Key Points
Combination treatment with RG7112 and Peg-IFNα 2a targets primitive JAK2V617F+ progenitor cells in myeloproliferative neoplasms.
The Philadelphia chromosomal–negative chronic myeloproliferative neoplasms (MPNs) originate at the level of the hematopoietic stem cell (HSC). The protracted clinical course of the MPNs has limited the use of potentially toxic treatment modalities, which may eliminate the responsible malignant clone. Treatment with low doses of RG7112, an orally available small-molecule inhibitor of p53-MDM2, both alone and combined with pegylated interferon α 2a (Peg-IFNα 2a), significantly decreased MPN colony-forming unit–granulocyte macrophage and burst-forming unit-erythroid numbers and preferentially eliminated the total number of JAKV617F+ MPN hematopoietic progenitor cells. The effects of RG7112 and Peg-IFNα 2a on MPN progenitor cells were dependent on blocking p53-MDM2 interactions and activating the p53 pathway, thereby increasing MPN CD34+ cell apoptosis. Treatment of polycythemia vera (PV) and primary myelofibrosis (PMF) CD34+ cells with low doses of RG7112 and Peg-IFNα 2a before their transplantation into immune-deficient mice decreased the degree of donor-derived chimerism as well as the JAK2V617F allele burden, indicating that these drugs can each alone or in combination deplete MPN HSCs. These results provide a rationale for the use of combinations of low doses of RG7112 and Peg-IFNα 2a for the treatment of PV or PMF patients with the intent of altering their natural history.
Introduction
The Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs), which include polycythemia vera (PV) essential thrombocythemia (ET), and primary myelofibrosis (PMF), are clonal hematologic malignancies thought to originate at the level of multipotent hematopoietic progenitor cells (HPCs) or hematopoietic stem cells (HSCs).1,,-4 To dramatically alter the natural history of MPN patients, drugs capable of eradicating or at least depleting the pool of MPN disease-initiating and disease-sustaining HSCs will likely be required. Of the drugs currently available that are used to treat patients with MPNs, only interferon-α (IFNα) has been shown to reproducibly reduce the JAK2V617F allele burden of PV and ET patients and to induce molecular remissions in 17% to 18% of patients.5,,,,,,-12 Although these remissions can be sustained for prolonged periods of time after cessation of interferon therapy, the reappearance of JAK2V617F+ cells can sometimes occur, suggesting that a pool of mutated HSCs survive. Our laboratory has attempted to target the p53 pathway with pharmacologic agents with the hope of reducing or eliminating the numbers of MPN stem cells.13 Because MPNs are likely the result of multiple genetic mutations as well as epigenetic events that are difficult to recapitulate in rodent models, we have used primary cells from MPN patients to evaluate these potential therapeutic agents.13,14
The tumor suppressor p53 plays an important role in the control of DNA repair, the cell cycle, apoptosis, and cancer surveillance.15,16 Most MPN patients have wild-type (WT) p53, whereas p53 mutations and heterozygous deletions have been almost exclusively identified in MPN patients who are undergoing transformation to acute leukemia.17,18 Although the therapeutic effects of IFNα in MPN patients have been attributed to its effect on several biological processes, interferon is also known to influence p53. IFNα binds to the type I IFN receptor, and activates the JAK/TYK/STAT pathway, leading to multiple downstream events.19 IFNα, however, also activates a p38 mitogen–activated protein kinase (MAPK), resulting in apoptosis of PV HPCs.20,21 Both of these pathways have been shown to act through the p53 tumor suppressor protein. p53 is also negatively regulated by MDM2,22,23 which is the master regulator of p53 and the p53-specific E3 ubiquitin ligase, which mediates the ubiquitin-dependent degradation of p53. MDM2 not only facilitates p53 degradation but also binds p53 and inhibits its transcriptional activity. Nakatake and coworkers demonstrated in human cell lines that JAK2V617F alters p53 responses to DNA damage through upregulation of La antigen, which increases MDM2 protein translation.24 We have recently reported that MDM2 levels are increased in primary PV CD34+ cells,13 whereas the p53 levels are reduced in CD34+ cells from both patients with PV and PMF. The cis-imidazoline compounds termed nutlins were the first potent and selective MDM2 inhibitors, and their discovery stimulated widespread interest in the design of small molecule p53-MDM2 inhibitors.22,25 We reported that combination treatment with low doses of Peg IFNα 2a and nutlin-3, an antagonist of MDM2, induced PV CD34+ apoptosis and inhibited PV colony formation significantly. The combination of these agents also decreased the number of JAK2V617F+ HPCs.13
The initial nutlins including the one used in our prior study were not suitable for use in man because of their limited potency, unfavorable physiochemical properties, and poor pharmacokinetic properties. RG7112, is a novel, orally bioactive member of the nutlin family, which is a more potent and selective small-molecule inhibitor of p53-MDM2 binding, which frees p53 and activates the p53 pathway. RG7112 is currently being evaluated in several phase 1 clinical trials of cancer patients.26 We hypothesized that RG7112 would be a promising candidate to be combined with IFNα, with the hope of reducing the numbers or actually eliminating MPN HPCs and HSCs.
