CD45 chimerism over time. N = 4-9 animals from ≥2 independent experiments for each group. Data presented as percentage or mean ± SEM. *P < .05 by ANOVA with Tukey’s comparison between doses of 2.5 to 10 µg and control.
Levels of chimerism were analyzed serially between 3 and 20 weeks. At 4 weeks, the levels of donor cell chimerism were significantly higher after ACK2 treatment at 2.5, 5, and 10 µg per fetus compared with controls (Figure 3B). Interestingly, ACK2 dose and peripheral blood chimerism were inversely correlated at 4 weeks (Pearson R = −0.96, P = .04), suggesting additional toxicity at the higher doses. Analysis of chimerism levels over time indicated a gradual increase in chimerism in treated mice, but a steady level of chimerism in control mice (Figure 3C). Peripheral blood chimerism at 24 weeks was evaluated in a subset of treated mice and confirmed steady long-term engraftment (data not shown). Thus, the levels of chimerism achieved with in utero ACK2 treatment were significantly higher at all time points beyond 4 weeks. Importantly, stable chimeric levels achieved after in utero ACK2 depletion are clinically meaningful and significantly higher than what we have previously reported after in utero transplantation of congenic cells without ACK2 depletion11 (ACK2 at 2.5 µg per fetus vs in utero alone, 24.2 ± 2.2 vs 9.9 ± 1.9 respectively, n = 4-14 per group, P = .002).
We next examined the lineage distribution of engrafted cells after host ACK2 treatment to determine whether there is preferential engraftment of a particular committed precursor or a true increase in HSC engraftment. We stained peripheral blood 4 weeks after transplantation with antibodies against granulocytes, B cells, and T cells and determined that there was multilineage engraftment of the transplanted cells (Figure 4A). We further determined that the increase in chimerism in ACK2-treated animals was secondary to equally increased engraftment in all lineages (Figure 4B). We next confirmed that ACK2 treatment led to increased KLS cell engraftment in the BM at both 1 week and 20 weeks compared with controls (Figure 4C). Peripheral blood chimerism levels were consistent with BM KLS chimerism levels in stably engrafted animals (Figure 4D). These findings support a model in which ACK2 depletion leads to increased engraftment of HSCs, rather than committed progenitors.
ACK2 treatment leads to increased multilineage and KLS chimerism. Peripheral blood was analyzed 4 weeks after transplantation and the lineage composition of donor cells was compared with the lineage composition of host cells. (A) Gating strategy to determine donor granulocyte (Gr-1+), B-cell (CD19+), and T-cell (CD3+) populations. (B) Lineage-specific chimerism for granulocytes, B cells, and T cells. N = 4-9 mice per group. *P < .05 and **P < .01 by ANOVA with Tukey’s multiple comparison test. (C) Total number of donor KLS cells harvested from long bones 1 and 20 weeks after transplantation. Treated mice were previously injected with 5 µg per fetus 7 days before neonatal transplantation (n ≥ 3 from ≥2 independent experiments for each group). Data presented as mean ± SEM. *P < .05 and **P < .01 by Student t test. (D) Peripheral blood CD45 chimerism compared with BM KLS chimerism in mice previously treated with ACK2 (5 µg per fetus) in utero at 1 and 20 weeks. N ≥ 7 per group. **P < .01 by Student t test.
ACK2 treatment leads to increased multilineage and KLS chimerism. Peripheral blood was analyzed 4 weeks after transplantation and the lineage composition of donor cells was compared with the lineage composition of host cells. (A) Gating strategy to determine donor granulocyte (Gr-1+), B-cell (CD19+), and T-cell (CD3+) populations. (B) Lineage-specific chimerism for granulocytes, B cells, and T cells. N = 4-9 mice per group. *P < .05 and **P < .01 by ANOVA with Tukey’s multiple comparison test. (C) Total number of donor KLS cells harvested from long bones 1 and 20 weeks after transplantation. Treated mice were previously injected with 5 µg per fetus 7 days before neonatal transplantation (n ≥ 3 from ≥2 independent experiments for each group). Data presented as mean ± SEM. *P < .05 and **P < .01 by Student t test. (D) Peripheral blood CD45 chimerism compared with BM KLS chimerism in mice previously treated with ACK2 (5 µg per fetus) in utero at 1 and 20 weeks. N ≥ 7 per group. **P < .01 by Student t test.
ACK2 treatment does not affect the immune barrier to transplantation
Although chimerism improves after in utero ACK2 treatment, clinical use of this strategy will likely involve transplantation of semiallogeneic or allogeneic cells. We therefore tested whether ACK2 treatment also improves engraftment after allogeneic transplantation. Contrary to in utero transplantation,11 neonatal transplantation of allogeneic cells into a wild-type host does not result in engraftment,30 likely because the host immune system is more mature. Furthermore, ACK2 treatment did not lead to detectable engraftment 4 weeks after neonatal allogeneic (BALB/c into B6) transplantation (control, n = 6; 2.5 µg, n = 4; 5 µg, n = 4; 10 µg, n = 5), supporting the observation that ACK2 depletes HSCs without significantly depleting mature progenitors. Thus, these experiments demonstrate a clear distinction between the “space” and “immune” barriers to engraftment.
Comparison of in utero to postnatal ACK2 administration and transplantation
In utero transplantation in mice can be followed by neonatal conditioning and repeat “booster” transplantations to improve levels of chimerism.31,32 However, improved engraftment using ACK2 in adult mice has required either low-dose irradiation25 or the use of Rag2−/−γc−/− or Rag2−/− animals,23 whereas our in utero approach was effective in wild-type mice without irradiation. To understand whether there is any advantage to ACK2 treatment in the fetal environment or whether this strategy can also be used after birth to boost engraftment, we performed experiments combining pre- and postnatal depletion and transplantation. We treated fetuses with ACK2 (5 µg/fetus) and transplanted the surviving neonates with congenic (CD45.1/CD45.2) FLMC at P1, followed by a second dose of ACK2 at 2 weeks of age, and then congenic (CD45.1+/+) FLMC transplantation 1 week later (Figure 5A). We first tested whether postnatal ACK2 treatment could effectively deplete HSCs and whether the antibody was also cleared at 1 week after injection. We found a 62 ± 7.0% reduction in KLS cells in the BM of treated mice compared with controls and undetectable levels of ACK2 in the serum by 7 days (Figure 5B-C). When we compared the chimerism levels from the first transplant with the second transplant, we found increased levels only from the first transplant, whereas the chimerism levels from the second transplant were not different between control and treated groups (1.1 ± 0.2% vs 1.6 ± 0.4%, P = .29, Figure 5D-E). In addition, the levels of chimerism at 8 weeks of age resulting from the second transplant were 10-fold lower than that of the first transplant in treated animals (P = .008). These results suggest that ACK2 treatment is likely most effective in the fetal environment and explain our ability to use this strategy in wild-type, nonirradiated fetal mice.
Increased efficacy of in utero ACK2 treatment compared with neonatal ACK2 treatment. (A) CD45.2 fetuses were injected with ACK2 in utero and transplanted at P1 with FLMCs from CD45.1/CD45.2 mice. They received a second ACK2 treatment at 2 weeks followed by transplantation of FLMC from CD45.1+/+ mice at 3 weeks of age. Control mice underwent transplantation without ACK2 treatment. (B) Total number of BM KLS cells within long bones 7 days after ACK2 treatment (25 µg/g) at 2 weeks of age. N = 5 per group; **P < .01 by Student t test. (C) Serum concentrations of ACK2 after ACK2 treatment at age 2 weeks. N = 6. (D) Gating strategy to detect host (CD45.2), first donor (CD45.1/CD45.2), and second donor (CD45.1+/+) leukocytes. (E) Levels of chimerism among mice that received multiple transplantations. N ≥ 5 per group. *P < .05 comparing peripheral blood CD45 chimerism from the first transplant between control and treated mice by Student t test. There were no differences in the levels of chimerism resulting from the second transplantation between control and treated mice.
Increased efficacy of in utero ACK2 treatment compared with neonatal ACK2 treatment. (A) CD45.2 fetuses were injected with ACK2 in utero and transplanted at P1 with FLMCs from CD45.1/CD45.2 mice. They received a second ACK2 treatment at 2 weeks followed by transplantation of FLMC from CD45.1+/+ mice at 3 weeks of age. Control mice underwent transplantation without ACK2 treatment. (B) Total number of BM KLS cells within long bones 7 days after ACK2 treatment (25 µg/g) at 2 weeks of age. N = 5 per group; **P < .01 by Student t test. (C) Serum concentrations of ACK2 after ACK2 treatment at age 2 weeks. N = 6. (D) Gating strategy to detect host (CD45.2), first donor (CD45.1/CD45.2), and second donor (CD45.1+/+) leukocytes. (E) Levels of chimerism among mice that received multiple transplantations. N ≥ 5 per group. *P < .05 comparing peripheral blood CD45 chimerism from the first transplant between control and treated mice by Student t test. There were no differences in the levels of chimerism resulting from the second transplantation between control and treated mice.
Discussion
In this study, we demonstrate that in utero depletion of host HSCs before transplantation leads to increased levels of chimerism and that using ACK2 in the fetus provides a unique window of opportunity to exploit the c-Kit/SCF axis for clinical transplantation. The levels of engraftment (15% to 20%) were higher than what we had previously achieved with IUHCTx alone11 and reached clinical significance for treating conditions such as sickle cell disease.33 Because IUHCTx has not been successful for any disease except for severe combined immunodeficiency6-8 because of low engraftment, it is possible that antibody-based clearance of fetal host HSCs could improve donor cell chimerism to clinically relevant levels.
Our experiments suggest a particular advantage of using ACK2 in the fetal environment compared with the adult. Notably, ACK2 does not enhance donor engraftment in the immunocompetent adult without irradiation augmentation,25 and fetal administration appears to be the only setting in which isolated ACK2 conditioning is sufficient to enhance engraftment of a wild-type animal. Fetal HSCs in the blood and BM may be more reliant on SCF-c-Kit signaling for survival and thus may be more effectively depleted with ACK2. Additionally, administration at E13.5 to E14.5 blocks c-Kit-SCF ligand signaling at a critical time point when HSCs are migrating from the fetal liver to the BM and ACK2 may limit their homing as fetal HSCs have been shown to be sensitive to the chemotactic effects of SCF for their migration.34 Thus, the use of ACK2 in the fetal environment may provide a “double hit” that further enhances its efficacy. The lack of depletion of fetal liver KLS cells on P1 is an intriguing finding that supports the hypothesis that fetal HSCs in the blood and BM may be more reliant on SCF for either sustained function or cell migration. In addition, we may not have detected the depletion in the liver on P1 because it is no longer the site of hematopoiesis.
In utero administration of ACK2 may also directly affect the BM niche, which could explain our finding of decreased engraftment at higher doses. At higher doses (20-50 µg/fetus), ACK2 is still detectable in the serum after 5 days (E18.5-19.5) and may affect the nascent BM niche itself, whereas lower doses of ACK2 are cleared earlier. Because the c-Kit receptor is expressed on cells of the BM niche, such as osteoblasts and osteoclasts,35,36 and vascular endothelial stem cells,37 high doses of ACK2 may have some toxicity on these components of the niche at the critical time point of their emergence. With low-dose fetal liver ACK2 injection, the antibody is cleared before formation of the BM niche and its seeding by the transplanted cells. Thus, use of ACK2 at a time when the niche is “shifting” provides an additional reason to consider the use of this strategy in utero.
We chose our experimental model of in utero depletion followed by neonatal transplantation because of the short gestational period in the mouse compared with the long half-life of ACK2. When extrapolated to large animals and humans, both ACK2 treatment and the subsequent transplantation can be performed in utero. However, the timing of depletion and of transplantation are both critical elements that need to be optimized in large animal models before clinical translation. Theoretically, such an optimized strategy could minimize toxicity on the ultimate BM niche for the transplanted cells. However, because the immune system matures earlier in humans, a host immune response could potentially limit engraftment of allogeneic cells. Additionally, if both the depletion and the transplantation are performed during the time when fetal liver is the site of hematopoiesis, the effects may be less pronounced.
The relative lack of effect of ACK2 observed in the postnatal environment of wild-type mice compared with fetal is noteworthy and may be influenced by several factors. There may be significant differences such as the variation in susceptibility of fetal HSCs compared with adult HSCs to SCF signaling.34,38 Fetal HSCs differ from adult HSCs in many ways, such as cell surface markers, proliferative capacity, and developmental potential.39-42 Although both fetal and adult murine HSCs express similar levels of c-Kit on the cell surface,38,43,44 they undergo a developmental “switch” shortly after birth, becoming more quiescent and less susceptible to SCF signaling.45 Gene expression differences downstream of the c-Kit receptor are also critically different between fetal and adult HSCs,38 and such changes in host HSCs can persist for some time after clearance of ACK2, giving even more of a competitive advantage for the transplanted cells. In addition, ACK2 may be toxic to the BM niche once it has formed. Alternatively, if the antibody affects migration of cells from the fetal liver to the BM, this benefit is not observed in the postnatal environment. The use of ACK2 in the fetal environment may also have the added benefit of targeting HSCs from the placenta, another important source of fetal hematopoiesis,46 especially because it has been shown that placental HSCs are also subject to SCF-c-Kit signaling for their survival.47
We observed some adverse effects of ACK2 at higher concentrations, highlighting the importance of proper titration in each model system. We think that mortality at high doses resulted from severe anemia and/or pancytopenia, which is known to cause death in fetuses because of heart failure.48 It is interesting that toxicity was not more widespread, because c-Kit is broadly expressed on many cell types such as melanocytes,28 mast cells,49 germ cells,50 myocardial stem cells,51 and interstitial cells of Cajal.29 The main off-target effect we observed at low doses was hypopigmentation, which was dependent upon the timing of ACK2 treatment, although we cannot rule out more subtle effects on other cell types. ACK2 has been shown to work through inhibition of SCF-c-Kit signaling and not direct depletion of c-Kit+ cells, and we speculate that HSCs are more reliant on SCF signaling for their survival than other cell types. The clinical efficacy and safety of ACK2 depletion will need to be examined in a relevant nonhuman primate model of the disease.
One limitation to our study is that we did not investigate the efficacy of engraftment of adult BM-derived cells, a more clinically relevant stem cell source. However, because ACK2 is cleared by the time the donor cells are transplanted, it should still be effective regardless of the donor cell source. In our experiments comparing prenatal with postnatal depletion, we again used fetal liver–derived HSCs for consistency between the experimental groups. One strength of our study is that we demonstrate that space and immune response are separate barriers for neonatal transplantation, a result that carries important implications for the design of clinical trials, which may require both HSC depletion and immunomodulation for optimum engraftment. For single gene disorders that can be treated by gene correction and retransplantation of autologous stem cells, such as inborn errors of metabolism, there will still be a need for fetal HSC depletion to improve the levels of engraftment.
Strategies that significantly increase donor cell engraftment with minimal toxicity will broaden the scope and success of IUHCTx. We demonstrate one such strategy with superior results in the fetal environment compared with postnatal, providing another compelling rationale for in utero treatment of congenital hematopoietic disorders.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of MacKenzie and Passegué laboratories and Drs Mike Harrison and Mike Longaker for helpful discussions. The authors also thank Ninnia Lescano for excellent mouse husbandry.
This work was supported by grants from National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant K08 A1085042-01) (T.C.M.), American Surgical Association (T.C.M.), California Institute of Regenerative Medicine (T.C.M.), and the University of California, San Francisco Liver Center flow cytometry core (NIH P30 DK026743). The contents of this publication are solely the responsibility of the authors.
Authorship
Contribution: S.C.D., A.C., and T.C.M. designed the research; S.C.D., C.K., P.W.M., and P.P.T. performed experiments; S.C.D., A.C., P.P.T., D.R., I.L.W., and T.C.M. analyzed results; and S.C.D. and T.C.M. wrote the paper with input from all of the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tippi C. MacKenzie, Campus Box 0570, University of California, San Francisco, 513 Parnassus Ave, San Francisco, CA 94143-0570; e-mail: tippi.mackenzie@ucsfmedctr.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal