Key Points
Staphylococcal enterotoxins stimulate benign T cells to induce activation of the immunoregulatory Stat3/IL-10 axis in malignant T cells.
Colonization with enterotoxin-producing Staphylococcus aureus may promote immune dysregulation in cutaneous T-cell lymphoma.
Patients with cutaneous T-cell lymphoma (CTCL) are frequently colonized with Staphylococcus aureus (SA). Eradication of SA is, importantly, associated with significant clinical improvement, suggesting that SA promotes the disease activity, but the underlying mechanisms remain poorly characterized. Here, we show that SA isolates from involved skin express staphylococcal enterotoxins (SEs) that induce crosstalk between malignant and benign T cells leading to Stat3-mediated interleukin-10 (IL-10) production by the malignant T cells. The SEs did not stimulate the malignant T cells directly. Instead, SEs triggered a cascade of events involving cell-cell and asymmetric cytokine interactions between malignant and benign T cells, which stimulated the malignant T cells to express high levels of IL-10. Much evidence supports that malignant activation of the Stat3/IL-10 axis plays a key role in driving the immune dysregulation and severe immunodeficiency that characteristically develops in CTCL patients. The present findings thereby establish a novel link between SEs and immune dysregulation in CTCL, strengthening the rationale for antibiotic treatment of colonized patients with severe or progressive disease.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a group of neoplastic diseases characterized by expansion of malignant T cells in the skin. The 2 predominant clinical forms of CTCL are mycosis fungoides (MF) and Sézary syndrome (SS). MF typically presents as patch, plaque, or tumor lesions, whereas SS is characterized by erythroderma and the presence of malignant T cells in the skin and blood.1,,-4 During disease progression, there is a decline in the number and activity of benign immune cells leading to suppression of cell-mediated immunity and ultimately severe immunodeficiency.5,,,,,,,,,,-16 Much evidence supports that malignant T cells drive the immune dysregulation to impede antitumor immunity and that suppression of the patient’s cellular immunity is associated with increased disease activity.17,,,,-22 Two factors that seem to hold a central position in driving the immune dysregulation are signal transducer and activator of transcription 3 (Stat3) and the immunoregulatory cytokine interleukin-10 (IL-10). In keeping, the malignant activity of Stat3 and the expression of IL-10 increase during disease progression in parallel with the evolving immune dysregulation.23,,-26 Activation of Stat3 in malignant cells can lead to secretion of soluble mediators facilitating activation of Stat3 in infiltrating benign immune cells, thereby suppressing cell-mediated cytotoxicity and promoting accumulation of immunosuppressive regulatory T cells. Furthermore, aberrant activation of Stat3 in malignant cells can induce expression of immunoregulatory factors including IL-10.27 IL-10 possesses strong immunosuppressive capacities and can dampen immune responses by several means. Among these, IL-10 promotes accumulation of tolerogenic macrophages and dendritic cells and represses Th1-mediated immune responses while favoring differentiation of anergic and immunosuppressive T cells.28,-30 IL-10 can, accordingly, suppress the antibacterial immune defense and increase the risk of septicemia.31 Supporting that IL-10 also plays an important role in cancer-associated immunosuppression, blocking IL-10 activity in combination with immunostimulatory agents can restore antitumor immune responses in animal models with resulting tumor inhibition or regression.28,-30 Indeed, IL-10 represses the expression of Th1 cytokines from CTCL cells, and malignant CTCL cells inhibit dendritic cell maturation as well as activation of benign T cells in an IL-10–dependent manner.32,,-35 More importantly, as in many other forms of cancer, high levels of IL-10 have been associated with progressive disease and resistance to therapy in CTCL.24,25,28,-30,36
As a consequence of the compromised skin barrier and evolving immune dysfunction, CTCL patients very frequently acquire bacterial infections, which comprise a major clinical problem.37 In particular, there is a high prevalence of Staphylococcus aureus (SA), which is present in approximately 40% of the patients.38,-40 Intriguingly, eradication of SA by antibiotics is associated with significant clinical improvement in colonized patients, including a reduced involved body-surface area as well as decreased redness and pruritus of the skin.38,39,41 It has further been reported that staphylococcal sepsis in SS patients is accompanied by increased disease activity often in absence of fever.38,42 Therefore, it has been suspected for decades that SA fosters the disease activity in CTCL, but the underlying mechanisms remain poorly characterized, and it is not common practice to initiate antibiotic treatment of colonized patients.38,39,41,43,,-46 One of the central means by which SA manipulates the host’s immune system is by secreting staphylococcal enterotoxins (SEs). SEs (and SE-like toxins) constitute a large family of secreted proteins (SEA-SEE, SEG-SEJ, SElK-R, SElU, and TSST-1) that functions as superantigens. Thus, SEs bypass the normal antigen-restricted activation of T cells by binding outside the antigen-binding groove of major histocompatibility complex class II (MHC-II) molecules on one cell and to the Vβ region of T-cell receptors (TCRs) on a T cell.47 Little is known about the functional differences of SEs, but each SE interacts with a restricted repertoire of MHC-II alleles and TCR Vβ segments47 and thereby targets specific T-cell subsets. The host’s reaction to SEs seems to be dependent on the site of exposure. Whereas SEs are best known for their ability to cause food poisoning after ingestion, cutaneous colonization with SA strains producing high levels of SEs is commonly observed in chronic inflammatory skin diseases as atopic dermatitis and psoriasis, where they are believed to exacerbate the disease by modulating the inflammatory environment.48,49 SA isolates from CTCL patients are, importantly, most often positive for 1 or more SE genes, and, although still controversial, a number of studies have provided evidence of a skewed TCR Vβ repertoire in CTCL, indicating that SEs are expressed and active in vivo.38,43,,-46,50 Accordingly, the TCR Vβ subtypes most frequently reported to be overrepresented (Vβ 5.1 and 8) in CTCL38,44,46,50 are responsive to SEs, and Jackow et al38 observed that patients colonized with TSST-1–positive SA (ET-41) had an overexpansion of T cells expressing the responsive TCR Vβ subtype (Vβ 2.1). Based on these findings, we hypothesized that SA might promote immune dysregulation in CTCL patients via secretion of SEs.
Here, we show that SEs induce cell-contact–dependent and independent crosstalk between malignant and benign T cells, which leads to robust Janus kinase 3 (Jak3)/Stat3-mediated expression of IL-10 by the malignant T cells. The present data thereby provide a novel mechanistic explanation of how SA colonization may promote immune dysregulation in CTCL.
Methods
Reagents
Western blotting antibodies against Jak3 and Erk1/2 were from Santa Cruz Biotechnology (Santa Cruz, CA) and the antibody against Stat3 from Cell Signaling Technology (Beverly, MA). Antibodies against MHC-I and MHC-II molecules were from Leinco Technologies (St. Louis, MO), and the IL-2 neutralizing antibody and isotype control antibodies were from R&D Systems (Minneapolis, MN). Fluorochrome-conjugated antibodies against CD4, CD7, CD26, the IL-10 receptor (IL-10R), MHC-II, and the respective fluorochrome-conjugated isotype control antibodies used for flow cytometry and fluorescence-activated cell sorting (FACS) were from R&D Systems, Biolegend (San Diego, CA), BD Biosciences (Franklin Lakes, NJ), and Leinco Technologies. The pan Jak inhibitor (JakI/P6) was from Calbiochem (San Diego, CA), and the Jak3 inhibitor Tofacitinib (CP-690550) was from Selleck Chemicals (Houston, TX). Finally, SEs and biotin-labeled SEA were from Toxin Technology (Sarasota, FL), and dimethylsulfoxide was from Sigma-Aldrich (St. Louis, MO).
Patients and SA isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of 9 patients diagnosed with SS in accordance with the World Health Organization–European Organization for Research and Treatment of Cancer classification.1 A characteristic of malignant T cells in SS patients is that they typically lack expression of the cell-surface markers CD7 and/or CD26 and often display reduced expression of CD4 when compared with benign T cells.1,51 As exemplified in supplemental Figure 1 (available at the Blood Web site), all SS patients included in the study displayed a clear abnormal accumulation of CD4+CD7− and/or CD4+CD26− T cells in their PBMCs. Accordingly, malignant (CD4low/+CD7−, CD4low/+CD26−) and benign (CD4+CD7+, CD4+CD26+) T-cell populations were defined on the basis of CD4, CD7, and/or CD26 surface expression. The malignant TCR Vβ usage was characterized with the IOTest Beta Mark kit (Beckman Coulter, Indianapolis, IN). In 4 patients, the malignant population expressed a single TCR Vβ subtype, whereas the malignant population expressed high levels of several TCR Vβ subtypes in 1 patient. We did not identify a predominant malignant TCR Vβ subtype in the remaining patients, which is probably because the IOTest Beta Mark kit only covers approximately 70% of the TCR Vβ repertoire. SA bacteria were isolated from 2 patients diagnosed with MF and 1 patient diagnosed with SS. In brief, bacteriologic samples were collected from involved and uninvolved skin surfaces using sterile cotton swabs wetted with 0.1% Triton X-100 in 0.075 M phosphate buffer (pH 7.9) and transferred to Stuart’s transport medium. Subsequently, the bacteriologic samples were cultivated on blood agar and incubated overnight at 37°C in air supplemented with 5% CO2. Representative colonies of the 10 most dominant colony types were isolated, subcultivated, and identified by matrix-assisted laser desorption/ionization time-of-flight spectroscopy. The SA isolates were examined for production of SEs (SEA-SEE and TSST-1) using the latex agglutination kits SET-RPLA and TST-RPLA (Oxoid, Basingstoke, Hampshire, United Kingdom) and the RIDASCREEN SET A,B,C,D,E kit (R-Biopharm AG, Darmstadt, Germany). In accordance with the Declaration of Helsinki, the samples were obtained with informed consent and after approval by the Committee on Health Research Ethics (#22559).
Cell lines
The malignant T-cell line, SeAx, and the benign T-cell line, MF1850, were established from patients diagnosed with CTCL.52,-54 The cell lines were cultured in human serum (HS) media (RPMI-1640 [Sigma-Aldrich], 2 mM l-glutamine [Sigma-Aldrich], 100 mg/mL penicillin/streptomycin [Sigma-Aldrich], and 10% HS [Blood Bank, State University Hospital, Copenhagen, Denmark]) supplemented with 103 U/mL IL-2 (Proleukin) from Chiron (Emeryville, CA). Prior to experimental setup, the cell lines were starved overnight in HS media (without IL-2), and, likewise, all experiments were performed in HS media (without IL-2). Alloantigen-specific CD4+ human T-cell lines from healthy donors have been described and characterized previously.54
ELISA
The concentration of IL-10 in cell culture supernatant was measured using the human IL-10 DuoSet enzyme-linked immunosorbent assay (ELISA) development kit from R&D Systems.
qPCR
Total cellular messenger RNA (mRNA) was purified and reverse transcribed into complementary DNA as described previously.55 Quantitative polymerase chain reaction (qPCR) was subsequently performed using the Brilliant II SYBR green qPCR kit from Stratagene (La Jolla, CA) and the samples analyzed on a Mx3000P (Stratagene). For amplification, 0.2 µM of the following primers was used: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-AAGGTGAAGGTCGGAGTCAA-3′; GAPDH reverse, 5′-AATGAAGGGGTCATTGATGG-3′; IL-10 forward, 5′-CTGGGGGAGAACCTGAAG-3′; IL-10 reverse, 5′-TGGCTTTGTAGATGCCTTTC-3′; IL-2 forward, 5′-CAAGAAGGCCACAGAACTGA-3′; and IL-2 reverse, 5′-GCTGTCTCATCAGCATATTCACA-3′.
Flow cytometry
For surface staining, cells were stained with antibodies for 30 minutes at 4°C and washed before flow cytometric analysis or FACS. IL-10 staining was performed using the IL-10 Secretion Assay Detection Kit from Miltenyi Biotech (Bergisch Gladbach, Germany). Data acquisition and flow cytometric analysis were done on LSRII and Fortessa flow cytometers (BD Biosciences) using FlowJo software (Tree Star, Ashland, OR).
Cell isolation and sorting
PBMCs were isolated from the blood of SS patients by Lymphoprep density gradient centrifugation (Axis-Shield, Oslo, Norway). For some experiments, the PBMCs were either used directly for flow cytometric analyses or cultured in HS media with PBS or SEA. For other experiments, the PBMCs were sorted into separate populations of malignant and benign T cells on the basis of CD4, CD7, and/or CD26 surface expression and then mono- and cocultured in HS media with PBS or SEA. For isolation of the malignant and benign T cells, the PBMCs were stained with fluorochrome-conjugated antibodies against CD4, CD7, and CD26 and subjected to FACS using a FACSAria (BD Biosciences). The purity of the sorted malignant and benign T cells was higher than 99% and 95%, respectively. Flow cytometric analysis of a representative purification is shown in supplemental Figure 2A.
In experiments where cocultured SeAx and MF1850 cells were sorted, the SeAx cells were stained prior culture with 5 µM carboxyfluorescein diacetate succinimidyl ester as previously described.56 Subsequently, the carboxyfluorescein diacetate succinimidyl ester–positive and negative cells were sorted by FACSAria, resulting in a purity of >98%. Flow cytometric analysis of a representative purification is shown in supplemental Figure 2B.
Transfection
Transient transfections were essentially performed as described previously23 using 0.25 µmol Jak3, Stat3, or nontargeting ON-TARGETplus SMARTpool small interfering RNA (siRNA) (Dharmacon, Chicago, IL).
Western blotting
Protein extraction and western blotting were performed as described earlier.57
Results
SE-producing SA from infected CTCL lesions induces expression of IL-10 in cocultures of malignant and benign T cells
To investigate the potential role of SA in CTCL immune dysregulation, we initially isolated SA from the skin of CTCL patients (Figure 1A) and examined their impact on IL-10 expression by malignant and benign CTCL T-cell lines. Importantly, SA isolates from involved (lesional) skin stimulated vigorous production of IL-10 in cocultures of malignant (SeAx) and benign (MF1850) T cells (Figure 1B-D), whereas SA isolates from uninvolved skin did not (Figure 1B-C). Separate cultures of malignant and benign T cells were unresponsive or responded weakly to SA isolates (Figure 1B-D), indicating that a vigorous IL-10 response depended on crosstalk between the malignant and benign T cells. Supernatant from SA obtained from involved skin also triggered IL-10 production in cocultures of malignant and benign T cells, demonstrating that the induction of IL-10 expression was at least partly mediated by soluble factors (Figure 1E). Both the malignant and benign T-cell lines expressed MHC-II molecules (Figure 1F), which are high-affinity receptors for SEs and are required for SE-mediated activation of T cells.47 Antibodies directed against MHC-II molecules profoundly inhibited the binding of SEA to the malignant and benign T cells (supplemental Figure 3) and the supernatant-induced IL-10 production (Figure 1E), strongly suggesting that the response was induced by SEs. Indeed, SEs were detected in the SA isolates from involved skin but not in the SA isolates from uninvolved skin (supplemental Table 1). Because all of the SA isolates from involved skin expressed SEA, we next examined the effect of the purified toxin on the T-cell lines (supplemental Table 1). Purified SEA mimicked the effect of SA and SA supernatant by inducing a potent increase in IL-10 mRNA and protein in cocultures, but not in monocultures, of malignant and benign T cells (Figure 1G-H). The response was not restricted to this particular set of T-cell lines, as analogous results were obtained with other combinations of malignant and benign T-cell lines (supplemental Figure 4A-B). SEA at concentrations as low as 0.5 ng/mL induced IL-10 production in cocultures, indicating a high potency of SEA to activate cellular crosstalk (supplemental Figure 4C). Moreover, the ability to induce an IL-10 response in cocultures was not restricted to SEA. Thus, SEE induced an almost identical IL-10 response (supplemental Figure 5A). SEB did not induce IL-10 production (supplemental Figure 5A), an expected result given the inability of the T-cell lines to respond to this type of SE.54 Only the benign T cells proliferated in response to SEA and SEE stimulation in monocultures, indicating that SEs had limited or no direct influence on the malignant T cells (supplemental Figure 5B). Consequently, it was important to establish if IL-10 was produced by the benign T cells, the malignant T cells, or both. We therefore sorted malignant and benign T cells after coculture with or without SEA and determined their relative expression of IL-10. Whereas SEA strongly increased the level of IL-10 mRNA in cocultured malignant T cells, the level of IL-10 mRNA was essentially unaffected in cocultured benign T cells (Figure 1I). These results provided evidence that SEs can induce high expression of IL-10 by malignant CTCL T-cell lines via a mechanism that requires the presence of benign T cells.
SEs stimulate malignant T cells to express IL-10 in the presence of benign T cells. (A) SA was isolated from the skin of CTCL patients. Shown are representative pictures of the involved skin lesions. (B-D) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured in the absence (−) or presence of SA isolated from involved and uninvolved skin of (B) an SS patient and (C-D) MF patients. After 24 hours, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. (E) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with media (−), supernatant from SA from involved skin (SA sup.) or SA sup. plus a pool of antibodies against HLA-DP, HLA-DQ, and HLA-DR (anti-MHC-II, 10 μg/mL) for 24 hours. Subsequently, the concentrations of IL-10 in the culture supernatants were determined by ELISA. Error bars represent standard error of the mean (SEM) of 3 replicate cultures. (F) Representative flow cytometric analysis of MHC-II expression on the malignant (SeAx) and benign (MF1850) T-cell lines. Dashed lines represent isotype control staining and solid lines with fill MHC-II staining. (G) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (phosphate-buffered saline [PBS]) or soluble egg antigens [SEA] (50 ng/mL) for 24 hours and the relative expression of IL-10 and GAPDH mRNA measured by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Error bars represent SEM of 3 independent experiments. (H) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 24 hours and the concentration of IL-10 in the cell culture supernatants analyzed by ELISA. Error bars represent SEM of 3 independent experiments. (I) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 16 hours. Subsequently, the cocultured malignant and benign T cells were sorted by FACS and the relative level of IL-10 and GAPDH mRNA in all samples determined by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Malign. (Benign) indicates IL-10 expression in malignant T cells that had been cocultured with benign T cells, and vice versa for Benign (Malign.). Error bars represent SEM of 3 independent experiments.
SEs stimulate malignant T cells to express IL-10 in the presence of benign T cells. (A) SA was isolated from the skin of CTCL patients. Shown are representative pictures of the involved skin lesions. (B-D) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured in the absence (−) or presence of SA isolated from involved and uninvolved skin of (B) an SS patient and (C-D) MF patients. After 24 hours, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. (E) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with media (−), supernatant from SA from involved skin (SA sup.) or SA sup. plus a pool of antibodies against HLA-DP, HLA-DQ, and HLA-DR (anti-MHC-II, 10 μg/mL) for 24 hours. Subsequently, the concentrations of IL-10 in the culture supernatants were determined by ELISA. Error bars represent standard error of the mean (SEM) of 3 replicate cultures. (F) Representative flow cytometric analysis of MHC-II expression on the malignant (SeAx) and benign (MF1850) T-cell lines. Dashed lines represent isotype control staining and solid lines with fill MHC-II staining. (G) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (phosphate-buffered saline [PBS]) or soluble egg antigens [SEA] (50 ng/mL) for 24 hours and the relative expression of IL-10 and GAPDH mRNA measured by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Error bars represent SEM of 3 independent experiments. (H) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 24 hours and the concentration of IL-10 in the cell culture supernatants analyzed by ELISA. Error bars represent SEM of 3 independent experiments. (I) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 16 hours. Subsequently, the cocultured malignant and benign T cells were sorted by FACS and the relative level of IL-10 and GAPDH mRNA in all samples determined by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Malign. (Benign) indicates IL-10 expression in malignant T cells that had been cocultured with benign T cells, and vice versa for Benign (Malign.). Error bars represent SEM of 3 independent experiments.
SEA induces secretion of IL-10 from primary malignant T cells in the presence of benign T cells
Having established that SEs induce expression of IL-10 by malignant T-cell lines when cocultured with benign T-cell lines, we next addressed whether SEA induced a similar response in primary CTCL cells. Indeed, SEA induced variable but potent secretion of IL-10 from PBMCs isolated from SS patients (Figure 2A). In line with the observations from the cell lines, the malignant T cells were the principal contributors to the SEA-mediated expression of IL-10 (Figure 2B), although SEA had no direct effect on the expression of IL-10 by purified primary malignant T cells (supplemental Figure 6). MHC-II molecules were expressed on the primary malignant and benign T cells, demonstrating that they had the capacity to bind SEs (Figure 2C and supplemental Figure 7). Substantiating that the induction of IL-10 was not mediated by direct stimulation of the malignant T cells, none of the malignant TCR Vβ subtypes identified in the patients were responsive to SEA (data not shown). Concordant with the findings made with the T-cell lines, these results indicated that SEs induced expression of IL-10 in primary malignant T cells but that the response required the presence of benign T cells. To confirm this conclusion, we purified primary malignant and benign T cells from SS patients and cultured them with or without SEA. The purified malignant and benign CTCL T cells generally exhibited no detectable or very low levels of spontaneous IL-10 expression in monocultures, and the expression was not significantly influenced by the addition of SEA (Figure 3A). Importantly, SEA induced a marked increase in the expression of IL-10 when the primary malignant T cells were cultured with benign SS T cells (Figure 3A). Likewise, SEA mediated a potent induction of IL-10 when primary malignant T cells were cultured together with the benign CTCL T-cell line MF1850 and benign CD4 T cells from healthy donors (Figure 3B-C). The SEA-induced secretion of IL-10 from primary malignant T cells was thus dependent on the presence of benign T cells but did not rely on a specific nature of benign T cells derived from CTCL patients.
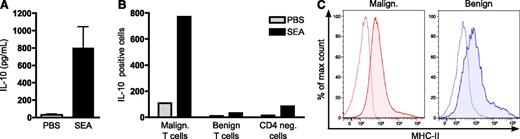
SEA induces expression of IL-10 from primary malignant T cells. (A) PBMCs were isolated from 7 SS patients and cultured with vehicle (PBS) or SEA (200 ng/mL). After 24 hours, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM. (B) Flow cytometric analysis of IL-10 expressing malignant (CD4+CD26−) T cells, benign (CD4+CD26+) T cells, and CD4-negative cells (CD4−) in SS PBMCs cultured with vehicle (PBS) or SEA (200 ng/mL) for 24 hours. (C) Representative flow cytometric analysis of MHC-II expression on malignant (CD4+CD26−) and benign (CD4+CD26+) T cells in PBMCs isolated from the blood of an SS patient. Dashed lines represent isotype control staining and solid lines with fill MHC-II staining. Flow cytometric analysis of MHC-II expression on malignant and benign T cells from 5 SS patients are summarized in supplemental Figure 7.
SEA induces expression of IL-10 from primary malignant T cells. (A) PBMCs were isolated from 7 SS patients and cultured with vehicle (PBS) or SEA (200 ng/mL). After 24 hours, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM. (B) Flow cytometric analysis of IL-10 expressing malignant (CD4+CD26−) T cells, benign (CD4+CD26+) T cells, and CD4-negative cells (CD4−) in SS PBMCs cultured with vehicle (PBS) or SEA (200 ng/mL) for 24 hours. (C) Representative flow cytometric analysis of MHC-II expression on malignant (CD4+CD26−) and benign (CD4+CD26+) T cells in PBMCs isolated from the blood of an SS patient. Dashed lines represent isotype control staining and solid lines with fill MHC-II staining. Flow cytometric analysis of MHC-II expression on malignant and benign T cells from 5 SS patients are summarized in supplemental Figure 7.
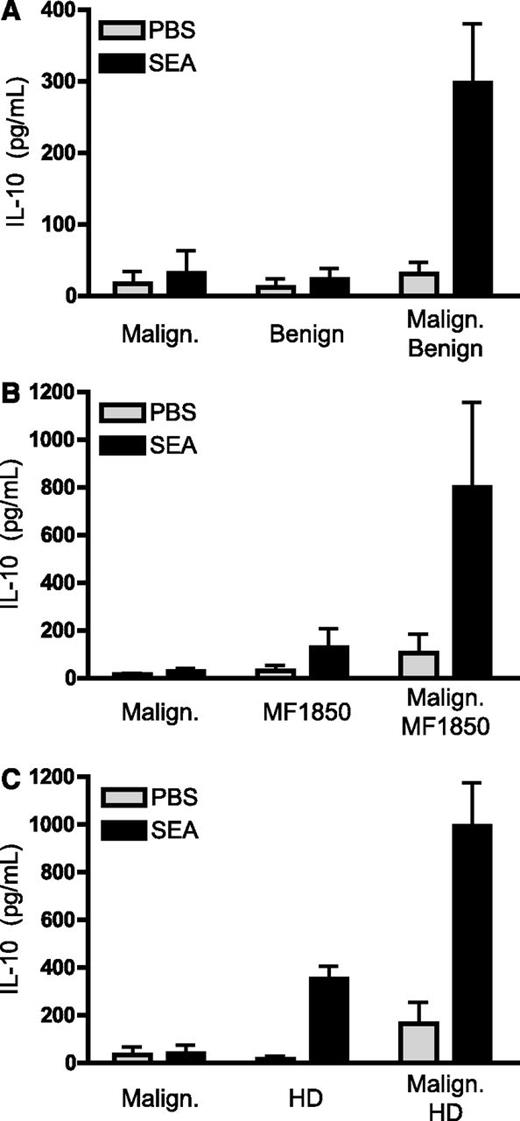
SE-mediated expression of IL-10 by primary malignant T cells requires the presence of benign T cells. (A-C) Malignant T cells were purified from the blood of SS patients and cultured for 24 hours with vehicle (PBS) or SEA (200 ng/mL) as well as (A) benign T cells purified from the blood of SS patients, (B) the benign CTCL T-cell line MF1850, or (C) a CD4 T-cell line established from the blood of a healthy donor (HD). Depicted is the concentration of IL-10 in the cell culture supernatants per 1 × 106 malignant T cells as measured by ELISA. Malignant T cells from 3 (A,C) and 4 (B) different patients were analyzed. Error bars represent SEM.
SE-mediated expression of IL-10 by primary malignant T cells requires the presence of benign T cells. (A-C) Malignant T cells were purified from the blood of SS patients and cultured for 24 hours with vehicle (PBS) or SEA (200 ng/mL) as well as (A) benign T cells purified from the blood of SS patients, (B) the benign CTCL T-cell line MF1850, or (C) a CD4 T-cell line established from the blood of a healthy donor (HD). Depicted is the concentration of IL-10 in the cell culture supernatants per 1 × 106 malignant T cells as measured by ELISA. Malignant T cells from 3 (A,C) and 4 (B) different patients were analyzed. Error bars represent SEM.
SEA induces expression of IL-10 via a mechanism that is dependent on cell-cell contact and IL-2
To investigate the mechanisms underlying the SE-induced expression of IL-10, malignant and benign T cells were cocultured in wells with or without cell-impermeable filters that allow transport of soluble messengers but prevent cell-cell contacts. As shown in Figure 4A, the SEA-induced expression of IL-10 was strongly inhibited in transwell cocultures as compared with cocultures without cell-impermeable filters, suggesting that cell-cell contact between the 2 cell types is required to induce robust IL-10 expression. SEs have previously been shown to induce secretion of IL-2 from benign CD4 T cells.58 In agreement, SEA stimulated benign CTCL cells to express IL-2 mRNA and protein (Figure 4B and supplemental Figure 8). Because IL-2 can promote the expression of IL-10 by malignant T cells, we speculated that IL-2 was involved in the SE-mediated induction of IL-10.59 Therefore, mono- and cocultured malignant and benign T cells were treated with SEA and the relative expression of IL-2 and IL-10 determined at different time points. SEA induced a quick and strong upregulation of IL-2 mRNA in the benign T cells in both mono- and cocultures, whereas SEA essentially had no effect on the expression of IL-2 in the malignant T cells (Figure 4B). Increased expression of IL-2 was already seen 1 hour following stimulation with SEA and reached a peak after 3 hours (Figure 4B). In parallel, 3 hours after stimulation with SEA, a steep increase in the level of IL-10 mRNA was observed in cocultured malignant T cells, while SEA had no significant impact on the IL-10 expression in monocultured malignant T cells (Figure 4B). The overlap in the expression of IL-2 and IL-10 prompted us to investigate the potential role of IL-2 in a more direct manner. To this end, malignant and benign T cells were cultured with SEA in the presence of an IL-2 neutralizing antibody, an isotype control antibody, or vehicle. Importantly, the IL-2 neutralizing antibody almost completely blocked the SEA-induced expression of IL-10 in transwell cocultures and profoundly inhibited it in direct cocultures when compared with the isotype control and vehicle (Figure 4C). These findings suggest that SEA promoted the malignant expression of IL-10 both by stimulating the benign T cells to produce IL-2 and by modulating cell-contact–dependent interactions between the malignant and benign T cells.
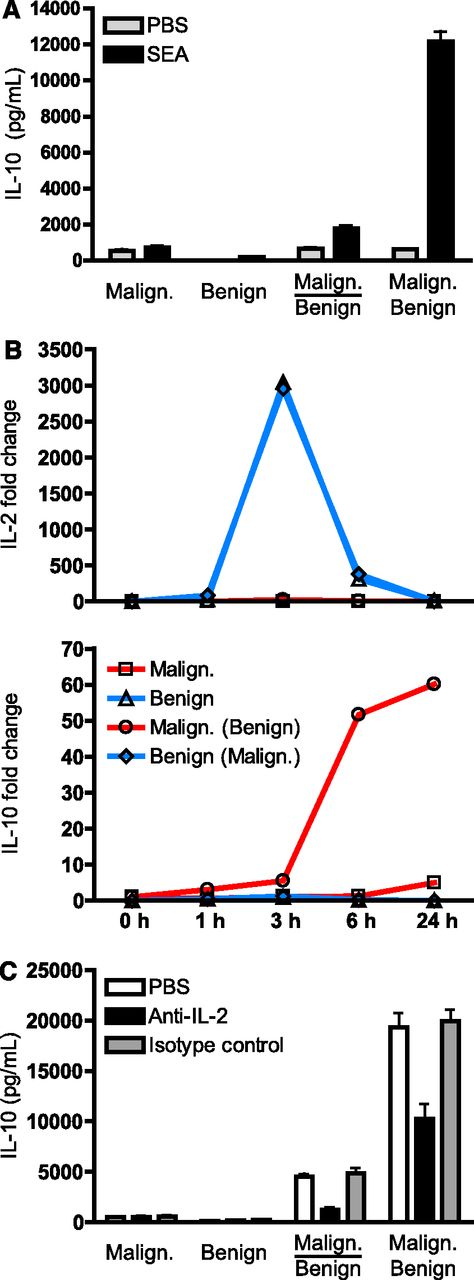
SEA induces IL-10 expression via a mechanism that is dependent on cell-cell contacts between malignant and benign T cells as well as secretion of IL-2 by benign T cells. (A) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL). In cocultures, the malignant and benign T cells were either cultured in transwells where the 2 subsets are separated by a cell-impermeable filter (indicated by a line) or together without a cell-impermeable filter (no line). The supernatants were harvested after 24 hours and the concentrations of IL-10 determined by ELISA. Error bars represent SEM of 3 independent experiments. (B) Malignant (SeAx) and benign (MF1850) T cells were cultured alone and together with SEA for different periods of time. At each given time point, the cells were harvested and the cocultured cells sorted into pure populations of malignant and benign T cells by FACS. Finally, the relative levels of IL-2, IL-10, and GAPDH mRNA in each sample were analyzed by qPCR. The levels of IL-2 and IL-10 mRNA were normalized to that of GAPDH and depicted as fold change when compared with benign and malignant T cells respectively at time point zero. Malign. (Benign) indicates cytokine expression in malignant T cells that had been cocultured with benign T cells, and vice versa for Benign (Malign.). Data are representative of 2 independent experiments. (C) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured in the presence of SEA (100 ng/mL) together with an IL-2 neutralizing antibody (Anti-IL-2, 1 μg/mL), an isotype control antibody (1 μg/mL), or vehicle (PBS) for 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM of 3 independent experiments.
SEA induces IL-10 expression via a mechanism that is dependent on cell-cell contacts between malignant and benign T cells as well as secretion of IL-2 by benign T cells. (A) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL). In cocultures, the malignant and benign T cells were either cultured in transwells where the 2 subsets are separated by a cell-impermeable filter (indicated by a line) or together without a cell-impermeable filter (no line). The supernatants were harvested after 24 hours and the concentrations of IL-10 determined by ELISA. Error bars represent SEM of 3 independent experiments. (B) Malignant (SeAx) and benign (MF1850) T cells were cultured alone and together with SEA for different periods of time. At each given time point, the cells were harvested and the cocultured cells sorted into pure populations of malignant and benign T cells by FACS. Finally, the relative levels of IL-2, IL-10, and GAPDH mRNA in each sample were analyzed by qPCR. The levels of IL-2 and IL-10 mRNA were normalized to that of GAPDH and depicted as fold change when compared with benign and malignant T cells respectively at time point zero. Malign. (Benign) indicates cytokine expression in malignant T cells that had been cocultured with benign T cells, and vice versa for Benign (Malign.). Data are representative of 2 independent experiments. (C) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured in the presence of SEA (100 ng/mL) together with an IL-2 neutralizing antibody (Anti-IL-2, 1 μg/mL), an isotype control antibody (1 μg/mL), or vehicle (PBS) for 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM of 3 independent experiments.
SE-induced expression of IL-10 is dependent on the Jak3/Stat3 pathway in malignant T cells
Because the Jak3/Stat3 pathway can promote the expression of IL-10 in malignant T cells,35,59 we investigated its possible involvement. Both a pan-Jak inhibitor (Figure 5A) and a Jak3 inhibitor (Figure 5B) efficiently blocked the SEA-mediated IL-10 expression. Likewise, transfection of the malignant T cells with Jak3-specific siRNA lead to efficient depletion of Jak3 (Figure 5C) and totally abrogated the SEA-induced expression of IL-10 in cocultures of malignant and benign T cells (Figure 5D). On the other hand, depleting Jak3 in the benign T cells had no effect on the expression of IL-10 (Figure 5C-D). Depletion of Stat3 in the malignant but not in the benign T cells also significantly inhibited the SEA-induced expression of IL-10 (Figure 5E-F). Altogether, these results demonstrate that the SE-induced expression of IL-10 was mediated via a Jak3/Stat3-dependent mechanism in malignant T cells.
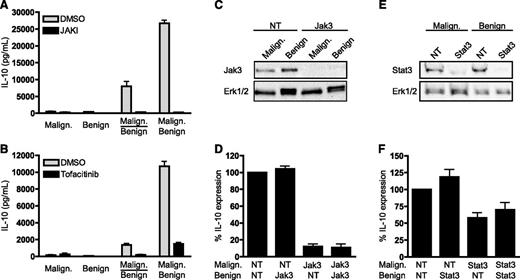
SEA induces IL-10 via a mechanism that is dependent on the Jak3/Stat3 pathway in malignant but not benign T cells. (A-B) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured in the presence of SEA (50 ng/mL) together with vehicle (dimethylsulfoxide), (A) a pan Jak inhibitor (JAKI, 1 µM) or (B) a Jak3 inhibitor (Tofacitinib, 0.3 µM). After 24 hours of culture, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM of 3 independent experiments. (C) Representative western blot of Jak3 and Erk1/2 expression after transient transfection of malignant (SeAx) and benign (MF1850) T cells with Jak3 or nontargeting (NT) siRNA. (D) Malignant (SeAx) and benign (MF1850) T cells were transiently transfected with NT or Jak3-specific siRNA and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Shown is the percent IL-10 expression relative to cocultures of malignant and benign cells transfected with NT siRNA. Error bars represent SEM of 3 independent experiments. (E) Representative western blot of Stat3 and Erk1/2 expression after transient transfection of malignant (SeAx) and benign (MF1850) T cells with Stat3 or NT siRNA. (F) Malignant (SeAx) and benign (MF1850) T cells were transiently transfected with NT or Stat3-specific siRNA and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Shown is the percent IL-10 expression relative to cocultures of malignant and benign T cells transfected with NT siRNA. Error bars represent SEM of 3 independent experiments. DMSO, dimethylsulfoxide.
SEA induces IL-10 via a mechanism that is dependent on the Jak3/Stat3 pathway in malignant but not benign T cells. (A-B) Malignant (SeAx) and benign (MF1850) T cells were mono- and cocultured in the presence of SEA (50 ng/mL) together with vehicle (dimethylsulfoxide), (A) a pan Jak inhibitor (JAKI, 1 µM) or (B) a Jak3 inhibitor (Tofacitinib, 0.3 µM). After 24 hours of culture, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Error bars represent SEM of 3 independent experiments. (C) Representative western blot of Jak3 and Erk1/2 expression after transient transfection of malignant (SeAx) and benign (MF1850) T cells with Jak3 or nontargeting (NT) siRNA. (D) Malignant (SeAx) and benign (MF1850) T cells were transiently transfected with NT or Jak3-specific siRNA and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Shown is the percent IL-10 expression relative to cocultures of malignant and benign cells transfected with NT siRNA. Error bars represent SEM of 3 independent experiments. (E) Representative western blot of Stat3 and Erk1/2 expression after transient transfection of malignant (SeAx) and benign (MF1850) T cells with Stat3 or NT siRNA. (F) Malignant (SeAx) and benign (MF1850) T cells were transiently transfected with NT or Stat3-specific siRNA and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. Shown is the percent IL-10 expression relative to cocultures of malignant and benign T cells transfected with NT siRNA. Error bars represent SEM of 3 independent experiments. DMSO, dimethylsulfoxide.