Materials and methods
Specimen collection and cell preparation
Units of whole blood were obtained from 28 PV patients being treated with therapeutic phlebotomy alone and/or aspirin and spleen samples from 8 PMF patients who required therapeutic splenectomy. Written informed consent was obtained from all patients according to guidelines established by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (ISMMS). The study was conducted in accordance with the Declaration of Helsinki. All patients met the World Health Organization diagnostic criteria for the diagnosis of PV and PMF.27 The JAK2V617F allele burden of each patient is provided in supplemental Tables 1 and 2 available on the Blood Web site. None of the patients had MPLW515L mutations. Single-cell suspensions were prepared from the PMF spleens that were surgically removed by methods previously described.28 The blood samples or single-cell suspensions of spleens were layered onto Ficoll-Hypaque (1.077g/mL; GE Healthcare, Piscataway, NJ) and low-density mononuclear cells were separated after centrifugation. The CD34+ cell population was isolated using a human CD34+ cell selection kit (StemCell Technologies, Vancouver, BC, Canada). Normal human bone marrow (BM) mononuclear cells and CD34+ cells were purchased from AllCells (Emeryville, CA). The experiments for which the CD34+ cells from each of the 36 patients were used are itemized in supplemental Tables 1 and 2.
Hematopoietic progenitor cell assays
CD34+ cells were assayed for HPC in semisolid media as previously described.29 Briefly, 500 CD34+ cells were plated in duplicate in tissue culture dishes (30-mm diameter containing 1 mL IMDM with 1.1% methylcellulose and 20% fetal bovine serum, to which stem cell factor [SCF], thrombopoietin [TPO], fms-like tyrosine kinase 3 ligand [Flt-3 ligand], interleukin-3 [IL-3], granulocyte macrophage-colony stimulating factor at 50 ng/mL, and 2 U/mL erythropoietin were added). Various doses of RG7112 (100 nM to 10 uM) (gift of Roche Pharamaceuticals, Nutley, NJ) alone and in combination with Peg-IFNα 2a (Roche Pharmaceuticals) (200 ng/mL) were added to the medium. Colonies were enumerated after 14 days of incubation, and individual colonies were plucked and genotyped for JAK2V617F as previously described.29 Similar doses of an inactive enantiomer, RG7112i, were used as a negative control (gift of Roche Pharmaceuticals). The total number of colonies plucked and analyzed for JAK2V617F from each case (>100) is provided in Tables 1 and 2.
Combination treatment with low doses of RG7112 and Peg-IFNα 2a reduced the number of JAKV617F+ hematopoietic colonies
| Patient number* . | JAK2 V617F allele burden (%) . | Total colonies genotyped . | Additions to culture . | |||||
|---|---|---|---|---|---|---|---|---|
| Cytokines only (control) . | Peg-IFNα 2a 200 ng/mL + RG7112 200 nM . | |||||||
| Homo % . | Hetero % . | WT† % . | Homo % . | Hetero % . | WT† % . | |||
| 2 | 14 | 127 | 8.3 | 33.3 | 58 | 11.7 | 17.6 | 70.6 |
| 15 | 70 | 129 | 76.2 | 9.5 | 14.3 | 55 | 5 | 40 |
| 5 | 49 | 144 | 21 | 9 | 70 | 21 | 0 | 79 |
| 6 | 22 | 132 | 50 | 14 | 38 | 29 | 21 | 50 |
| 13 | 75 | 180 | 25 | 54 | 21 | 20 | 30 | 50 |
| 14 | 20 | 168 | 30 | 39 | 31 | 15 | 40 | 45 |
| 3 | 40 | 189 | 17 | 4 | 79 | 11 | 2 | 87 |
| 16 | 50 | 121 | 44 | 50 | 4 | 58 | 18 | 24 |
| 22 | 70 | 121 | 59 | 35 | 6 | 64 | 9 | 27 |
| 21 | 45 | 112 | 4.5 | 45.5 | 50 | 0 | 16.7 | 83.3‡ |
| 19 | + | 105 | 12.5 | 75 | 12.5 | 9.1 | 0 | 91‡ |
| Patient number* . | JAK2 V617F allele burden (%) . | Total colonies genotyped . | Additions to culture . | |||||
|---|---|---|---|---|---|---|---|---|
| Cytokines only (control) . | Peg-IFNα 2a 200 ng/mL + RG7112 200 nM . | |||||||
| Homo % . | Hetero % . | WT† % . | Homo % . | Hetero % . | WT† % . | |||
| 2 | 14 | 127 | 8.3 | 33.3 | 58 | 11.7 | 17.6 | 70.6 |
| 15 | 70 | 129 | 76.2 | 9.5 | 14.3 | 55 | 5 | 40 |
| 5 | 49 | 144 | 21 | 9 | 70 | 21 | 0 | 79 |
| 6 | 22 | 132 | 50 | 14 | 38 | 29 | 21 | 50 |
| 13 | 75 | 180 | 25 | 54 | 21 | 20 | 30 | 50 |
| 14 | 20 | 168 | 30 | 39 | 31 | 15 | 40 | 45 |
| 3 | 40 | 189 | 17 | 4 | 79 | 11 | 2 | 87 |
| 16 | 50 | 121 | 44 | 50 | 4 | 58 | 18 | 24 |
| 22 | 70 | 121 | 59 | 35 | 6 | 64 | 9 | 27 |
| 21 | 45 | 112 | 4.5 | 45.5 | 50 | 0 | 16.7 | 83.3‡ |
| 19 | + | 105 | 12.5 | 75 | 12.5 | 9.1 | 0 | 91‡ |
24 to 48 colonies were plucked and examined in each condition. Homo, homozygous; hetero, heterozygous.
The number indicates the individual patients listed in supplemental Table 2.
A 1-tailed paired Student t test was used for statistical analysis. Every individual case was compared with treatment with cytokines alone, and all cases were included in the statistical analysis; P = .019.
Peg-IFNα 2a 200 ng/ mL + RG7112 100 nM.
Effects of treatment with low doses of RG7112 and Peg-IFNα 2a alone or in combination on the number of WT JAK2 hematopoietic colonies
| Patient number* . | JAK2 V617F allele burden (%) . | Total of colonies genotyped . | % Of hematopoietic colonies with WT JAK2 . | |||
|---|---|---|---|---|---|---|
| Cytokines only (control) . | Peg-IFNα 2a . | RG7112 . | RG7112 + IFNα . | |||
| 2 | 14 | 127 | 58 | 56.5 | 46 | 70.6† |
| 15 | 70 | 129 | 14.3 | 26.1 | 23 | 40* |
| 5 | 49 | 144 | 70 | 67 | 71 | 79 |
| 6 | 22 | 132 | 38 | 47 | 45 | 50 |
| 13 | 75 | 180 | 21 | 32 | 25 | 50† |
| 14 | 20 | 168 | 31 | 44 | 28 | 45 |
| 3 | 40 | 189 | 79 | 83 | 79 | 87 |
| 16 | 50 | 121 | 4 | 6 | 6 | 24† |
| 22 | 70 | 121 | 6 | 0 | 0 | 27† |
| 21 | 45 | 112 | 50 | 80 | 58.3 | 83.3 |
| 19 | + | 105 | 12.5 | 20 | 64 | 90.9† |
| Patient number* . | JAK2 V617F allele burden (%) . | Total of colonies genotyped . | % Of hematopoietic colonies with WT JAK2 . | |||
|---|---|---|---|---|---|---|
| Cytokines only (control) . | Peg-IFNα 2a . | RG7112 . | RG7112 + IFNα . | |||
| 2 | 14 | 127 | 58 | 56.5 | 46 | 70.6† |
| 15 | 70 | 129 | 14.3 | 26.1 | 23 | 40* |
| 5 | 49 | 144 | 70 | 67 | 71 | 79 |
| 6 | 22 | 132 | 38 | 47 | 45 | 50 |
| 13 | 75 | 180 | 21 | 32 | 25 | 50† |
| 14 | 20 | 168 | 31 | 44 | 28 | 45 |
| 3 | 40 | 189 | 79 | 83 | 79 | 87 |
| 16 | 50 | 121 | 4 | 6 | 6 | 24† |
| 22 | 70 | 121 | 6 | 0 | 0 | 27† |
| 21 | 45 | 112 | 50 | 80 | 58.3 | 83.3 |
| 19 | + | 105 | 12.5 | 20 | 64 | 90.9† |
24 to 48 colonies were plucked and examined in each condition.
Patient numbers are listed in supplemental Table 2.
Benefits of the combination therapy are defined as the appearance of at least a 10% greater increase in WT colonies after combination therapy compared with the colonies treated with a single agent.
Nested allele-specific polymerase chain reaction for JAK2V617F
Genomic DNA was isolated from randomly plucked colonies using the Extract-N-Amp Blood PCR Kits (Sigma, St Louis, MO). JAK2V617F was detected by using a nested allele-specific polymerase chain reaction (PCR). The final PCR products were analyzed in 2.0% agarose gels. The nested PCR product had a size of 453 bp. A 279-bp product indicated allele-specific JAK2V617F positivity, whereas a 229-bp product denoted allele-specific WT product. Colonies were classified as homozygous for JAK2V617F if they contained only the 279-bp band, whereas heterozygous colonies were identified based on the presence of both the 279-bp and 229-bp bands.29
Flow cytometric assays of apoptosis
CD34+ cells from patients with PV and PMF, as well as normal CD34+ cells, were cultured in serum-free medium containing SCF, TPO, Flt-3 ligand, and IL-3 at 50 ng/mL and cells were treated with 200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a alone and in combination. After 2 days, an aliquot of cells was collected and washed in phosphate-buffered saline for staining with CD34 monoclonal antibody and Annexin V (BD Biosciences) directly, or they were fixed in 4% formaldehyde and permeabilized with 90% cold methanol and stained with monoclonal antibodies to CD34, p21, PUMA, and Bax (Cell Signaling Technologies, Danvers, MA). An isotope IgG antibody was used as a negative control. The staining with cleaved-caspase-3 monoclonal antibodies was performed with another aliquot of cells obtained after 4 days of incubation. Data were acquired on a FACS Calibur analyzer (Becton Dickinson, Franklin Lakes, NJ).
Western blot analysis
CD34+ cells were purified from the PV patients and normal donors and cultured in serum-free expansion medium (SFEM) containing cytokines as described before. The cells were treated with either a low dose of RG7112 or Peg-IFNα 2a alone, or in combination, for 24 hours. The cells were then harvested and the whole-cell protein extracts were prepared with RIPA lysis buffer (Boston Bio Products, Worcester, MA) containing a protease inhibitor cocktail (Thermo Scientific, Rockford, IL) for western blotting. p21, PUMA, and GAPDH antibodies were purchased from Cell Signaling Technologies.
NOD-SCID marrow repopulating assay
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and the experiments were approved by the Animal Care Committee of the ISMMS. Individual 6- to 8-week-old female NSG mouse recipients were sublethally irradiated with 240 cGy of total body radiation with a cobalt radiation source. CD34+ cells isolated from the blood of 2 patients with PV and the spleens from 2 patients with PMF were cultured in SFEM containing cytokines and treated with RG7112 or Peg-IFNα 2a alone or in combination for 5 days as described before. The studies with the PMF CD34+ cells were repeated on a second occasion. The cells were then washed in phosphate-buffered saline and injected into the tail veins of NSG mice. After 4 to 7 months, the mice were sacrificed and the BM and spleen were harvested for analysis of human cell engraftment. The spleens were weighted with precision balance (Denver Instrument, Bohemia, NY). Cells from the BM and spleens were stained with monoclonal antibodies against the human CD45, CD34, CD33, CD19, CD235, and CD41a (Becton Dickinson, San Jose, CA). The degree of human cell chimerism present in mice receiving CD34+ cells treated with cytokines alone was compared with mice receiving CD34+ cells treated with RG7112 or Peg-IFNα 2a alone or in combination. To examine the JAK2V617F mutational status of the human hematopoietic cells, gDNA was extracted from human CD45+ cells isolated from the NSG BM cells by hCD45 beads (Miltenyi Biotec, Auburn, CA), and JAK2V617F allele burden was assessed using quantitative real-time PCR.
Statistical analysis
Results were reported as the mean ± standard deviation of individual data points obtained from the varying number of individual experiments. Statistical significance was determined using Student t test or 1-tailed paired-samples t test.
Results
RG7112, but not RG7112i, decreases PV CFU-GM– and BFU-E–derived colony formation in a dose-dependent fashion
The effect of RG7112 on the ability of PV CD34+ cells to form hematopoietic colonies in vitro was assessed. An inactive enantiomer RG7112i served as negative control treatment. RG7112, but not RG7112i, was capable of suppressing PV BFU-E– and CFU-GM–derived colony formation in a dose-dependent fashion (P < .01) (supplemental Figure 1).
Combination treatment with RG7112 and Peg-IFNα 2a decreases PV and PMF CFU-GM– and BFU-E–derived colony formation
CD34+ cells from 22 PV and 7 PMF patients as well as 5 normal controls were treated with suboptimal doses of these agents (200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a) alone or in combination. These doses of RG7112 and Peg-IFNα 2a alone or in combination did not decrease the numbers of normal CFU-GM– and BFU-E–derived colonies (Figure 1A). By contrast, treatment with 200 ng/mL of Peg-IFNα 2a alone decreased PV CFU-GM– and BFU-E–derived colony formation by 30 and 60% respectively, and PMF CFU-GM– and BFU-E–derived colony formation by 40% and 60%, respectively, whereas treatment with RG7112 alone at a dose of 200 ng/mL modestly (P > .05) decreased hematopoietic colony formation (Figure 1B-C). Combination treatment with 200 nM of RG7112 and 200 ng/mL of IFNα 2a, however, resulted in an even further reduction of the numbers of PV and PMF CFU-GM and BFU-E colonies than was observed with Peg-IFNα 2a alone (Figure 1B-C). These data suggest that treatment with low doses of RG7112 and Peg-IFNα 2a preferentially target both PV and PMF CD34+ cells.
Low doses of RG7112 and Peg IFNα reduce PV and PMF CFU-GM– and BFU-E–derived colony formation. (A) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by normal BM CD34+ cells (n = 5). (B) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by PV CD34+ cells (n = 22). (C) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by PMF CD34+ cells (n = 7).
Low doses of RG7112 and Peg IFNα reduce PV and PMF CFU-GM– and BFU-E–derived colony formation. (A) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by normal BM CD34+ cells (n = 5). (B) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by PV CD34+ cells (n = 22). (C) Effects of 200 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a on CFU-GM– and BFU-E–derived colony formation by PMF CD34+ cells (n = 7).
Low doses of RG7112 and Peg-IFNα 2a reduce the numbers of JAK2V617F+ HPC
To evaluate the potential of a combination of low doses of RG7112 and Peg-IFNα 2a to eliminate malignant HPC, individual hematopoietic colonies were randomly plucked and the JAK2V617F allele status was determined. CD34+ cells from 11 of 22 different PV patients were evaluated (Table 1); RG7112 combined with Peg-IFNα 2a therapy significantly decreased the numbers of JAK2V617F+ colonies and increased the numbers of JAK2 WT colonies (P = .019, 1-tailed paired t test). In 4 of 9 PV cases, treatment with the combination of 200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a led to a ≥20% increase in the numbers of WT JAK2 hematopoietic colonies compared with untreated cells. In 2 cases, treatment with 200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a led to the eradication of all assayable HPCs. We therefore used the 100-nM RG7112 dose for these 2 patient samples so there would be colonies available for genotyping. In these cases, treatment with 100 nM of RG7112 combined with 200 ng/mL of Peg-IFNα 2a increased WT JAK2 colonies by 33% and 78%, respectively (Table 1). In Table 1, one can also see that combination treatment primarily depleted JAK2V617F heterozygous colonies. In 3 of 9 PV cases, combination treatment with 200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a decreased JAK2V617F heterozygous colonies by at least 20% (Table 2), and in 2 additional PV cases, treatment with 100 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a decreased JAK2V617F heterozygous colonies by 29% and 75%. These data suggest that there is a patient-to-patient heterogeneity with regard to response to the RG7112, and that a combination of these agents is more effective than either agent alone in preferentially decreasing malignant PV HPC numbers.
RG7112 and Peg-IFNα 2a in combination promote apoptosis of MPN CD34+ cells by activating the p53 pathway
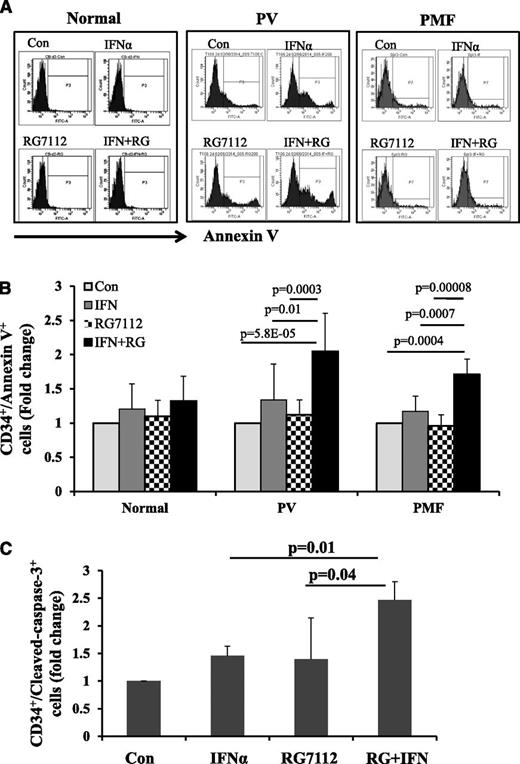
Annexin V staining of PV and PMF CD34+ cells showed that exposure to low doses of RG7112 or Peg-IFNα 2a alone slightly increased the proportion of PV and PMF CD34+ cells undergoing apoptosis, but that a combination of the 2 drugs induced CD34+ cell apoptosis to a statistically significantly greater degree (Figure 2A-B). By contrast, neither treatment with RG7112 or Peg-IFNα 2a alone or in combination at the doses tested significantly induced apoptosis of normal CD34+ cells (Figure 2A-B). These data were then confirmed with staining for c-caspase 3 antibody. As is seen in Figure 2C, combination treatment increased the proportion of MPN c-caspase-3+ CD34+ cells to a greater extent than either drug alone (P < .05).
Low doses of RG7112 and Peg-IFNα 2a in combination promote MPN CD34+ cell apoptosis. (A) Flow cytometric analysis showing the results of Annexin V staining on normal, PV, and PMF CD34+ cells after 2 days of treatment with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (B) Histogram showing the effects of these treatments on the CD34+/AnnexinV+ cell population in normal (n = 5), PV (n = 8), and PMF (n = 6) CD34+ cells after 2 days of treatment with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (C) Histogram showing the effects of 4 days of drug treatment on PV CD34+/cleaved caspase-3+ cells (n = 6, P < .05).
Low doses of RG7112 and Peg-IFNα 2a in combination promote MPN CD34+ cell apoptosis. (A) Flow cytometric analysis showing the results of Annexin V staining on normal, PV, and PMF CD34+ cells after 2 days of treatment with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (B) Histogram showing the effects of these treatments on the CD34+/AnnexinV+ cell population in normal (n = 5), PV (n = 8), and PMF (n = 6) CD34+ cells after 2 days of treatment with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (C) Histogram showing the effects of 4 days of drug treatment on PV CD34+/cleaved caspase-3+ cells (n = 6, P < .05).
To further understand the mechanism by which RG7112 combined with Peg-IFNα 2a specifically affects MPN CD34+ cells, p21, PUMA, and Bax protein levels, which are downstream of p53, were evaluated in CD34+ cells from 8 PV patients and 5 normal individuals. Flow cytometric analyses showed that treatment with either RG7112 or Peg-IFNα 2a alone resulted in a modest increase in p21, whereas combination treatment led to a far greater increase in p21 protein levels in PV CD34+ cells (Figure 3A-B). Although Peg-IFNα 2a alone increased p21 protein in normal CD34+ cells, combination treatment did not further increase p21 levels (Figure 3A-B). We next evaluated PUMA and Bax levels in both PV and normal CD34+ cells after treatment with a low dose of RG7112 or Peg-IFNα 2a alone or in combination. Treatment with RG7112 or Peg-IFNα 2a alone increased PUMA and Bax protein levels to a limited degree, whereas combination treatment resulted in a greater increase of these proapoptotic proteins (Figure 3C-D). Although treatment with RG7112 or Peg-IFNα 2a alone increased Bax protein in PV CD34+ cells to a statistically significant level, combination treatment increased Bax protein levels to an even greater degree (Figure 3E-F). By contrast, treatment of normal CD34+ cells with RG7112 or Peg-IFNα 2a alone or in combination did not statistically significantly increase either PUMA or Bax protein levels (Figure 3C-F). These data were confirmed using western blot analysis. The results show that combination treatment with low doses of RG7112 and Peg-IFNα 2a increased p21 and PUMA protein levels in PV CD34+ cells to a greater degree than treatment with either reagent alone (Figure 4A-B). Neither RG7112 nor Peg-IFNα 2a alone, nor combination treatment at the doses tested, had similar effects on normal CD34+ cells (Figure 4C-D).
Low doses of RG7112 and Peg-IFNα 2a increase p53 activity. Normal (n = 5) and PV (n = 6) CD34+ cells were treated with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (A) Results of flow cytometric analysis of CD34+/p21+ cells in normal and PV CD34+ cells. (B) The fold change of numbers of CD34+/p21+ cells in normal and PV CD34+ cells after drug treatment. (C) Results of flow cytometric analysis of CD34+/PUMA+ cells after drug treatment of normal and PV CD34+ cells. (D) The fold change of CD34+/PUMA+ cell numbers after drug treatment of normal and PV CD34+ cells. (E) Flow cytometric analysis of CD34+/Bax+ cells after drug treatment of normal and PV CD34+ cells. (F) The fold change of numbers of CD34+/Bax+ cells after treatment of normal and PV CD34+ cells.
Low doses of RG7112 and Peg-IFNα 2a increase p53 activity. Normal (n = 5) and PV (n = 6) CD34+ cells were treated with 200 nM of RG7112 or 200 ng/mL of Peg-IFNα 2a alone or in combination. (A) Results of flow cytometric analysis of CD34+/p21+ cells in normal and PV CD34+ cells. (B) The fold change of numbers of CD34+/p21+ cells in normal and PV CD34+ cells after drug treatment. (C) Results of flow cytometric analysis of CD34+/PUMA+ cells after drug treatment of normal and PV CD34+ cells. (D) The fold change of CD34+/PUMA+ cell numbers after drug treatment of normal and PV CD34+ cells. (E) Flow cytometric analysis of CD34+/Bax+ cells after drug treatment of normal and PV CD34+ cells. (F) The fold change of numbers of CD34+/Bax+ cells after treatment of normal and PV CD34+ cells.
Western blot results show that low doses of RG7112 and Peg-IFNα 2a increase p21 and PUMA protein levels in PV CD34+ cells. (A) Western blotting shows p21 and PUMA protein levels in 2 individual PV samples after treatment with low doses of RG7112 or Peg-IFNα 2a alone or in combination. (B) The ratio of the degree of p21/GAPDH in PV samples. (C) Western blotting of p21 and PUMA protein levels in 2 individual normal samples after treatment with low doses of RG7112 or Peg-IFNα 2a alone or in combination. (D) The ratio of the degree of p21/GAPDH in normal samples.
Western blot results show that low doses of RG7112 and Peg-IFNα 2a increase p21 and PUMA protein levels in PV CD34+ cells. (A) Western blotting shows p21 and PUMA protein levels in 2 individual PV samples after treatment with low doses of RG7112 or Peg-IFNα 2a alone or in combination. (B) The ratio of the degree of p21/GAPDH in PV samples. (C) Western blotting of p21 and PUMA protein levels in 2 individual normal samples after treatment with low doses of RG7112 or Peg-IFNα 2a alone or in combination. (D) The ratio of the degree of p21/GAPDH in normal samples.
Treatment with RG7112 and Peg-IFNα 2a decreases the ability of MPN CD34+ cells to repopulate the BM and spleens of NSG mice
CD34+ cells from 4 MPN patients (2 PMF, 2 PV) were treated with 200 nM of RG7112 and 200 ng/mL of Peg-IFNα 2a alone or in combination for 5 days, and then transplanted into NSG mice as previously described.28 One of the PMF specimens (PMF1) was JAK2V617F+ and the other (PMF2) was JAK2 WT. After 4 to 7 months, the mice were sacrificed, the spleens were weighed, and the degree of human cell chimerism in the BM and spleen was evaluated. The transplantation of PMF CD34+ from one patient that had not been treated with either drug led to splenomegaly in 2 recipient mice 7 months after transplantation (Figure 5A). Both human CD45+ and CD34+ cells were observed in the recipient spleens (supplemental Figure 2A). NSG mice receiving CD34+ cells treated with either RG7112 or Peg-IFNα 2a alone, or in combination, had smaller spleens with reduced degrees of human cell chimerism (Figure 5A and supplemental Figure 2A). Human CD45+ cells were isolated from the spleens of mice and the JAK2V617F allele burden was quantitated. Treatment with RG7112 or Peg-IFNα 2a alone or in combination decreased the JAK2V617F allele burden by 90% (supplemental Figure 2B). The transplantation of MPN CD34+ cells treated with cytokines alone resulted in human cell chimerism ranging between 0.8% and 31.3% in the BM of NSG mice (Figure 5B). Prior treatment of MPN CD34+ cells with 200 ng/mL of Peg-IFNα 2a alone decreased human MPN CD45+ cells chimerism by 50% to 100%. Treatment with 200 nM of RG7112 alone decreased human CD45+ cell chimerism by 3% to 100%, whereas combination treatment reduced the human CD45+ cells chimerism by 91% to 100% (Figure 5B). This inhibitory effect was seen with both PV and PMF CD34+ cells (Figure 5B and supplemental Table 1). The effect of treatment with RG7112 and Peg-IFNα 2a alone or in combination decreased the proportion of cells belonging to human myeloid and lymphoid cell lineages (CD19, CD33, CD41a, and CD235) in NSG mouse BM (Figure 5B and supplemental Table 3). A similar reduction of human cell chimerism was observed when transplanting JAKV617F+ or WT JAK2 PMF CD34+ cells treated with the various drugs. Similar data were obtained when the same 2 PMF specimens were transplanted into a second set of NSG mice (supplemental Table 3). Treatment with RG7112 or Peg-IFNα 2a alone decreased the JAK2V617F allele burden by 50%, whereas combination treatment decreased the JAK2V617F allele by 80% (Figure 6A). In the PV cases, treatment with RG7112 or Peg-IFNα 2a alone decreased the JAK2V617F allele burden by 10% to 20%, whereas combination treatment led to a 75% reduction in JAK2V617F+ marrow cells (Figure 6B). These data indicate that treatment with RG7112 combined with Peg-IFNα 2a can eliminate MPN HSCs.
Treatment with low doses of RG7112 and Peg-IFNα 2a decreases the ability of MPN CD34+ cells to repopulate the BM and spleen of NSG mice. (A) Prior treatment of MF spleen CD34+ cells with low doses of RG7112 and Peg-IFNα 2a reduced the spleen size and weight in a recipient NSG mouse. The transplantation of untreated PMF CD34+ led to an enlarged spleen 7 months after transplantation, whereas recipient mice transplanted with CD34+ treated with either RG7112 or Peg-IFNα 2a alone or in combination had smaller spleens. Similar results were observed when CD34+ cells from this same patient were transplanted into a second set of NSG mice. (B) Treatment with RG7112 and Peg-IFNα 2a alone or in combination decreased both donor-derived myeloid and lymphoid cells (CD19, CD33, CD41a, and CD235) in the BM of recipient NSG mice.
Treatment with low doses of RG7112 and Peg-IFNα 2a decreases the ability of MPN CD34+ cells to repopulate the BM and spleen of NSG mice. (A) Prior treatment of MF spleen CD34+ cells with low doses of RG7112 and Peg-IFNα 2a reduced the spleen size and weight in a recipient NSG mouse. The transplantation of untreated PMF CD34+ led to an enlarged spleen 7 months after transplantation, whereas recipient mice transplanted with CD34+ treated with either RG7112 or Peg-IFNα 2a alone or in combination had smaller spleens. Similar results were observed when CD34+ cells from this same patient were transplanted into a second set of NSG mice. (B) Treatment with RG7112 and Peg-IFNα 2a alone or in combination decreased both donor-derived myeloid and lymphoid cells (CD19, CD33, CD41a, and CD235) in the BM of recipient NSG mice.
Treatment with low doses of RG7112 and Peg-IFNα 2a reduced the numbers of JAK2V617F+ cells in NOD/SCID mice. Combination treatment of PMF (A) and PV (B). Donor CD34+ cells treated with RG7112 and Peg-IFNα 2a in combination led to a greater reduction in JAK2V617F allele burden than that observed in recipient NSG mice transplanted with CD34+ cells treated with either agent alone.
Treatment with low doses of RG7112 and Peg-IFNα 2a reduced the numbers of JAK2V617F+ cells in NOD/SCID mice. Combination treatment of PMF (A) and PV (B). Donor CD34+ cells treated with RG7112 and Peg-IFNα 2a in combination led to a greater reduction in JAK2V617F allele burden than that observed in recipient NSG mice transplanted with CD34+ cells treated with either agent alone.
Discussion
Therapies for MPN patients have almost universally involved the use of agents that normalize blood counts and reduce the degree of splenomegaly, the incidence of disease-related symptoms, and the tendency to develop thrombotic events.30,-32 None of these strategies, however, has been shown in a systematic fashion to delay disease progression or to lessen the rate of transformation to acute leukemia. Drug therapies that would affect the evolution of the MPNs would be anticipated to deplete malignant HSCs or affect the microenvironment that supports the residence of MPN HSCs. We previously reported that p53 was a target for the development of drugs that could be used to deplete MPN HPCs.13,33 In vitro studies revealed that treatment with both low doses of Peg-IFNα 2a and nutlin-3, a small-molecule antagonist of MDM2 alone and in combination, significantly inhibited PV HPC numbers, induced apoptosis, preferentially eliminated malignant HPC, and activated the p53 pathway.13
RG7112 is the first small-molecule p53-MDM2 inhibitor that has proceeded along the clinical development pathway. It has already been evaluated in clinical trials including one phase 1 study in patients with advanced solid tumors and another in patients with acute leukemia.26 In the present study, we attempted to determine whether this drug was effective in eliminating MPN HPCs and HSCs, with the anticipation that such data would provide a rationale for the pursuit of clinical trials using this class of compounds to not only normalize blood counts in patients with PV but to also affect the burden of malignant HPCs and HSCs. We observed that RG7112, but not RG7112i, decreased PV CFU-GM– and BFU-E–derived colony formation in a dose-dependent fashion; the IC50 for both CFU-GM and BFU-E was approximately 1 uM (supplemental Figure 1). Furthermore, the combination of low doses of RG7112 combined with Peg-IFNα 2a significantly inhibited the ability of both PV and PMF CD34+ to form hematopoietic colonies in vitro to a greater extent than that observed with normal CD34+ cells, and that combination therapy significantly decreased the numbers of JAK2V617F+ hematopoietic colonies. In the previous reports, we have shown that higher doses of interferon decreased both JAK2V617F homozygous and heterozygous colonies. The exclusivity of the inhibitory effect to JAK2V617F heterozygous colonies in this report is likely a result of the extremely low doses of interferon used. These results indicate that both RG7112 and Peg-IFNα 2a can preferentially decrease PV and PMF HPCs.
Treatment of PV and ET patients with Peg-IFNα 2a alone has resulted in elimination of JAK2V617F allele burden in the granulocytes of 17% to 18% of patients, as well as elimination of hematopoietic cells with marker cytogenetic abnormalities and restoration of polyclonal hematopoiesis in some patients.9,,-12,34 These findings suggest that Peg-IFNα 2a treatment depletes the pool of malignant primitive HPCs and possibly HSCs in a limited number of PV and ET patients. Mullaly and coworkers previously investigated the effects of IFNα on MPN HSCs using a murine model of JAK2V617+ MPNs.35 They demonstrated that MPN HSCs were depleted by treatment with IFNα by promoting the exit of JAK2V617F HSC from a quiescent state with cell cycle activation as well as commitment to a predetermined erythroid differentiation program. These studies indicated that IFNα may not be sufficient to completely eradicate JAK2V617F HSCs, and those additional drugs or combinations of drugs would be required for such purposes. We have demonstrated using primary MPN CD34+ cells that Peg-IFNα 2a treatment depletes not only MPN HPCs but also HSCs, and that a second drug, RG7112, has similar activity against primitive HPCs and HSCs. Both Peg-IFNα 2a and RG7112 are available in a formulation that is presently available for the treatment of MPN patients. The actions of these drugs are additive against MPN HSCs and HPCs, providing the rationale for a therapeutic strategy in which the low doses of these 2 drugs would be not only more tolerable but more effective in eliminating PV HPCs and HSCs. Evidence has been provided that both of these drugs act in part by activating the p53 pathway and promoting CD34+ cell apoptosis. These observations do not exclude the possibility that IFNα might also promote HSC cycling and erythroid differentiation, as suggested by Mullaly and coworkers.35 Hasan and coworkers, using knock-in mice with conditional expression of JAK2 (V617F) in hematopoietic cells, developed a murine model mimicking PV, which they used to study the actions of IFNα. This study shows that JAK2 (V617F) in mice amplifies not only late but also early hematopoietic cells, giving them a proliferative advantage through high cell cycling and low rates of apoptosis that sustains the emergence of the MPN but is lost with IFNα treatment. Their results showed that BM and spleen stem cells were less apoptotic in knock-in mice than in WT mice and that IFNα slightly increased apoptosis, especially in the spleens of knock-in cells.36 The findings of Hassan and colleagues more closely resemble the data reported in this report, in that both laboratories demonstrated the importance of apoptosis as a mediator of the effects of IFNα on MPN cells.
The action of RG7112, however, appears to be specific to the p53 pathway because its enantomer lacked the ability to affect PV HPC colony formation. Experiments using flow cytometric assay and western blotting indicated that the p53 downstream proteins p21, PUMA, and Bax were increased after combination drug treatment but not when either of these drugs were used alone at the same doses. Furthermore, this drug treatment did not have a similar effect on normal CD34+ cells, indicating that normal HPCs and HSCs would be able to survive in such treatment and reestablish normal hematopoiesis once the malignant HSC pool was depleted or eliminated. The effects of both Peg-IFNα 2a and RG7112 at lower doses varied considerably from patient to patient and did not appear to be dependent on the patient’s baseline JAK2V617F allele burden. These individual drugs as well as their use in combination were able to deplete both JAK2V617F homozygous and heterozygous HPCs, but only the combination of these 2 drugs on occasion was able to totally eliminate either homozygous or heterozygous HPCs.
We were able to examine the effects of RG7112 and Peg-IFNα 2a on MPN marrow repopulating cells using the NSG assay system, which serves as a surrogate assay for MPN HSCs. For these purposes, we studied the behavior of CD34+ cells from 2 PV patient and 2 PMF patients. Three of these patients had JAK2V617+ MPNs, which allowed us to use this molecular marker to track the burden of malignant cells. Peg-IFNα 2a alone proved to be more effective than RG7112 at the doses tested in affecting MPN HSCs. Remarkably, however, the combination of the 2 drugs decreased the degree of human chimerism by at least 90%. In the 2 experiments in which JAK2V617F+ CD34+ cells were assayed, we demonstrated that a combination of the drugs was also able to dramatically reduce the JAK2V617F allele burden within the donor-derived cells. As was previously reported by our group, engraftment of PMF CD34+ cells can lead to splenomegaly in NSG mice.28 Treatment of CD34+ cells with each of these compounds alone and in combination reduced the degree of splenomegaly and the degree of human cell chimerism within the spleen of NSG mice transplanted with PMF splenic CD34+ cells. These data indicate that the splenic microenvironment does not protect PMF CD34+ cells from the effects of either RG7112 or Peg-IFNα 2a.
Both the MDM2 antagonist, RG7112, and Peg-IFNα 2a alone were also able to deplete PMF and PV HPCs, and the combination of low doses of these drugs was effective in eliminating PMF and PV HSCs. Peg-IFNα 2a has been proposed to be an effective treatment strategy for patients in the early stages but not the more advanced forms of PMF. Peg-IFNα 2a therapy of early-stage PMF patients has not been reported to reduce the malignant clone as monitored with the JAK2V6717F allele burden.37,38 The effectiveness of RG7112 and Peg-IFNα 2a treatment alone and in combination to deplete PMF HSCs, however, provides a rationale for the evaluation of this drug combination in PMF patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the Leukemia and Lymphoma Society (R.H.).
Authorship
Contribution: M.L. designed and performed the research, collected and analyzed data, and wrote the manuscript; L.X. performed the research and collected the data; Y.L. and X.W. assisted in performing the experiments; and R.H. designed and analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Tisch Cancer Institute, Department of Medicine, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal