Key Points
mDia1 deficiency led to a cell-autonomous overexpression of CD14 on granulocytes and a hypersensitive innate immune response.
mDia1 heterozygous and knockout mice developed age-dependent MDS that was accelerated by chronic stimulation of the innate immunity.
The myelodysplastic syndromes (MDSs) include a spectrum of stem cell malignancies characterized by an increased risk of developing acute myeloid leukemia. Heterozygous loss of chromosome 5q (del[5q]) is the most common cytogenetic abnormality in MDS. DIAPH1 is localized to 5q31 and encodes one of the formin proteins, mDia1, which is involved in linear actin polymerization. Mice with mDia1 deficiency develop hematologic features with age mimicking human myeloid neoplasm, but its role in the pathogenesis of MDS is unclear. Here we report that mDia1 heterozygous and knockout mice develop MDS phenotypes with age. In these mice, CD14 was aberrantly overexpressed on granulocytes in a cell-autonomous manner, leading to a hypersensitive innate immune response to lipopolysaccharide (LPS) stimuli through CD14/Toll-like receptor 4 signaling. Chronic stimulation with LPS accelerated the development of MDS in mDia1 heterozygous and knockout mice that can be rescued by lenalidomide. Similar findings of CD14 overexpression were observed on the bone marrow granulocytes of del(5q) MDS patients. Mechanistically, mDia1 deficiency led to a downregulation of membrane-associated genes and a specific upregulation of CD14 messenger RNA in granulocytes, but not in other lineages. These results underscore the significance of mDia1 heterozygosity in deregulated innate immune responses in del(5q) MDS.
Introduction
The myelodysplastic syndromes (MDSs) are a heterogeneous group of clonal bone marrow disorders characterized by dysplastic or ineffective production of myeloid cells.1,-3 The incidence of MDS increases with age. Clinically, patients with MDS often present with anemia, cytopenia(s), dysplasia in 1 or more of the myeloid lineages, ineffective hematopoiesis, and increased risk of developing acute myeloid leukemia (AML).4 Various genomic abnormalities have been discovered to be associated with MDS. Deletion of chromosome 5q (del[5q]) is the most common cytogenetic defect in MDS and is more frequently found in therapy-related MDS.5
There are 2 common deleted regions (CDRs) on 5q: a distal locus that is commonly deleted in 5q− syndrome (sole chromosome 5q deletion) with good prognosis, and a proximal locus that is deleted in patients with higher risk of MDS including therapy-related MDS.6 Loss of certain genes located on these 2 regions was confirmed to be contributory to the pathogenesis of MDS. For example, short hairpin RNA knockdown of RPS14, which is located on the distal CDR, mimics erythroid abnormality in 5q− syndrome.7,8 Early growth response 1 and α-catenin, with their encoding genes within the proximal CDR, are also found to be important for the pathogenesis of MDS.9,-11 In addition, heterozygosity of miR-145 and miR-146a, 2 microRNAs located on the distal CDR, mediates the dysplastic phenotype in megakaryocytes in 5q− syndrome.12,13 These data support the contention that MDS is a complex disease in which deregulation of multiple genes including those on the 5q region contributes to its pathogenesis.
Besides these 2 CDRs, array-based comparative genomic hybridization studies revealed that patients with MDS or AML often have large deletions on 5q that are beyond the 2 CDRs.6 DIAPH1, which encodes mDia1, is located on 5q31.3, which is flanked by these 2 CDRs.14 mDia1 belongs to the formin protein family and is important for the polymerization of linear actin filaments.15 In hematopoietic cells, mDia1 plays important roles in the activation of T cells, macrophages, dendritic cells, and neutrophils.16,-18 mDia1 heterozygous and knockout mice were reported to develop myeloproliferative neoplasm with age.19 However, whether and how mDia1 is involved in the pathogenesis of MDS is unknown. Here we show that mDia1 heterozygous and knockout mice mimic human MDS with granulocytopenia and age-dependent myelodysplasia. Strikingly, CD14 was aberrantly overexpressed on the bone marrow and peripheral blood granulocytes of mDia1 heterozygous and knockout mice in a cell-autonomous manner, which led to a hypersensitivity of the innate immune response to lipopolysaccharide (LPS) stimuli through CD14/Toll-like receptor (TLR) 4 signaling pathway. Chronic stimulation of LPS accelerated the development of MDS in mDia1 heterozygous and knockout mice that can be rescued by lenalidomide, an immunomodulatory drug commonly used for the treatment of del(5q) MDS. Mechanistically, mDia1 deficiency led to a downregulation of membrane-associated genes and a specific upregulation of CD14 in granulocytes, but not in other cell types. Thus, our study reveals an important role of mDia1 in the regulation of the innate immune signaling and loss of mDia1 in the pathogenesis of del(5q) MDS.
Methods
Materials
The biotin mouse lineage panel was purchased from BD Pharmingen to mark lineage positive cells or to positively or negatively select lineage positive cells from mouse bone marrow cells. The phycoerythrin (PE)–conjugated antibodies rat anti-mouse Ly-6G (Gr1 clone 1A8), which is used to specifically mark neutrophils, and PE rat anti-mouse CD11b/Mac1 and CD8a were obtained from BD Biosciences. Allophycocyanin-conjugated anti-mouse SLAM/CD150 and PerCP/PE Cy5.5-conjugated anti-mouse CD117/cKit were purchased from Biolegend. All other conjugated anti-mouse protein antibodies were purchased from eBiosciences. Cy5-Annexin V was purchased from BioVision. Hoechst 33342, 4,6 diamidino-2-phenylindole, and rhodamine phalloidin were purchased from Molecular Probes, Invitrogen. LPS from Escherichia coli 055:B5 was from Sigma.
Animals
The mDia1 knockout mice with a mixed 129/C57B6 genetic background were obtained from Arthur S. Alberts of Van Andel Institute (Grand Rapids, MI). The mice were backcrossed to C57B6 for 10 generations to obtain a pure C57B6 genetic background. These mice harbor a green fluorescent protein (GFP) reporter gene in the targeting construct so that the heterozygous and knockout mice had GFP expression in their hematopoietic cells. Hence, fluorescein isothiocyanate or GFP channel-based tools and experiments were avoided while investigating these mice. All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at Northwestern University.
Flow cytometric analysis
Detailed protocol for flow cytometric analysis is in the supplemental Methods available on the Blood Web site.
Bone marrow transplantation
Detailed protocol for bone marrow transplantation is in the supplemental material.
LPS injection and lenalidomide treatment
LPS injection and lenalidomide treatment are described in the supplemental Methods.
MDS patient data and institutional review board approval
MDS patient data were obtained following informed consent under institutional review board–approved protocols at H. Lee Moffitt Cancer Center and Research Institute. The study was conducted in accordance with the Declaration of Helsinki.
In vitro myeloid cell differentiation
The purification of bone marrow lineage negative cells is detailed in the supplemental Methods. The purified cells were plated in a myeloid differentiation media (Iscove modified Dulbecco medium containing 15% fetal bovine serum, 0.1 mM β-mercapto-ethanol, 1% penicillin-streptomycin, 2 mM l-glutamine, 50 ng/mL stem cell factor, 10 ng/mL interleukin [IL] 3, and 10 ng/mL granulocyte macrophage colony-stimulating factor for 3 days). The cells were then harvested for flow cytometric analysis.
Results
Mice with mDia1 deficiency exhibited granulocytopenia and age-dependent myelodysplasia
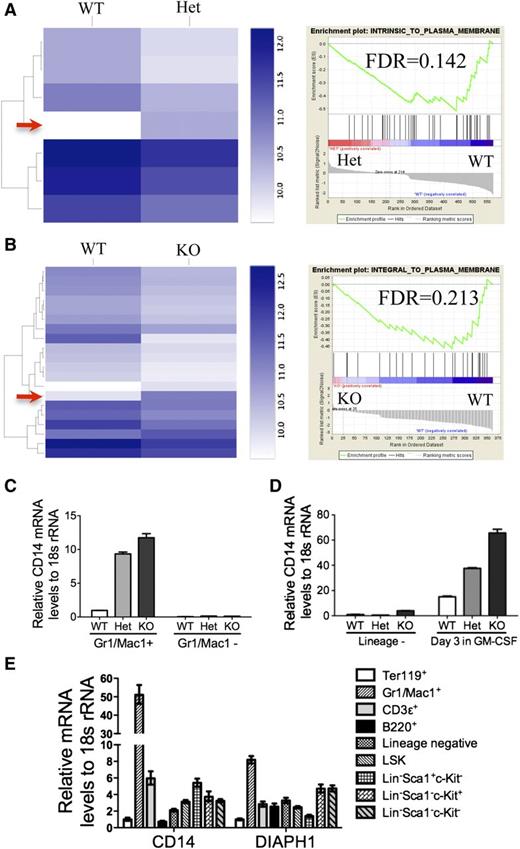
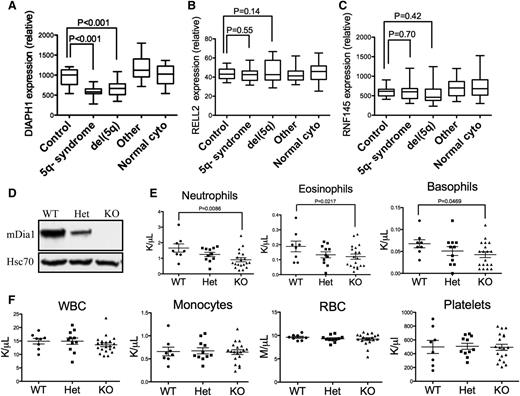
To explore the role of mDia1 deficiency in the pathogenesis of MDS, we first analyzed the gene expression profiles of DIAPH1 in patients with various subtypes of MDS, which showed that DIAPH1 was significantly decreased in MDS patients with 5q− syndrome and del(5q) associated with other types of cytogenetic abnormalities (Figure 1A). The expression levels of genes that are in close proximity to DIAPH1 on 5q, such as RELL2 and RNF145, were not decreased (Figure 1B-C), which suggests an association of mDia1 deficiency in del(5q) MDS.
Mice with mDia1 deficiency showed granulocytopenia at young ages. (A-C) Relative DIAPH1, RELL2, and RNF145 mRNA expressions in CD34+ bone marrow cells of patients with or without chromosome 5q deletion. Control: samples from orthopedic surgery, N = 17; 5q− syndrome: sole 5q deletion, N = 17; del(5q): del(5q) MDS with other cytogenetic abnormalities, N = 21; other: MDS with cytogenetic abnormalities other than del(5q), N = 18; normal cyto: MDS without cytogenetic abnormalities, N = 19. Data were obtained from a microarray gene expression analysis (Gene Expression Omnibus accession number: GSE19429). (D) Western blot analysis of total bone marrow cells from the indicated mice. (E) Peripheral blood absolute neutrophil, eosinophil, and basophil counts from mDia1 wild-type (WT, N = 8), heterozygous (Het, N = 11), and knockout (KO, N = 19) mice. All the mice were aged between 6 and 8 weeks. (F) Complete blood count (CBC) of indicated lineages as in panel E.
Mice with mDia1 deficiency showed granulocytopenia at young ages. (A-C) Relative DIAPH1, RELL2, and RNF145 mRNA expressions in CD34+ bone marrow cells of patients with or without chromosome 5q deletion. Control: samples from orthopedic surgery, N = 17; 5q− syndrome: sole 5q deletion, N = 17; del(5q): del(5q) MDS with other cytogenetic abnormalities, N = 21; other: MDS with cytogenetic abnormalities other than del(5q), N = 18; normal cyto: MDS without cytogenetic abnormalities, N = 19. Data were obtained from a microarray gene expression analysis (Gene Expression Omnibus accession number: GSE19429). (D) Western blot analysis of total bone marrow cells from the indicated mice. (E) Peripheral blood absolute neutrophil, eosinophil, and basophil counts from mDia1 wild-type (WT, N = 8), heterozygous (Het, N = 11), and knockout (KO, N = 19) mice. All the mice were aged between 6 and 8 weeks. (F) Complete blood count (CBC) of indicated lineages as in panel E.
To further explore the potential role of mDia1 in MDS, we used mDia1 knockout mice in a pure C57/B6 background to analyze if they have any signs or symptoms of myelodysplasia. The protein levels of mDia1 in total bone marrow cells were confirmed to be downregulated in heterozygous mice and depleted in knockout mice (Figure 1D). The mDia1 heterozygous and knockout mice showed prominent gene-dosage dependent granulocytopenia including neutropenia, eosinopenia, and basopenia at young ages (Figure 1E), which are some of the features of MDS in humans.3 The granulocytopenia persisted when they aged (supplemental Figure 1A). The other circulation blood cell lineages were not affected in these mice (Figure 1F). These include monocytes, which often increase with decreased neutrophils.
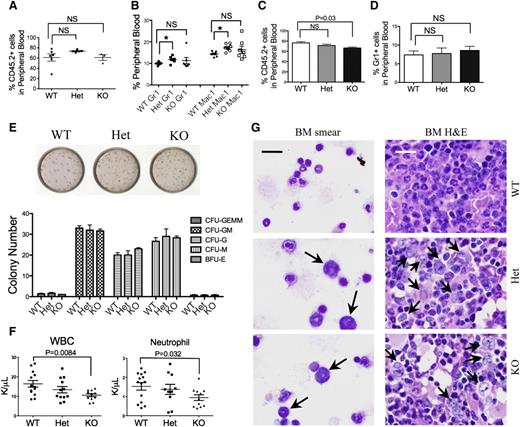
To determine the etiology of granulocytopenia, we performed a flow cytometric analysis of the mDia1 mouse bone marrow, which showed that there were no significant changes in the composition of various hematopoietic lineages, nor in immunophenotypically defined stem cells in young mice (supplemental Figure 1B). Bone marrow morphologic examination also revealed neither dysplastic changes nor myeloproliferative features in mDia1 heterozygous or knockout mice at young ages (supplemental Figure 1C). Transplantation of total bone marrow cells (Figure 2A-B) or lineage negative, Sca1+, c-Kit+ (LSK) bone marrow progenitor/stem cells (Figure 2C-D) from mDia1 wild-type, heterozygous, and knockout mice to lethally irradiated recipient mice showed no significant engraftment defect with mDia1 deficiency. Furthermore, an in vitro colony-forming assay of lineage negative cells showed no differentiation defects of various lineages with mDia1 deficiency (Figure 2E). These results demonstrated that mDia1 is largely dispensable for normal hematopoiesis in young mice. However, neutropenia was present in mice transplanted with mDia1 knockout total bone marrow cells (Figure 2F), which indicates that neutropenia in these mice is cell autonomous. This was confirmed by a reverse transplantation assay in which lethally irradiated mDia1-deficient mice transplanted with wild-type bone marrow cells showed no granulocytopenia (supplemental Figure 2A). mDia1 is known to be required for neutrophil migration,16 which could contribute to granulocytopenia in mDia1 heterozygous and knockout mice. Immunostains of Gr1/Mac1 double-positive bone marrow granulocytes illustrated abnormally polarized actin filaments in mDia1 heterozygous and knockout cells, which is consistent with the role of mDia1 in the regulation of actin cytoskeleton integrity (supplemental Figure 2B). These cells also exhibited increased adhesion to fibronectin, which could lead to decreased bone marrow mobilization (supplemental Figure 2C). Furthermore, cultured granulocytes showed slightly increased rate of apoptosis (supplemental Figure 2D-E). These results suggest that loss of mDia1 causes hematologic defects mainly in differentiated granulocytes or their committed progenitors.
Mice with mDia1 deficiency developed age-related MDSs. (A) Transplantation of total bone marrow cells from indicated mice (CD45.2+) to lethally irradiated mice (CD45.1+). Flow cytometric analysis of CD45.2+ cells in the peripheral blood was performed 1 month after transplantation. WT: N = 6, Het: N = 5, KO: N = 3. (B) Same as in panel A except that Gr1/Mac1 double-positive cells were analyzed. N = 7 in each group. (C) Transplantation of LSK cells (CD45.2+) from indicated mice to lethally irradiated recipient mice (CD45.1+). The percentage of peripheral blood CD45.2+ cells was determined 1 month after transplantation. N = 3 in each group. (D) Same as in panel C except that Gr1+ cells were analyzed by flow cytometry. (E) Colony-forming unit (CFU) assay of various hematopoietic lineages derived from LSK cells of indicated mice (6-8 weeks of age). BFU-E, burst-forming unit, erythrocytes; G, granulocytes; GEMM, granulocytes, erythrocytes, monocytes/macrophages; GM, granulocytes, monocytes; M, monocytes. Data were representative of at least 3 independent experiments. (F) White blood cell and neutrophil counts of mice 1 month after transplantation with mDia1 wild-type, heterozygous, and knockout total bone marrow cells. (G) Wright-Giemsa stains of bone marrow smears (left panels) and hematoxylin and eosin (H&E) stains of bone marrow sections (right panels) of indicated mice (>1.5 years). Arrows indicate dysplastic granulocytes. Scale bars represent 30 μm. The stains were representatives of 3 animals from each genotype.
Mice with mDia1 deficiency developed age-related MDSs. (A) Transplantation of total bone marrow cells from indicated mice (CD45.2+) to lethally irradiated mice (CD45.1+). Flow cytometric analysis of CD45.2+ cells in the peripheral blood was performed 1 month after transplantation. WT: N = 6, Het: N = 5, KO: N = 3. (B) Same as in panel A except that Gr1/Mac1 double-positive cells were analyzed. N = 7 in each group. (C) Transplantation of LSK cells (CD45.2+) from indicated mice to lethally irradiated recipient mice (CD45.1+). The percentage of peripheral blood CD45.2+ cells was determined 1 month after transplantation. N = 3 in each group. (D) Same as in panel C except that Gr1+ cells were analyzed by flow cytometry. (E) Colony-forming unit (CFU) assay of various hematopoietic lineages derived from LSK cells of indicated mice (6-8 weeks of age). BFU-E, burst-forming unit, erythrocytes; G, granulocytes; GEMM, granulocytes, erythrocytes, monocytes/macrophages; GM, granulocytes, monocytes; M, monocytes. Data were representative of at least 3 independent experiments. (F) White blood cell and neutrophil counts of mice 1 month after transplantation with mDia1 wild-type, heterozygous, and knockout total bone marrow cells. (G) Wright-Giemsa stains of bone marrow smears (left panels) and hematoxylin and eosin (H&E) stains of bone marrow sections (right panels) of indicated mice (>1.5 years). Arrows indicate dysplastic granulocytes. Scale bars represent 30 μm. The stains were representatives of 3 animals from each genotype.
Because MDS is an age-related disease, we asked whether old mDia1 heterozygous and knockout mice would develop myelodysplasia that is best evaluated from bone marrow. Indeed, bone marrow smears and H&E sections of old (>1.5 years) mDia1 heterozygous and knockout mice showed prominent dysplasia with a significant accumulation of large myeloid cells with open chromatin and irregular nuclear contour (Figure 2G), which together with neutropenia closely mimics features of human MDS. Although flow cytometric analysis of different bone marrow hematopoietic lineages in old mDia1-deficient mice showed no difference compared with wild-type ones (supplemental Figure 3A), it is consistent with the fact that no immunophenotypic parameters have been proved to be diagnostic of MDS.20 In addition, we did not observe features of myeloproliferative neoplasm in these mice, which could be attributable to the different mouse genetic background from previous study.19
CD14 was overexpressed on granulocytes of mDia1 heterozygous and knockout mice
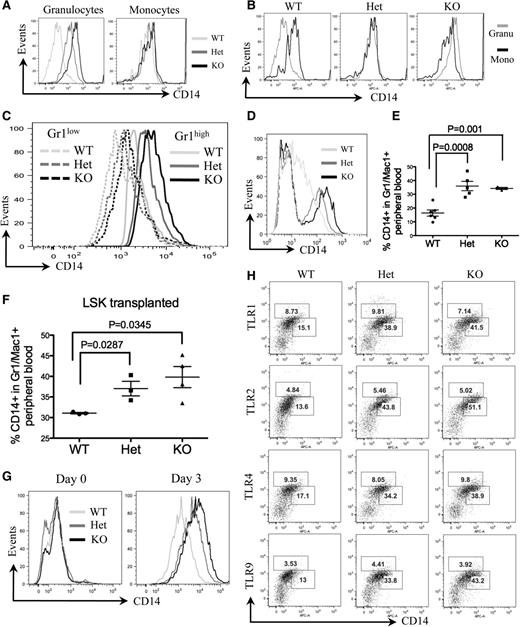
We further analyzed a group of myeloid markers that are commonly tested in patients with MDS or AML. Surprisingly, CD14, but not other myeloid markers (data not shown), was highly expressed in the Gr1 and Mac1 double-positive bone marrow granulocytes of mDia1 heterozygous and knockout mice (Figure 3A). CD14 is a glycosylphosphatidylinositol-anchored membrane protein that is generally expressed on the cell surface of monocytes but at a lower level on granulocytes.21 To determine in which cell types CD14 is overexpressed, we performed a flow cytometric analysis of CD14 of the bone marrow granulocytes (Gr1/Mac1+) and monocytes (Mac1+), which showed that CD14 was dramatically increased on granulocytes of mDia1 heterozygous and knockout mice. On the other hand, their monocyte levels of CD14 were similar to those in the wild-type mice (Figure 3A). Strikingly, CD14 on granulocytes reached an equal or higher level than that on monocytes of mDia1 heterozygous and knockout mice, respectively (Figure 3B). Bone marrow Gr1/Mac1+ cells include mature granulocytes and committed granulocytic progenitor cells (relatively low Gr1 expression). These progenitor cells also showed increased surface CD14 expression in mDia1-deficient mice, albeit the levels were relatively lower than those in the mature forms (Figure 3C). Similar to the bone marrow, CD14 also showed high expression on peripheral blood neutrophils (defined by Ly6G [Gr1 clone 1A8]) in these mice (Figure 3D). These data indicate that mDia1 loss is associated with aberrant CD14 signaling, which could be related to the phenotypes in the mDia1-deficient mice.
CD14 was aberrantly overexpressed on granulocytes of mDia1 heterozygous and knockout mice. (A) Flow cytometric analysis of CD14 expression in bone marrow granulocytes and monocytes of indicated mice. Data are representative of 3 independent experiments. (B) Flow cytometric analysis to compare CD14 levels in granulocytes vs monocytes of indicated mice. (C) Flow cytometric analysis of CD14 levels on the gated bone marrow Gr1low and Gr1high populations from indicated mice. (D) Flow cytometric analysis of CD14 levels on the gated Gr1 (Ly6G clone 1A8) positive peripheral blood granulocytes from indicated mice. (E) Same as Figure 2A. The percentages of CD14 positive cells in the peripheral blood Gr1/Mac1 double-positive granulocytes were analyzed. WT: N = 6, mean ± standard error of the mean (SEM) = 20.9 ± 2.02; Het: N = 5, mean ± SEM = 39.06 ± 3.25; KO: N = 3, mean ± SEM = 37.97 ± 0.52. (F) Same as Figure 2C. The percentages of CD14 positive cells in the peripheral blood Gr1/Mac1 double-positive granulocytes were analyzed. N = 3 in each group. WT: mean ± SEM = 31.07 ± 0.18; Het: mean ± SEM = 37.03 ± 1.75; KO: mean ± SEM = 39.83 ± 2.57. (G) Lineage negative bone marrow cells from indicated mice were purified and cultured in granulocyte differentiation medium for 3 days. The cells were harvested on day 0 and day 3 in culture for flow cytometric analysis of CD14. (H) Flow cytometric analysis of CD14 and indicated TLRs in peripheral blood Gr1/Mac1 double-positive cells of mice with indicated genotypes. The percentages of TLRs (upper gate) and CD14 (lower gate) positive cells are presented. Mice involved in this figure were all 6-8 weeks old.
CD14 was aberrantly overexpressed on granulocytes of mDia1 heterozygous and knockout mice. (A) Flow cytometric analysis of CD14 expression in bone marrow granulocytes and monocytes of indicated mice. Data are representative of 3 independent experiments. (B) Flow cytometric analysis to compare CD14 levels in granulocytes vs monocytes of indicated mice. (C) Flow cytometric analysis of CD14 levels on the gated bone marrow Gr1low and Gr1high populations from indicated mice. (D) Flow cytometric analysis of CD14 levels on the gated Gr1 (Ly6G clone 1A8) positive peripheral blood granulocytes from indicated mice. (E) Same as Figure 2A. The percentages of CD14 positive cells in the peripheral blood Gr1/Mac1 double-positive granulocytes were analyzed. WT: N = 6, mean ± standard error of the mean (SEM) = 20.9 ± 2.02; Het: N = 5, mean ± SEM = 39.06 ± 3.25; KO: N = 3, mean ± SEM = 37.97 ± 0.52. (F) Same as Figure 2C. The percentages of CD14 positive cells in the peripheral blood Gr1/Mac1 double-positive granulocytes were analyzed. N = 3 in each group. WT: mean ± SEM = 31.07 ± 0.18; Het: mean ± SEM = 37.03 ± 1.75; KO: mean ± SEM = 39.83 ± 2.57. (G) Lineage negative bone marrow cells from indicated mice were purified and cultured in granulocyte differentiation medium for 3 days. The cells were harvested on day 0 and day 3 in culture for flow cytometric analysis of CD14. (H) Flow cytometric analysis of CD14 and indicated TLRs in peripheral blood Gr1/Mac1 double-positive cells of mice with indicated genotypes. The percentages of TLRs (upper gate) and CD14 (lower gate) positive cells are presented. Mice involved in this figure were all 6-8 weeks old.
We further analyzed CD14 levels in the peripheral blood granulocytes of the mice transplanted with total bone marrow cells from mDia1 mice. Similar to the nontransplanted cells, the CD14 level was significantly higher on granulocytes of mice engrafted with mDia1 heterozygous and knockout bone marrow cells than those engrafted with wild-type ones (Figure 3E). The same results were obtained with bone marrow LSK progenitor/stem cell transplanted mice (Figure 3F). These data demonstrate that CD14 aberrant overexpression on granulocytes is cell autonomous. This was further confirmed by an in vitro differentiation assay in which the bone marrow lineage negative progenitor cells from mDia1 heterozygous and knockout mice showed higher CD14 levels than wild-type ones after 3 days culture in a medium that facilitated granulocyte differentiation (Figure 3G). The levels of TLR4, which is known to be associated with CD14 to mediate inflammatory cytokine secretion in the innate immune response,22 as well as other TLRs tested, remained unchanged (Figure 3H).
CD14 overexpression in mDia1 heterozygous and knockout mice sensitized the innate immune response and accelerated the development of MDS
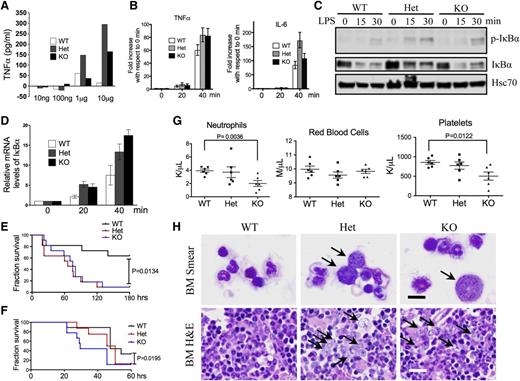
What is the functional significance of the aberrant CD14 overexpression on granulocytes? The CD14/TLR4 signaling pathway is well established in the innate immune response to the stimuli of LPS,22,23 which acts as an endotoxin and is found in the outer membrane of gram-negative bacteria. We therefore tested whether mDia1 mice would have increased sensitivity to LPS. Indeed, mDia1 heterozygous and knockout mice showed a dosage-dependent overexpression of tumor necrosis factor (TNF) α to LPS injection compared with the wild-type ones (Figure 4A). The messenger RNA (mRNA) levels of TNFα, as well as IL-6, were also more significantly increased in Gr1/Mac1 double-positive granulocytes of mDia1 heterozygous and knockout mice compared with those from the wild-type ones upon in vitro LPS stimulation (Figure 4B). However, the TNFα mRNA level showed no difference in the Mac1high/Gr1low (monocytic) population in mice with different genotypes (supplemental Figure 3B), which supports that the hypersensitive innate immune response was attributed to the granulocytes. LPS treatment also significantly upregulated the nuclear factor κB signaling pathway as demonstrated by the increased phospho-IκBα (Figure 4C) and IκBα mRNA levels (Figure 4D) in mDia1 heterozygous and knockout Gr1/Mac1 double-positive cells. Furthermore, injection of a high-dose LPS led to a significantly increased mortality in mDia1 heterozygous and knockout mice (Figure 4E), as well as mice transplanted with total bone marrow cells from mDia1-deficient mice (Figure 4F). These results demonstrate that aberrant overexpression of CD14 on granulocytes of mDia1 heterozygous and knockout mice is functionally significant in that it sensitizes the innate immune responses in these mice.
Aberrant CD14 overexpression on granulocytes was functionally significant in the pathogenesis of del(5q) MDS. (A) The levels of serum TNFα after intraperitoneal injection of LPS with different doses into indicated mice. (B) Real-time polymerase chain reaction (PCR) analysis of TNFα and IL-6 mRNA levels in Gr1/Mac1 double-positive granulocytes treated with LPS for different times. (C) Western blot analysis of phospho–inhibitory nuclear factor κB (IκB) α and total IκBα levels in cells from panel B at indicated time points. Heat shock cognate protein 70 (Hsc70) was used as a loading control. (D) Real-time PCR analysis of IκBα mRNA level in cells from panel B at indicated time points. (E) Injection of high-dose LPS (30 µg/g) into indicated mice (6-8 weeks old, whole body knockout). The fraction of survival over time was plotted using Kaplan-Meier survival analysis. N = 11 in each group. Data were from 2 independent experiments. (F) Same as in panel E except that WT mice 1 month after transplantation of total bone marrow cells from the indicated mDia1 mice were used. N = 8 in each group. (G) CBC for neutrophils, red blood cells, and platelets 6 months after weekly intraperitoneal injection of LPS (2 µg/g). N = 6 in each genotype group. Mice were 6-8 weeks old at the time of first injection. (H) Wright-Giemsa stains of bone marrow smears (top panels) and H&E stains of bone marrow sections (lower panels) of indicated mice from panel F. Arrows indicate dysplastic granulocytes. Scale bars represent 10 μm (upper) and 50 μm (lower).
Aberrant CD14 overexpression on granulocytes was functionally significant in the pathogenesis of del(5q) MDS. (A) The levels of serum TNFα after intraperitoneal injection of LPS with different doses into indicated mice. (B) Real-time polymerase chain reaction (PCR) analysis of TNFα and IL-6 mRNA levels in Gr1/Mac1 double-positive granulocytes treated with LPS for different times. (C) Western blot analysis of phospho–inhibitory nuclear factor κB (IκB) α and total IκBα levels in cells from panel B at indicated time points. Heat shock cognate protein 70 (Hsc70) was used as a loading control. (D) Real-time PCR analysis of IκBα mRNA level in cells from panel B at indicated time points. (E) Injection of high-dose LPS (30 µg/g) into indicated mice (6-8 weeks old, whole body knockout). The fraction of survival over time was plotted using Kaplan-Meier survival analysis. N = 11 in each group. Data were from 2 independent experiments. (F) Same as in panel E except that WT mice 1 month after transplantation of total bone marrow cells from the indicated mDia1 mice were used. N = 8 in each group. (G) CBC for neutrophils, red blood cells, and platelets 6 months after weekly intraperitoneal injection of LPS (2 µg/g). N = 6 in each genotype group. Mice were 6-8 weeks old at the time of first injection. (H) Wright-Giemsa stains of bone marrow smears (top panels) and H&E stains of bone marrow sections (lower panels) of indicated mice from panel F. Arrows indicate dysplastic granulocytes. Scale bars represent 10 μm (upper) and 50 μm (lower).
Aberrant innate immune signaling is involved in megakaryocytic dysplasia in MDS.12,24 Inflammatory cytokines also contribute to the defects in hematopoietic cells and ineffective hematopoiesis.25,-27 To determine if aberrant innate immune signaling could accelerate the development of MDS in mDia1 heterozygous and knockout mice, we injected low doses of LPS chronically and monitored the hematologic phenotypes over time. At 6 months, neutropenia, although not exacerbated compared with the untreated mice (supplemental Figure 3C), persisted in mDia1 heterozygous and knockout mice. In addition, the red blood cell counts were relatively decreased in heterozygous mice compared with the wild-type controls. The platelet counts were significantly decreased in mDia1 knockout mice. These CBC indices indicate the development of MDS (Figure 4G). Indeed, bone marrow morphologic examination revealed prominent myeloid dysplasia (Figure 4H), similar to that observed in the old mDia1 heterozygous and knockout mice (Figure 2G). Flow cytometric analysis of Gr1/Mac1 double-positive cells in these mice was unremarkable (supplemental Figure 3D), which is also similar to that discovered in old mDia1-deficient mice.
Lenalidomide rescued LPS-induced MDS in mDia1-deficient mice
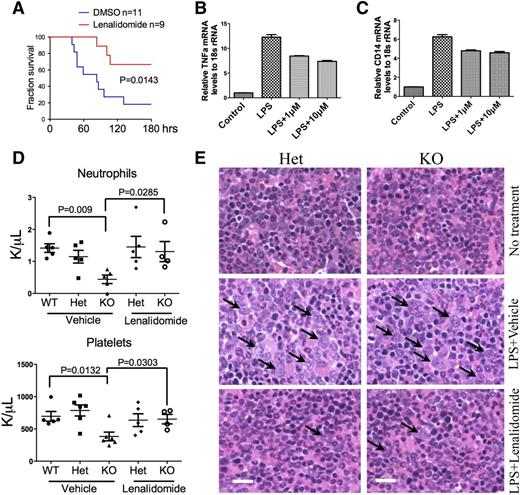
Lenalidomide is the drug of choice for the treatment of del(5q) MDS.28,-30 It has a well-established role of anti-inflammatory function by reducing the expression of proinflammtory cytokines such as TNFα and IL-6.31 We therefore injected the mDia1 heterozygous mice with lenalidomide and a high dose of LPS to determine if lenalidomide could rescue the LPS-induced mortality. As expected, lenalidomide significantly increased the survival rate of heterozygous mDia1 mice caused by LPS (Figure 5A). It also reduced the expression of TNFα and CD14 in a dose-dependent manner (Figure 5B-C).
Lenalidomide rescued LPS-induced MDS phenotypes in mDia1 heterozygous and knockout mice. (A) Kaplan-Meier survival curve of mDia1 heterozygous mice pretreated with lenalidomide (10 mg/kg per day for 5 days) followed by a lethal dose of LPS (30 µg/g). Data were from 3 independent experiments. Mice used were 6-8 weeks old. (B-C) Real-time PCR analysis of TNFα (B) and CD14 (C) mRNA levels in Gr1/Mac1 double-positive bone marrow cells from mDia1 heterozygous mice (6-8 weeks old) treated with dimethylsulfoxide (DMSO), LPS (2 µg/mL) alone, or LPS plus the indicated dosage of lenalidomide. (D) Neutrophil and platelet counts of indicated mice treated with chronic low-dose LPS (10 µg/g every 20 days) plus lenalidomide or vehicle control for 4 months. N = 5 in each group except mDia1 knockout mice treated with LPS and lenalidomide (N = 4). Mice were 6-8 weeks old at the time of first injection. (E) H&E stains of bone marrow sections of indicated mice from panel D. Arrows indicate dysplastic granulocytes. Scale bars represent 50 μm.
Lenalidomide rescued LPS-induced MDS phenotypes in mDia1 heterozygous and knockout mice. (A) Kaplan-Meier survival curve of mDia1 heterozygous mice pretreated with lenalidomide (10 mg/kg per day for 5 days) followed by a lethal dose of LPS (30 µg/g). Data were from 3 independent experiments. Mice used were 6-8 weeks old. (B-C) Real-time PCR analysis of TNFα (B) and CD14 (C) mRNA levels in Gr1/Mac1 double-positive bone marrow cells from mDia1 heterozygous mice (6-8 weeks old) treated with dimethylsulfoxide (DMSO), LPS (2 µg/mL) alone, or LPS plus the indicated dosage of lenalidomide. (D) Neutrophil and platelet counts of indicated mice treated with chronic low-dose LPS (10 µg/g every 20 days) plus lenalidomide or vehicle control for 4 months. N = 5 in each group except mDia1 knockout mice treated with LPS and lenalidomide (N = 4). Mice were 6-8 weeks old at the time of first injection. (E) H&E stains of bone marrow sections of indicated mice from panel D. Arrows indicate dysplastic granulocytes. Scale bars represent 50 μm.
To determine whether lenalidomide could rescue MDS phenotypes induced by chronic LPS injection in mDia1 heterozygous and knockout mice, we retroperitoneally injected lenalidomide to mice before each low-dose LPS injection. Indeed, mDia1-deficient mice treated with lenalidomide after 4 months showed a significant improvement of neutrophil and platelet counts (Figure 5D), which was accompanied by a dramatic alleviation of myelodysplasia in these mice (Figure 5E).
CD14 was overexpressed on bone marrow granulocytes of patients with 5q− syndrome
As previously mentioned, the phenotypic similarity of the heterozygous to the knockout mice is significant because patients with del(5q) MDS typically retain an intact 5q allele. To determine if similar findings are present in MDS patients with 5q− syndrome, we analyzed CD45 positive bone marrow granulocytes defined by high forward and side scatter on flow cytometric analysis. Consistent with the discovery in the mDia1 mice, granulocytes from patients with 5q− syndrome expressed significantly high CD14 levels compared with MDS patients without del(5q) and normal individuals (Figure 6A-B). However, CD14 levels were very low in the CD34 positive blast population in these patients (supplemental Figure 3E), which supports the significance of CD14 aberrant overexpression on granulocytes. The percentage of CD11b positive bone marrow granulocytes in patients with 5q− syndrome was similar to that in the control patients (Figure 6A; supplemental Figure 3F), which was also similarly observed in old mDia1-deficient mice (supplemental Figure 3A). Importantly, immunohistochemistry studies demonstrated that the bone marrow granulocytes in most patients with 5q− syndrome expressed significantly lower levels of mDia1 compared with controls and non-del(5q) MDS patients (Figure 6C). Because not all these patient showed high CD14 expression on their granulocytes (Figure 6B), the heterogeneous genetics and disease stage of human MDS could also play a role in the regulation of CD14 expression. Clinically, these patients with CD14 overexpression showed frequent infections and prolonged duration of the infections, indicating a functional correlation of CD14 overexpression with a hypersensitive innate immune system (supplemental Table 1).
CD14 overexpression on granulocytes of patients with del(5q) MDS. (A) Flow cytometric analysis of CD14 and CD11b levels in bone marrow granulocytes from patients with 5q− syndrome and healthy controls. (B) Quantitative analysis of CD14 levels of bone marrow granulocytes from indicated patient groups. 5q− syndrome: N = 11, mean ± SEM = 18.84 ± 7.97; other MDS (without del[5q]): N = 12, mean ± SEM = 2.25 ± 0.66; control: N = 21, mean ± SEM = 3.10 ± 0.30. (C) Immunohistochemistry stains of mDia1 of bone marrow sections from representative patients of indicated groups in panel B. Granulocytes are indicated by arrows. Megakaryocytes and lymphocytes with high mDia1 expression levels served as internal controls. Scale bars represent 30 μm.
CD14 overexpression on granulocytes of patients with del(5q) MDS. (A) Flow cytometric analysis of CD14 and CD11b levels in bone marrow granulocytes from patients with 5q− syndrome and healthy controls. (B) Quantitative analysis of CD14 levels of bone marrow granulocytes from indicated patient groups. 5q− syndrome: N = 11, mean ± SEM = 18.84 ± 7.97; other MDS (without del[5q]): N = 12, mean ± SEM = 2.25 ± 0.66; control: N = 21, mean ± SEM = 3.10 ± 0.30. (C) Immunohistochemistry stains of mDia1 of bone marrow sections from representative patients of indicated groups in panel B. Granulocytes are indicated by arrows. Megakaryocytes and lymphocytes with high mDia1 expression levels served as internal controls. Scale bars represent 30 μm.
Loss of mDia1 led to a downregulation of membrane-associated genes and a specific upregulation of CD14 mRNA in granulocytes
mDia1 was implicated to influence gene expression through the regulation of the cytoplasmic actin monomer pool.14 To explore whether mDia1 deficiency affects gene expression patterns in granulocytes, we performed an RNA sequencing analysis of the Gr1/Mac1 double-positive bone marrow granulocytes of the mDia1 mice. Compared with the wild-type cells, mDia1 heterozygous and knockout cells showed a significant decrease of many genes that encode proteins integral to the plasma membrane shown by the gene set enrichment analysis (Figure 7A-B; supplemental Tables 2 and 3), which confirms the important roles of mDia1 in the maintenance of cytoskeleton. Interestingly, there were not many genes upregulated with mDia1 deficiency except CD14, which was increased in both heterozygous and knockout mDia1 granulocytes (Figure 7A-B, arrows). We confirmed the RNA-sequencing data with real-time PCR (Figure 7C), which also showed that CD14 overexpression was cell-type specific because it was not increased in Gr1/Mac1 negative cells in these mice. CD14 mRNA level was also increased in granulocytes differentiated from cultured bone marrow lineage negative cells derived from mDia1 heterozygous and knockout mice (Figure 7D). In addition, real-time PCR analysis of various blood lineages showed that CD14 mRNA level in Gr1/Mac1 double-positive cells dramatically exceeded that in other cells, which was also correlated with their high mDia1 mRNA level (Figure 7E). Taken together, these data further confirm that mDia1 deficiency induced a granulocyte specific overexpression of CD14 and demonstrate that mDia1 deficiency affected the transcriptional regulation of CD14 in granulocytes.
Loss of mDia1 led to a downregulation of membrane-associated genes and a specific upregulation of CD14 mRNA in granulocytes. (A-B) Heat map comparing RNA sequencing gene expression profiles of granulocytes (Gr1+, Mac1+) of bone marrow WT vs Het (A, left panel), and WT vs KO mDia1 mice (B, left panel). Arrows indicate CD14 overexpression in mDia1 Het and KO Gr1/Mac1+ cells. Gene set enrichment analysis shows decreased expression of a plasma membrane signature (right panels). N = 4 in each genotype group. Mice used were 6-8 weeks old. (C) Real-time PCR analysis of CD14 mRNA levels from the indicated cells. (D) The cells were cultured as in Figure 3G. CD14 mRNA levels of the indicated cells were analyzed using real-time PCR. (E) Real-time PCR analysis of CD14 and DIAPH1 levels in indicated blood lineages from WT mouse (6-8 weeks old).
Loss of mDia1 led to a downregulation of membrane-associated genes and a specific upregulation of CD14 mRNA in granulocytes. (A-B) Heat map comparing RNA sequencing gene expression profiles of granulocytes (Gr1+, Mac1+) of bone marrow WT vs Het (A, left panel), and WT vs KO mDia1 mice (B, left panel). Arrows indicate CD14 overexpression in mDia1 Het and KO Gr1/Mac1+ cells. Gene set enrichment analysis shows decreased expression of a plasma membrane signature (right panels). N = 4 in each genotype group. Mice used were 6-8 weeks old. (C) Real-time PCR analysis of CD14 mRNA levels from the indicated cells. (D) The cells were cultured as in Figure 3G. CD14 mRNA levels of the indicated cells were analyzed using real-time PCR. (E) Real-time PCR analysis of CD14 and DIAPH1 levels in indicated blood lineages from WT mouse (6-8 weeks old).
Discussion
In this study, we demonstrate that aberrant CD14 expression on bone marrow and peripheral granulocytes sensitized the innate immune response in mDia1 heterozygous and knockout mice. The same CD14 overexpression was observed on bone marrow granulocytes in patients with 5q− syndrome. Our study suggests an important role of the innate immune signaling in the pathogenesis of MDS with del(5q). Specifically, heterozygosity or loss of mDia1 led to a cell-autonomous hypersensitivity of the innate immune response in granulocytes on LPS stimulation that is associated with myeloid dysplasia. On the other hand, damage-associated molecular pattern molecules, which are often produced by apoptotic cells commonly seen in MDS and the elderly, could serve as the in vivo ligand to activate CD14/TLR and lead to myeloid dysplasia.32 Together with previously reported evidence of dyserythropoiesis attributable to loss of RPS14,7,8 and dysmegakaryopoiesis attributable to loss of miR-145/146a,12,13 the combined loss of RPS14, miR-145/146a, and mDia1 may recapitulate the pathogenesis of trilineage dysplasia in MDS with del(5q).
Importantly, the aberrant CD14 expression is not attributable to the increased cell number of monocytes in mDia1 heterozygous and knockout mice. Monocytes are often increased in patients with decreased granulocytes,33 such as congenital neutropenia. In mDia1 heterozygous and knockout mice, although there was a significant decrease of the absolute granulocyte count, the monocyte count remained unchanged compared with the wild-type controls. This was further confirmed with a specific granulocyte surface marker (Figure 3D). In these granulocytes, CD14 level was significantly higher in mDia1 heterozygous and knockout mice than those from the wild-type mice. In addition, real-time PCR analysis demonstrated that CD14 levels were very low in lineages other than Gr1/Mac1 double-positive cells, which could help explain why CD14 was specifically upregulated in these cells with loss of mDia1.
Our study also demonstrated that CD14 overexpression, hypersensitivity to LPS injection, and neutropenia in mDia1-deficient mice were cell autonomous. Neutropenia in these mice could be explained by several possible mechanisms. These include the reported neutrophil migration defect with loss of mDia1,16 increased apoptosis in the Gr1/Mac1 double-positive population, and increased adhesion of these cells to the bone marrow niche. Our unpublished results indicated that the Gr1/Mac1 double-positive bone marrow granulocytes in mDia1-deficient mice showed increased expression of certain integrins, which could contribute to their enhanced adhesion in bone marrow and reduced mobilization.
Excessive production of proinflammatory cytokines such as TNFα has long been recognized as one of the pathogenic mechanisms that leads to apoptosis in the bone marrow cells and peripheral cytopenias in MDS.34,35 Our study highlights the significance of the activated CD14/TLR4 signaling pathway in proinflammatory cytokine overproduction in del(5q) MDS. The CD14/TLR4 signaling pathway is one of the well-studied pattern-recognition receptors in the innate immune system.36,37 One of the downstream effector proteins of the CD14/TLR4 pathway, TNF receptor-associated factor 6 (TRAF6), is targeted by miR-146a on chromosome 5q. Mice with loss of miR-146a or overexpression of TRAF6 mimic MDS with megakaryocytic dysplasia.12 Therefore, patients with del(5q) MDS with heterozygosity for both DIAPH1 and miR-146a would have increased expression of CD14 and TRAF6 resulting in hypersensitive innate immune system.
One of the interesting findings in our study is that some of the phenotypes were more severe in the heterozygous mice than the knockout ones (see Figure 4A-B; supplemental Figure 2B), which could be explained by the relatively higher granulocyte count in the heterozygous mice but near equivalent CD14 levels in each granulocyte compared with the knockout mice. This phenotype further underlines the importance of mDia1 heterozygosity in these processes. It also closely mimics the genomic abnormalities in MDS because most patients with del(5q) MDS retain a wild-type 5q allele38 where genetic mutations are rare.6 This indicates that genes involved in MDS likely harbor epigenetic changes that lead to their reduced expression. Indeed, a recent study showed that epigenetic suppression of CTNNA1, which is located on 5q, contributes to the myeloid cell transformation in MDS.10 This could also be the case for DIAPH1, which encodes mDia1, because in patients with del(5q) MDS, the mDia1 mRNA level is significantly decreased, but not the levels of genes with close proximity to DIAPH1.
Our study also suggests that lenalidomide, the drug of choice for 5q− syndrome, could partially function through the reduction of the aberrant innate immune responses in del(5q) MDS. Lenalidomide also downregulated the mRNA level of CD14, which is consistent with a similar function of its analog thalidomide.39 The significant improvement of myelodysplasia after lenalidomide treatment in chronic LPS–treated mDia1-deficient mice further indicates that it not only alleviates the symptoms, but also changes the dysplastic features of the bone marrow diseased cell population.
With loss of 5q as the most common cytogenetic abnormalities in patients with MDS, discovery of its specific biomarkers is a much-needed task with a dearth of effective and specific therapeutic treatments.40 The identification of aberrant overexpression of CD14 on granulocytes of del(5q) MDS provides such a kind of biomarker for the management of del(5q) MDS. Correlation of CD14 overexpression with the natural histories of disease in these patients revealed frequent infections and prolonged duration of the infections, indicating a functional correlation of CD14 overexpression with a dysfunctional innate immune system. Therefore, therapeutic management targeting CD14/TLR4 signaling would be beneficial not only for the inhibition of MDS progression, but also the symptomatic alleviation of infections related to the compromised innate immune system in these patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs John Crispino and William Muller for critical reading of the manuscript; Drs Jing Zhang, Art Alberts, Vijay Sankaran, Jiahai Shi, LoAnn Peterson, Charles Goolsby, William Karpus, Deyu Fang, and Edward Thorp for helpful comments; and Jeffery Nelson at Northwestern University and Flow Cytometry laboratory at Moffitt Cancer Center for the help with the flow cytometric assays.
This work was supported by a National Institutes of Health, National Heart, Lung and Blood Institute pathway to independence award (R00HL102154) (P.J.), a National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant (DK062757-12) (D.A.W.), an American Society of Hematology scholar award (P.J.), and the Leukaemia & Lymphoma Research of 779 the United Kingdom (J.B.).
Authorship
Contribution: G.K., Y.M., B.Z., C.E.H., D.A.W., and P.J. designed the research; G.K., Y.M., B.Z., C.E.H., J.Y., J.M., and P.J. performed the experiments; L.Z., J.G., A.A.B., A.K.V., A.P., J.B., A.F.L., and P.J. analyzed the clinical data; M.J.S. analyzed the RNA sequencing data; G.K., Y.M., B.Z., C.E.H., D.A.W., and P.J. analyzed all the remaining data; and G.K. and P.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peng Ji, Department of Pathology, Feinberg School of Medicine, Northwestern University, 303 East Chicago Ave, Ward 3-210, Chicago, IL 60611; e-mail: peng-ji@fsm.northwestern.edu.
References
Author notes
G.K. and Y.M. contributed equally to this study.

![Figure 6. CD14 overexpression on granulocytes of patients with del(5q) MDS. (A) Flow cytometric analysis of CD14 and CD11b levels in bone marrow granulocytes from patients with 5q− syndrome and healthy controls. (B) Quantitative analysis of CD14 levels of bone marrow granulocytes from indicated patient groups. 5q− syndrome: N = 11, mean ± SEM = 18.84 ± 7.97; other MDS (without del[5q]): N = 12, mean ± SEM = 2.25 ± 0.66; control: N = 21, mean ± SEM = 3.10 ± 0.30. (C) Immunohistochemistry stains of mDia1 of bone marrow sections from representative patients of indicated groups in panel B. Granulocytes are indicated by arrows. Megakaryocytes and lymphocytes with high mDia1 expression levels served as internal controls. Scale bars represent 30 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2014-01-552463/2/m_780f6.jpeg?Expires=1769107388&Signature=tryOb~6F2QtWjJKQ8LNqnA4Z~Xn4gcOvbE30QOwUX5ELl93yaLZJfAYW6puRlSjpek6BqnjwxG5d8V~TfroCHlp12ctVJ5jJZDTY1KQokMd1B-TJlrOisW18sLkwLx6gZbCX7dTSpMiDLWkcJWtsQXg~YAhbTuF4fnf0tgYg8K38UwJXUQFbQVN4V3dsuxcFjW2YXFdNN2DWRi74so1M5KFfZC-ubfC8rPos01O72nq96Lzrw3ZVFaNlcrmwu5iZhNeJ4FvMTrcFP9EZlwf1NerPzgbdRZWlPtP0BmAig3CQIkopMa81HgRs4JzFEvLJ0QfJEtIC2MPcIKFNoLhLMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)