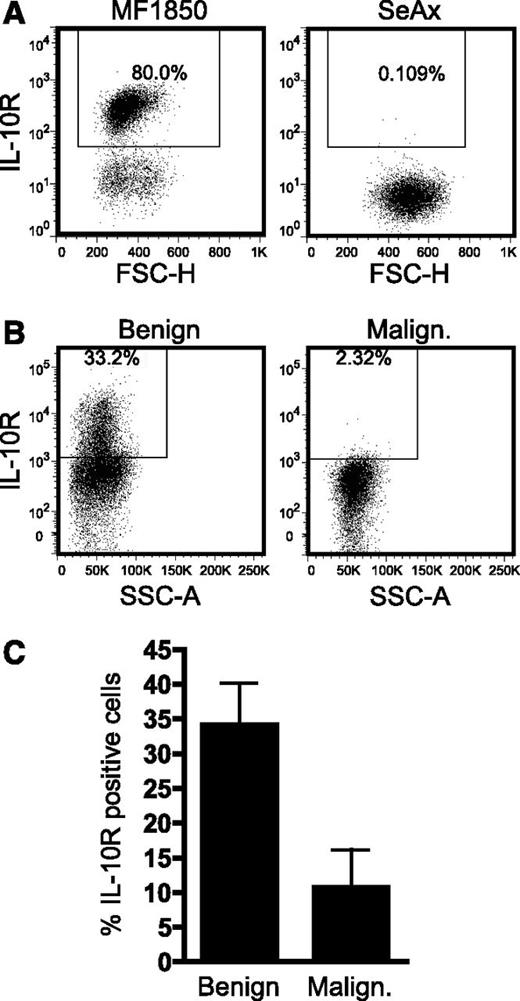
Expression of the IL-10 receptor is low or absent in malignant T cells
It has previously been shown that malignant T cells can suppress the activation of benign T cells in an IL-10–dependent manner,35 raising the question whether IL-10 could also mediate autocrine suppression of the malignant T cells. Therefore, we analyzed surface expression of IL-10R on malignant and benign T cells. IL-10R was expressed in the benign T-cell line, MF1850, but not in the malignant T-cell line, SeAx (Figure 6A). In a similar manner, IL-10R expression was absent or strongly decreased on malignant T cells when compared with benign T cells in PBMCs isolated from the blood of SS patients (Figure 6B-C and data not shown), indicating that malignant T cells generally are resistant to IL-10 stimulation.
IL-10R expression is low or absent on the malignant T cells. (A) Representative flow cytometric analysis of IL-10 receptor (IL-10R) expression on the benign T-cell line, MF1850, and the malignant T-cell line, SeAx. (B) Representative flow cytometric analysis of IL-10R expression on benign (CD4+CD26+) and malignant (CD4+CD26−) T cells in PBMCs from an SS patient. (C) Graph showing the average percentage of IL-10R–positive benign (CD4+CD26+) and malignant (CD4+CD26−) T cells in PBMCs isolated from 5 SS patients. Bars represent SEM. FSC-H, forward scatter height; SSC-A, side scatter area.
IL-10R expression is low or absent on the malignant T cells. (A) Representative flow cytometric analysis of IL-10 receptor (IL-10R) expression on the benign T-cell line, MF1850, and the malignant T-cell line, SeAx. (B) Representative flow cytometric analysis of IL-10R expression on benign (CD4+CD26+) and malignant (CD4+CD26−) T cells in PBMCs from an SS patient. (C) Graph showing the average percentage of IL-10R–positive benign (CD4+CD26+) and malignant (CD4+CD26−) T cells in PBMCs isolated from 5 SS patients. Bars represent SEM. FSC-H, forward scatter height; SSC-A, side scatter area.
Discussion
It has long been suspected that SA via expression of SEs can influence the malignant expansion and evolving immune dysregulation in CTCL patients.38,39,41,43,,-46,50 Indeed, prior studies have reported that SEs in some cases can stimulate the proliferation of malignant T cells.43,54 However, a mechanistic connection between SEs and lymphoma-associated immune dysregulation has remained elusive. Here, we demonstrate that SEs can induce vigorous expression of the immunoregulatory cytokine IL-10 in cocultures of malignant and benign T cells, thereby providing a novel link between SEs and immune dysregulation in CTCL. Remarkably, SEs did not induce high expression of IL-10 in monocultured malignant or benign T cells. Instead, SEs modulated the interactions between malignant and benign T cells to induce high malignant expression of IL-10 (Figure 7A-C). Our results suggest a mechanism where SEs induce cell-cell contact–dependent crosstalk between malignant and benign T cells and stimulate the benign T cells to produce IL-2 (Figure 7B). These interactions in turn promote activation of the Jak3/Stat3 pathway in the malignant T cells, which induces expression of IL-10 and subsequently leads to the inhibition of cell-mediated immunity and antitumor responses (Figure 7C). Because both strong activation of Stat3 and high expression of IL-10 have been identified as markers of poor response to treatment in CTCL, the present data indicate that elimination of SA may not only lead to clinical improvement but also enhance the patient’s response to treatment with other therapeutics.36,60
Simplified schematic showing the proposed mechanism of how SEs may suppress cellular immunity and antitumor responses. (A) In contrast to benign T cells, malignant T cells typically express a monoclonal TCR Vβ chain and often exhibit decreased expression or function of the TCR complex. SEs may therefore in many cases not stimulate malignant T cells directly but rather indirectly through activation of benign T cells. (B) Upon colonization with enterotoxin-producing SA bacteria, SEs bind MHC-II molecules expressed on malignant T cells, benign T cells, and antigen-presenting cells. SEs bound to MHC-II molecules subsequently crosslink TCRs on benign T cells, which elicits cell-cell contact–dependent interactions between the benign and malignant T cells and triggers secretion of IL-2 from the benign T cells. (C) These signals in turn induce high expression of IL-10 from the malignant T cells via a Jak3/Stat3-dependent pathway. IL-10 secreted from the malignant T cells can dampen cellular immunity by several means. For example, IL-10 impairs the maturation of dendritic cells, represses the expression of Th1 cytokines (interferon-γ, IL-12), inhibits T-cell activation, and promotes the function of regulatory T cells as well as the development of immunoregulatory M2 macrophages (MΦ), collectively contributing to suppression of cellular immunity and antitumor immune responses. The malignant T cells, however, display deficient expression of IL-10R and are therefore protected against the suppressive effects of IL-10. DC, dendritic cell; IFN-γ, interferon-γ.
Simplified schematic showing the proposed mechanism of how SEs may suppress cellular immunity and antitumor responses. (A) In contrast to benign T cells, malignant T cells typically express a monoclonal TCR Vβ chain and often exhibit decreased expression or function of the TCR complex. SEs may therefore in many cases not stimulate malignant T cells directly but rather indirectly through activation of benign T cells. (B) Upon colonization with enterotoxin-producing SA bacteria, SEs bind MHC-II molecules expressed on malignant T cells, benign T cells, and antigen-presenting cells. SEs bound to MHC-II molecules subsequently crosslink TCRs on benign T cells, which elicits cell-cell contact–dependent interactions between the benign and malignant T cells and triggers secretion of IL-2 from the benign T cells. (C) These signals in turn induce high expression of IL-10 from the malignant T cells via a Jak3/Stat3-dependent pathway. IL-10 secreted from the malignant T cells can dampen cellular immunity by several means. For example, IL-10 impairs the maturation of dendritic cells, represses the expression of Th1 cytokines (interferon-γ, IL-12), inhibits T-cell activation, and promotes the function of regulatory T cells as well as the development of immunoregulatory M2 macrophages (MΦ), collectively contributing to suppression of cellular immunity and antitumor immune responses. The malignant T cells, however, display deficient expression of IL-10R and are therefore protected against the suppressive effects of IL-10. DC, dendritic cell; IFN-γ, interferon-γ.
IL-10 has been reported to promote CTCL immune dysregulation by several means, including direct inhibition of benign T cells.17,32,,-35 It could thus be speculated that IL-10 might similarly suppress the proliferation and cytokine production of malignant T cells. We found that the expression of IL-10R was very low or completely absent on malignant T cells, indicating that they are protected against suppressive effects mediated by IL-10. Likewise, malignant CTCL cells express the immunosuppressive cytokine transforming growth factor β (TGF-β) but often lose surface expression of TGF-β receptor II.35,59,61,-63 This suggests that the malignant T cells elegantly manipulate and use the immune system while escaping its control. Similar to IL-10R and TGF-β receptor II, malignant T cells from advanced disease typically display decreased surface expression of CD3 and/or exhibit resistance to TCR stimulation and apoptosis.51,64 By targeting the benign T cells, SEs may still influence the malignant T cells even as they become resistant to TCR stimulation. Moreover, the proposed indirect mechanism implies that the pathogenic role of SEs is not limited to cases of patients expressing a SE-responsive TCR Vβ on the malignant clone. On the contrary, patients harbor benign T cells with a wider repertoire of TCR Vβ families, making them vulnerable to this indirect mode of action.
Infections comprise a major clinical problem in CTCL, and patients with advanced disease frequently die of infections rather than complications from the tumor burden.37 We hypothesize that SEs are part of a vicious circle where initial deterioration of the skin barrier leads to colonization with SE-producing SA. The SEs then modulate the interactions between malignant and benign immune cells leading to enhanced suppression of the host’s cellular immunity and increased disease activity, thereby contributing to further deterioration of the skin barrier. In turn, weakening of the host’s defenses stabilizes the SA colonization, facilitates its propagation, and increases the susceptibility to secondary infections.
In conclusion, we identify a mechanistic link between colonization with SE-producing SA and immune dysregulation in CTCL, thus strengthening the rationale for antibiotic treatment of colonized patients with severe or progressive disease. Furthermore, the present study supports accumulating evidence that bacterial toxins may promote cancer65,,,,-70 and conceptually demonstrates how bacterial toxins may contribute to cancer by influencing the crosstalk between tumor and immune cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Kaltoft for generously providing the CTCL cell lines.
This work was supported in part by research funding from the Danish Cancer Society, the Carlsberg Foundation, Dansk Kræftforsknings Fond, the Danish Research Councils, the Copenhagen Cluster of Immunology, the Lundbeck Foundation, the Novo Nordic Foundation, the University of Copenhagen, and the National Cancer Institute (R01-CA96856).
Authorship
Contribution: T.K. and A.W.-O. performed the experiments; T.K., A.W.-O., and N.O. analyzed and made the figures; L.M.L., R.G., M.K., and L.I. provided essential materials and patient samples; and T.K., A.W.-O., L.M.L., C.M.B., S.K., C.G., M.A.W., R.G., M.K., L.I., A.W., and N.O. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Odum, Department of International Health, Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3c, DK-2200 Copenhagen N, Denmark; e-mail: ndum@sund.ku.dk.
References
Author notes
T.K. and A.W.-O. contributed equally to this study.

![Figure 1. SEs stimulate malignant T cells to express IL-10 in the presence of benign T cells. (A) SA was isolated from the skin of CTCL patients. Shown are representative pictures of the involved skin lesions. (B-D) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured in the absence (−) or presence of SA isolated from involved and uninvolved skin of (B) an SS patient and (C-D) MF patients. After 24 hours, the concentrations of IL-10 in the cell culture supernatants were determined by ELISA. (E) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with media (−), supernatant from SA from involved skin (SA sup.) or SA sup. plus a pool of antibodies against HLA-DP, HLA-DQ, and HLA-DR (anti-MHC-II, 10 μg/mL) for 24 hours. Subsequently, the concentrations of IL-10 in the culture supernatants were determined by ELISA. Error bars represent standard error of the mean (SEM) of 3 replicate cultures. (F) Representative flow cytometric analysis of MHC-II expression on the malignant (SeAx) and benign (MF1850) T-cell lines. Dashed lines represent isotype control staining and solid lines with fill MHC-II staining. (G) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (phosphate-buffered saline [PBS]) or soluble egg antigens [SEA] (50 ng/mL) for 24 hours and the relative expression of IL-10 and GAPDH mRNA measured by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Error bars represent SEM of 3 independent experiments. (H) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 24 hours and the concentration of IL-10 in the cell culture supernatants analyzed by ELISA. Error bars represent SEM of 3 independent experiments. (I) Malignant (SeAx) and benign (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or SEA (50 ng/mL) for 16 hours. Subsequently, the cocultured malignant and benign T cells were sorted by FACS and the relative level of IL-10 and GAPDH mRNA in all samples determined by qPCR. In each sample, the level of IL-10 mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with malignant T cells cultured with PBS. Malign. (Benign) indicates IL-10 expression in malignant T cells that had been cocultured with benign T cells, and vice versa for Benign (Malign.). Error bars represent SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2014-01-551184/2/m_761f1.jpeg?Expires=1769168274&Signature=AmQCUZms06ETV-OcAtWhwvvQ0jMOdEYVvfR2NSaEsmtM0x2qkOI6j5qnDopxEToJXwJ0Nvz7WHcNIVFLeGSRZ5F2LnfA6VhjEsyUPCgOuU9g8TwISiA-v0P~r-QMxyHBaKCRVVqH8NLuiCFijifyQPL8oyFSk~HG~FX3Ysoz0mQH-KYTSAh3uc-AjUII~DqJuW~xP8zHUNzeMfv9oH2tnEf1xQIglL3vE--oLApVyk1ufpBwEfsrQPz3OITpytE6ArHOS0BtOK5WmKPu4pzSNAPYcYbv-g7Q45Zk4uQDyeog1Kb4OpvVfAwvj~Kw7alMkar~zErs6tkrMjSomiPGFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal