Key Points
SL-401 was well tolerated, and a single course of treatment produced a high rate of objective responses in BPDCN patients.
Abstract
This is the first prospective study of treatment of patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), an aggressive hematologic malignancy derived from plasmacytoid dendritic cells that typically involves the skin and rapidly progresses to a leukemia phase. Despite being initially responsive to intensive combination chemotherapy, most patients relapse and succumb to their disease. Because BPDCN blasts overexpress the interleukin-3 receptor (IL3R), the activity of SL-401, diptheria toxin (DT)388IL3 composed of the catalytic and translocation domains of DT fused to IL3, was evaluated in BPDCN patients in a phase 1-2 study. Eleven patients were treated with a single course of SL-401 at 12.5 μg/kg intravenously over 15 minutes daily for up to 5 doses; 3 patients who had initial responses to SL-401 received a second course in relapse. The most common adverse events including fever, chills, hypotension, edema, hypoalbuminemia, thrombocytopenia, and transaminasemia were transient. Seven of 9 evaluable (78%) BPDCN patients had major responses including 5 complete responses and 2 partial responses after a single course of SL-401. The median duration of responses was 5 months (range, 1-20+ months). Further studies of SL-401 in BPDCN including those involving multiple sequential courses, alternate schedules, and combinations with other therapeutics are warranted. This trial is registered at clinicaltrials.gov as #NCT00397579.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy derived from plasmacytoid dendritic cells (PDCs).1 The disease may occur at any age, but most patients are elderly men who present with skin lesions and/or involved lymph nodes, spleen, and bone marrow.2 Diagnosis rests on the blastic or plasmacytoid morphology, expression of CD4/CD56/CD123/other markers restricted to PDCs, and lack of expression of lymphoid, natural killer, or myeloid lineage-associated antigens.1 Although transient responses are seen to combination chemotherapy regimens used to treat acute leukemia or lymphoma, most patients relapse with drug-resistant disease. Durable responses have been reported with allogeneic stem cell transplant (ASCT).3 Median overall survival is 9 to 13 months, irrespective of the initial presentation of the disease.4 Molecular profiling has revealed multiple abnormalities in the genes affecting DNA methylation and chromatin remodeling and downstream activation of the nuclear factor-κB pathway.5-7 We sought to identify and target a gene product overexpressed on most BPDCN blasts.
High CD123 or interleukin 3 receptor (IL3R)α surface expression is ubiquitous in BPDCN.8 Furthermore, cultivation of primary BPDCN blasts in vitro required IL3 supplementation for growth and survival.9 These observations support the expression of both IL3R subunits (α and β) on the cell surface, thereby yielding the high affinity IL3R along with a functional downstream signaling pathway. Based on the ubiquitous expression of the IL3R and its functionality, IL3R represents a rational target for therapeutic development in BPDCN and other IL3R-expressing malignancies. Further, there is negligible expression of IL3Rα on normal hematopoietic stem cells.10
SL-401 is a recombinant fusion protein composed of the catalytic and translocation domains of diptheria toxin (DT) fused via a Met-His linker to IL3.11 The IL3 domain of SL-401 binds to its natural receptor, which is then internalized, leading to translocation of the DT A fragment to the cytosol, followed by ADP ribosylation of elongation factor 2, inactivation of protein synthesis, and cell death.12 The mechanism of SL-401 cytotoxicity differs from other available therapeutics, and because SL-401 inhibits protein synthesis, the agent is able to kill relatively dormant cells.13 Furthermore, the SL-401 payload DT is not a substrate for P-glycoprotein and other drug efflux pumps that are associated with multidrug resistance.14 The unique mechanism of action of SL-401 coupled with the biology of BPDCN were the impetus for Delettre et al to evaluate the effects of SL-401 on primary BPDCN blasts sampled from patients and BPDCN cell lines.15 The investigators demonstrated that BPDCN blasts were ultrasensitive to SL-401, with femtomolar IC50 values. SL-401 also increased overall survival in an animal model of BPDCN.
The current study represents an expansion stage of a phase 1-2 study of SL-401, in which 92 patients with advanced hematologic malignancies received a single course of SL-401 as a 15-minute intravenous infusion over 15 minutes.16,17 The maximum tolerated dose was 16.6 μg/kg/day for 5 daily doses, with transient transaminasemia and manifestations of vascular leak as consistent dose-limiting toxicities at the 22.1 μg/kg/day dose level. A dose of 12.5 μg/kg/day appeared to have the most favorable risk/benefit profile, with a low incidence of dose-limiting toxicity and several durable complete responses (CRs) observed in patients with BPDCN and acute myelogenous leukemia (AML). Based on the above rationale and preclinical results, this expansion arm of SL-401 in patients with recurrent/refractory BPDCN or those deemed not candidates for standard chemotherapy was initiated.
Patients and methods
This BPDCN study represents an expansion arm of a phase 1-2 trial of SL-401 in patients with advanced hematologic malignancies. The drug was prepared by one of the authors (AEF) under cGMP conditions and underwent good laboratory practice toxicology tests in rodents and nonhuman primates.18,19 The study was performed under investigational new drug #11314, which was held by the principal investigator (AEF), registered in clinical trials.gov as NCT00397579, and approved by the Baylor Scott & White Health Temple, Texas Institutional Review Board (IRB) #50047 and the University of Texas Southwestern, Dallas, TX, IRB #01913. The study was conducted in accordance with the Declaration of Helsinki. In the phase 1 stage of the study, which will be reported separately, cohorts of new patients were treated with a single course of SL-401 as a 15-minute infusion at doses ranging from 4 to 22 μg/kg intravenously. Patients were treated on 1 of 2 schedules: every other day for up to 6 infusions (regimen A) or daily for 5 infusions (regimen B). The maximum tolerated dose for regimen B was 16.6 μg/kg/day, but the 12.5-μg/kg/day dose level was selected for treatment of BPDCN patients in this expansion arm due to a favorable risk/benefit profile. Most patients received a single course of SL-401, but the protocol was amended with approval of the US Food and Drug Administration and IRB to permit retreatment. Three patients, all of whom had response following treatment with SL-401, were retreated with a second course following disease progression.
Eligibility and diagnosis
Patients with BPDCN diagnosed by morphologic, histochemical, and cell surface marker criteria were eligible for study. Patients had to have either recurrent/refractory BPDCN or were deemed not candidates for standard chemotherapy. Patients had to have an Eastern Cooperative Oncology Group performance status of <2 and give informed consent according to institutional and federal guidelines. Other eligibility requirements included the following: age >18 years, bilirubin <1.5 mg/dL, average 24-hour creatinine and urea clearance of >60 mL/min, transaminases <2.5 × upper limit of normal, albumin >3 g/dL, cardiac ejection fraction >50%, and willingness to use an approved form of birth control while on study. Patients with serious concurrent medical problems, active central nervous system leukemia, or history of congestive heart failure or myocardial infarction within 6 months were excluded.
Treatment
Patients were treated at the Baylor Scott & White Health Medical Center or the University of Texas Southwestern Health Science Center. Premedications administered 30 to 60 minutes prior to each daily dose of SL-401 included acetaminophen, 325 mg orally; diphenhydramine, 25 mg intravenously; methylprednisolone, 40 mg intravenously; famotidine, 20 mg intravenously; and 1 L normal saline intravenously. SL-401, 12.5 μg/kg, was administered as a 15-minute infusion daily for a maximum of 5 daily doses, which was considered a single course of treatment. During the treatment period, individual SL-401 infusions could be delayed to allow for resolution of relevant toxicities, but all 5 infusions were required to be administered within 10 days. Treatment was permitted if the serum albumin was ≥3 g/dL. Patients could receive albumin, 25 g intravenously, daily if serum albumin decreased to <3 g/dL during treatment days or in the immediate post-treatment period.
Toxicity and response evaluation
Toxicities were determined before treatment and daily for 10 days and then on days 15 and 30, and at follow-up visits by history, physical exams, complete blood cell counts with differential, serum chemistries, serum lactate dehydrogenase, and serum uric acid, and graded using the revised National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.0; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
Responses were assessed by examinations/photographs/biopsies of the skin, lymph node examinations/biopsies, bone marrow aspirate and biopsy, and positron emission tomography and computed tomography (PET/CT) performed before treatment, at 1 month, and then every 3 months and at times of disease progression. BPDCN response criteria were based on published recommendations from Cheson and colleages and Pagano and colleagues.4,20,21 CR required normalization of peripheral blood and bone marrow, absence of disease on PET/CT imaging, normal liver and spleen size without nodules, and absence of skin involvement documented by examination and biopsy of previously affected areas. A partial response (PR) was defined as a >50% decrease in bone marrow blasts, >50% decrease in the sum of the product of the diameters (SPDs) of up to 6 of the largest dominant nodal masses, no increase in the size of other lymph nodes, >50% decrease in SPD of spleen or liver nodules, no increase in the size of the liver or spleen, and >50% decrease in skin lesions. Stable disease (SD) was defined as the failure to achieve a PR in patients without bone marrow involvement and without evidence of disease progression in skin/lymph nodes/liver/spleen, whereas progressive disease (PD) was death during treatment or disease progression with persistence of >5% bone marrow blasts or any new lymph nodes or new skin lesions or increase from nadir by >50% of SPD of any single previously involved lymph node or total assessed lymph node masses or >50% increase from nadir in the SPD of liver or spleen nodules or >50% increase in liver or spleen size.
Pharmacology/immune response
Venous blood samples were drawn before treatment and 5, 30, and 90 minutes after infusion on the first and last day of treatment and on day 15 and 30. Sera were harvested and frozen at −75°C until assayed. A previously described bioassay was used to quantitate circulating SL-401 with TF1/H-Ras target cells.22 Cmax was the maximal measured drug level, and the half-life was calculated based on a simple 1-compartment exponential decay model. Humoral immune response to SL-401 was measured on serum by using a sandwich enzyme immunoassay following the methods we reported previously using SL-401 as the well-bound antigen.18
Statistical analyses
Toxicities are dichotomized as none vs any or none and mild vs moderate to severe. The rates of toxicity, overall response, and CR, as well as their 95% confidence intervals using an exact binomial method, were estimated. The mean + standard deviation values of the pharmacokinetic (PK) parameters including maximum concentration(Cmax) and half-life (t1/2) were reported.
Results
Patients
Eleven patients were treated with 14 courses of SL-401; 3 patients received a second course of treatment on disease recurrence. All 11 patients were evaluable for safety, whereas 9 were evaluable for objective response. Seven patients received all 5 doses in their first course, whereas 2 patients received a single dose of SL-401 and 1 patient each had 2 or 3 doses. The reasons for patients receiving <5 doses during the treatment period included the following: clinical deterioration, possibly due to underlying BPDCN after 1 dose (1 patient); transient grade 2 uremia/elevated creatinine (1 patient); transient grade 2 hypoalbuminemia and deep vein thrombosis (1 patient); and transient grade 3 transaminasemia (2 patients).
Relevant patient demographic and prior treatment information is detailed in Table 1. All patients were men, the median age was 70 years, and the mean age was 68 years, with a range of 40 to 77 years. Four patients were treatment naïve and deemed not candidates for standard chemotherapy, whereas 3 and 4 patients had a single or multiple prior chemotherapy regimens, respectively. Three patients were previously treated with intensive chemotherapy followed by ASCT (Table 1). Six patients had gross evidence of disease limited to the skin, whereas 5 had gross bone marrow disease with or without skin, nodal, or splenic involvement. One patient died of rapidly progressive BPDCN 1 week after receiving a single dose of SL-401. There were no treatment-related deaths.
Clinical characteristics
| Subject . | Age (years)/gender . | Prior therapy . | Sites of disease . | Marrow BPDCN blasts (course 2) (%) . |
|---|---|---|---|---|
| 1, 1B | 40/M | Cytarabine/daunorubicin/etoposide, ASCT, DLI | Bone marrow, spleen, lymph nodes | 2 (90) |
| 2 | 72/M | Cytarabine/idarubicin, gemcitabine, ASCT × 2 | Skin | 0 |
| 3 | 65/M | Etoposide/doxorubicin/vincristine/prednisone/cyclophosphamide, fludarabine, ASCT | Skin | 1 |
| 4 | 70/M | Decitabine | Skin, bone marrow | 8 |
| 5, 5B | 70/M | None | Skin | 0 (10) |
| 6 | 74/M | Cyclophosphamide/doxorubicin/vincristine/prednisone | Lymph nodes, bone marrow | 1.4 |
| 7 | 70/M | None | Skin | 0.2 |
| 8, 8B | 73/M | None | Skin | 1 (1) |
| 9 | 68/M | Cytarabine/daunorubicin, cytarabine | Skin | 1 |
| 10 | 77/M | None | Skin | 2 |
| 11 | 67/M | Daunorubicin/cytarabine, ASCT, fludarabine, cyclosphosphamide/etoposide/doxorubicin | Bone marrow | 90 |
| Subject . | Age (years)/gender . | Prior therapy . | Sites of disease . | Marrow BPDCN blasts (course 2) (%) . |
|---|---|---|---|---|
| 1, 1B | 40/M | Cytarabine/daunorubicin/etoposide, ASCT, DLI | Bone marrow, spleen, lymph nodes | 2 (90) |
| 2 | 72/M | Cytarabine/idarubicin, gemcitabine, ASCT × 2 | Skin | 0 |
| 3 | 65/M | Etoposide/doxorubicin/vincristine/prednisone/cyclophosphamide, fludarabine, ASCT | Skin | 1 |
| 4 | 70/M | Decitabine | Skin, bone marrow | 8 |
| 5, 5B | 70/M | None | Skin | 0 (10) |
| 6 | 74/M | Cyclophosphamide/doxorubicin/vincristine/prednisone | Lymph nodes, bone marrow | 1.4 |
| 7 | 70/M | None | Skin | 0.2 |
| 8, 8B | 73/M | None | Skin | 1 (1) |
| 9 | 68/M | Cytarabine/daunorubicin, cytarabine | Skin | 1 |
| 10 | 77/M | None | Skin | 2 |
| 11 | 67/M | Daunorubicin/cytarabine, ASCT, fludarabine, cyclosphosphamide/etoposide/doxorubicin | Bone marrow | 90 |
B, second course administered following disease recurrence; DLI, donor lymphocyte infusion; M, male.
Toxicities
Adverse events (AEs) attributed to drug treatment at the 12.5-μg/kg dose level as listed in Table 2 were generally mild (grade 1) to moderate (grade 2) in severity. Several patients did have transient grade 3 to 4 AEs including thrombocytopenia (grade 3, 4 patients; grade 4, 1 patient), transaminase elevations (grade 3, 5 patients), neutropenia (grade 3, 1 patient), and hyponatremia (grade 4, 1 patient). All treatment-related AEs were brief and resolved completely. One patient was diagnosed with an advanced renal cancer several months following treatment.
Dose number and drug-related adverse events
| Patient no. . | No. doses received . | Drug-related adverse effects (CTCAE v4.03 toxicity grade) . |
|---|---|---|
| 1 | 5 | Gr 2 chills, Gr 2 fever, Gr 1 nausea, Gr 3 AST, Gr 1 ALT, Gr 2 hypoalbuminemia, |
| 1B | 5 | None |
| 2 | 3 | Gr 1 fever, Gr 2 hypoalbuminemia, Gr 1 thrombocytopenia, Gr 2 edema |
| 3 | 5 | Gr 2 chills, Gr 2 fever, headache, Gr 3 AST, Gr 2 ALT, Gr 1 hypoalbuminemia, Gr 1 tachycardia, Gr 1 facial flushing |
| 4 | 5 | Gr 1 edema, Gr 2 hypoalbuminemia, Gr 3 thrombocytopenia, Gr 3 neutropenia, Gr 1 AST, Gr 1 ALT |
| 5 | 5 | Gr 1 fever, Gr 1 fatigue, Gr 1 hypoalbuminemia, Gr 2 thrombocytopenia, Gr 2 AST, Gr 2 ALT |
| 5B | 5 | Gr 1 thrombocytopenia, Gr 1 hypoalbuminemia |
| 6 | 5 | Gr 4 thrombocytopenia, Gr 2 hypoalbuminemia, Gr 1 hypocalcemia |
| 7 | 5 | Gr 3 AST, Gr 3 ALT, Gr 3 thrombocytopenia, Gr 4 hyponatremia, Gr 1 nausea, Gr1 anorexia, Gr 1 edema |
| 8 | 1 | Gr 2 uremia/elevated creatinine, Gr 1 hypotension, Gr 3 thrombocytopenia, Gr 1 ALT, Gr 1 AST |
| 8B | 2 | Gr 1 elevated prothrombin time, Gr 2 thrombocytopenia, Gr 2 neutropenia, Gr 1 anemia, Gr 1 uremia |
| 9 | 2 | Gr 3 ALT, Gr 2 AST, Gr 3 thrombocytopenia, Gr 1 nausea, Gr 1 hypoalbuminemia, Gr 1 fever, Gr 1 chills |
| 10 | 5 | Gr 3 ALT, Gr 3 AST, Gr 3 thrombocytopenia, Gr 1 anemia, Gr 2 nausea, Gr 1 hyponatremia, Gr 2 nausea, Gr 1 anorexia, Gr 2 fatigue, Gr 1 hypocalcemia, Gr 2 hypoalbuminemia |
| 11 | 1 | Gr 2 hypoalbuminemia, Gr 1 ALT |
| Patient no. . | No. doses received . | Drug-related adverse effects (CTCAE v4.03 toxicity grade) . |
|---|---|---|
| 1 | 5 | Gr 2 chills, Gr 2 fever, Gr 1 nausea, Gr 3 AST, Gr 1 ALT, Gr 2 hypoalbuminemia, |
| 1B | 5 | None |
| 2 | 3 | Gr 1 fever, Gr 2 hypoalbuminemia, Gr 1 thrombocytopenia, Gr 2 edema |
| 3 | 5 | Gr 2 chills, Gr 2 fever, headache, Gr 3 AST, Gr 2 ALT, Gr 1 hypoalbuminemia, Gr 1 tachycardia, Gr 1 facial flushing |
| 4 | 5 | Gr 1 edema, Gr 2 hypoalbuminemia, Gr 3 thrombocytopenia, Gr 3 neutropenia, Gr 1 AST, Gr 1 ALT |
| 5 | 5 | Gr 1 fever, Gr 1 fatigue, Gr 1 hypoalbuminemia, Gr 2 thrombocytopenia, Gr 2 AST, Gr 2 ALT |
| 5B | 5 | Gr 1 thrombocytopenia, Gr 1 hypoalbuminemia |
| 6 | 5 | Gr 4 thrombocytopenia, Gr 2 hypoalbuminemia, Gr 1 hypocalcemia |
| 7 | 5 | Gr 3 AST, Gr 3 ALT, Gr 3 thrombocytopenia, Gr 4 hyponatremia, Gr 1 nausea, Gr1 anorexia, Gr 1 edema |
| 8 | 1 | Gr 2 uremia/elevated creatinine, Gr 1 hypotension, Gr 3 thrombocytopenia, Gr 1 ALT, Gr 1 AST |
| 8B | 2 | Gr 1 elevated prothrombin time, Gr 2 thrombocytopenia, Gr 2 neutropenia, Gr 1 anemia, Gr 1 uremia |
| 9 | 2 | Gr 3 ALT, Gr 2 AST, Gr 3 thrombocytopenia, Gr 1 nausea, Gr 1 hypoalbuminemia, Gr 1 fever, Gr 1 chills |
| 10 | 5 | Gr 3 ALT, Gr 3 AST, Gr 3 thrombocytopenia, Gr 1 anemia, Gr 2 nausea, Gr 1 hyponatremia, Gr 2 nausea, Gr 1 anorexia, Gr 2 fatigue, Gr 1 hypocalcemia, Gr 2 hypoalbuminemia |
| 11 | 1 | Gr 2 hypoalbuminemia, Gr 1 ALT |
ALT, alanine transaminase; AST, asparate transaminase; B, second course; Gr, grade.
Acute AEs, including fever, chills, and nausea, were usually experienced within the first several hours after infusion. All were mild to moderate in severity, possibly mitigated by the premedication regimen. However, these AEs were occasionally noted 4 to 8 hours after treatment and responded to supplemental acetaminophen, meperidine, antiemetics, and/or H1- and H2-histamine antagonists. Asymptomatic hypoxemia or hypotension was infrequently noted on mandated monitoring of vital signs; however, these resolved rapidly after administration of oxygen or fluids, respectively. Mild to moderate decrements in serum albumin and blood pressure, often detected during vital signs monitoring, along with occasional peripheral edema (grades 1-2), were sometimes observed within hours following SL-401 treatment. In these patients whose serum albumin did not resolve to levels required for retreatment, parenteral albumin supplementation resulted in normalization of serum albumin and resumption of further treatment without sequelae. One 72-year-old patient with preexisting hyponatremia, likely secondary to his diuretic medication, developed transient grade 4 hyponatremia during treatment with SL-401; hydration and additional diuretics were used to manage edema.
Most patients experienced isolated elevations of hepatic transaminases without hyperbilirubinemia. The onset was generally on days 5 to 10, with complete resolution typically by days 15 to 21.
Several patients experienced thrombocytopenia, neutropenia, and/or anemia. However, many affected patients were heavily pretreated and/or had preexistent depression of blood cell counts. Further decrements of blood cell counts during treatment were short lived, and neither bleeding nor infection was noted.
Pharmacologic and immunologic studies
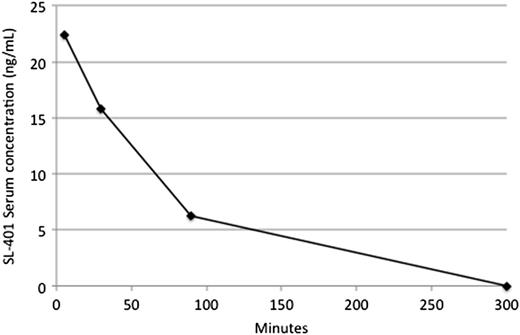
Plasma sampling for pharmacokinetic studies was performed on days 1 and 5 of course 1 in 11 and 6 patients, respectively. One of 3 patients in fulminant disease relapse who received compassionate treatment with a second course also had plasma sampling performed on day 1 of their second course. The results of relevant pharmacologic and immunologic studies are shown in Table 3. Cmax values averaged 25 ± 24 and 22 ± 18 ng/mL after treatment on days 1 and 5, respectively. The clearance of SL-401 generally fit a mono-exponential model on both days 1 and 5. Drug clearance was highly variable, with t1/2 values averaging 52 ± 20 and 51 ± 29 minutes on days 1 and 5, respectively. A typical plasma concentration disposition curve is shown in Figure 1. Cmax and t1/2 values were not significantly different between days 1 and 5. Neither SL-401 Cmax nor t1/2 values were related to response or toxicity in this small study.
Pharmacokinetic values and anti-SL-401 antibody titer
| Subject number . | Cmax values on day1/day5 (ng/mL) . | Half-life values on day 1/day5 (minutes) . | Anti-SL-401 antibody pretreatment/days15-39 (μg/mL) . |
|---|---|---|---|
| 1 | 0/7 | –/50 | 6/34 |
| 2 | 0/– | −/− | 8/4 590 |
| 3 | 22/2 | 40/10 | 0.5/35 |
| 4 | 8/18 | 30/30 | 1/– |
| 5 | 0/ND | −/− | 4/10 000 |
| 5B | 0/0 | −/− | 4 400/5 300 |
| 6 | 23/20 | 49/47 | 0.5/1 847 |
| 7 | 70/50 | 54/82 | 0.6/2 700 |
| 8 | 47/– | 89/– | 34/– |
| 9 | 55/– | 74/– | 11/– |
| 10 | 14/35 | 35/84 | 0.26/– |
| 11 | 40/– | 44/– | 2/– |
| Mean (SD) | 25 (24)/22 (18) | 52 (20)/51 (29) | 6 (10)/3 201 (3 749) |
| Subject number . | Cmax values on day1/day5 (ng/mL) . | Half-life values on day 1/day5 (minutes) . | Anti-SL-401 antibody pretreatment/days15-39 (μg/mL) . |
|---|---|---|---|
| 1 | 0/7 | –/50 | 6/34 |
| 2 | 0/– | −/− | 8/4 590 |
| 3 | 22/2 | 40/10 | 0.5/35 |
| 4 | 8/18 | 30/30 | 1/– |
| 5 | 0/ND | −/− | 4/10 000 |
| 5B | 0/0 | −/− | 4 400/5 300 |
| 6 | 23/20 | 49/47 | 0.5/1 847 |
| 7 | 70/50 | 54/82 | 0.6/2 700 |
| 8 | 47/– | 89/– | 34/– |
| 9 | 55/– | 74/– | 11/– |
| 10 | 14/35 | 35/84 | 0.26/– |
| 11 | 40/– | 44/– | 2/– |
| Mean (SD) | 25 (24)/22 (18) | 52 (20)/51 (29) | 6 (10)/3 201 (3 749) |
Patient 5B excluded from mean and standard deviation calculation.
SD, standard deviation; –, either patient sample not obtained or patient did not receive fifth daily dose so day 5 PK not evaluable; ND, not determined.
SL-401 concentration-time curve for patient 3 on day 1. Drug concentrations assayed by a previously described bioassay.22 The Cmax and t1/2 values were 22 ng/mL and 40 minutes, respectively.
SL-401 concentration-time curve for patient 3 on day 1. Drug concentrations assayed by a previously described bioassay.22 The Cmax and t1/2 values were 22 ng/mL and 40 minutes, respectively.
Pretreatment concentrations of circulating antibodies ranged from 0.5 to 34 μg/mL, most likely reflecting prior immunization with diphtheria toxoid in childhood (Table 3). In all 6 patients who had both pre- and postcourse 1 studies performed, antibody titers increased to values ranging from 34 to 10 000 μg/mL, with a mean of 3201 ± 3749/mL on days 15 to 39 after treatment. A single patient 5B who had SL-401 antibody titers measured at the time of retreatment with a second course of SL-401 had pretreatment and post-treatment antibody titer values of 4400 and 5300 μg/mL, respectively. High-dose chemotherapy followed by ASCT was not a determinant of pretreatment antibody titer. Neither pretreatment nor post-treatment antibody titer values related to response or toxicity in this small study.
Clinical response
Seven (78%; 95% confidence interval, 40-97%) of the 9 BPDCN patients evaluable for response had objectives responses, including 5 CRs and 2 PRs after a single cycle of treatment. Table 4 details the patient characteristics and relevant details pertaining to the response for each subject. The median response duration was 5 months, with a range of 1 to 20+ months. Responses occurred in all sites of disease, including lymph nodes, bone marrow, spleen, and skin. The onset of response was generally rapid; diminishment of skin lesions and lymphadenopathy were often noted during the course of drug administration or within 1 to 2 weeks (Figure 2). Patients 3 and 4 had complete disease clearance by day 15, with recurrence observed by day 30, suggesting the incremental value of treatment with multiple sequential treatments. In patients with cytopenias, presumptively due to bone marrow involvement, rapid normalization of blood counts was observed, often within 4 weeks, without an interim period of deep myelosuppression. The extent of prior therapy did not appear to be a determinant either for response or response duration. Overall, patients who received ≥3 sequential days of SL-401 without disease progression or toxicity were more likely to have a major or durable response.
Best response and follow-up in BPDCN
| Subject number . | Dose/schedule . | Cause for treatment interruption or lack of completion . | Overall response . | Length of response (months) . | Cause of death . |
|---|---|---|---|---|---|
| 1 | Five consecutive daily doses | CR | 5 | See 1B | |
| 1B | Five consecutive daily doses | PD | — | Progressive disease | |
| 2 | Three consecutive daily doses | Low albumin + DVT | CR | 20+ | Alive in remission |
| 3 | Two consecutive daily doses; then 3 consecutive daily doses 1 week later | ALT elevation | PD | — | Alive with progressive disease |
| 4 | Five consecutive daily doses | PD | — | Progressive disease | |
| 5 | Five consecutive daily doses | CR | 3 | See 5B | |
| 5B | Five consecutive daily doses | PD | — | Progressive disease + advanced RCC | |
| 6 | Five consecutive daily doses | CR | 3+ | Alive in remission | |
| 7 | Five doses over 6 days | ALT elevation | CR | 7+ | Alive in remission |
| 8 | One dose | Creatinine elevation | PR | 1 | See 8B |
| 8B | Two consecutive daily doses | Creatinine elevation | PD | — | Alive with progressive disease |
| 9 | Two consecutive daily doses | ALT elevation | NE | — | Alive with progressive disease |
| 10 | Five consecutive daily doses | PR | 4+ | Alive with partial remission | |
| 11 | One dose | Clinical deterioration possibly due to BPDCN | NE | — | Presumed progressive disease |
| Subject number . | Dose/schedule . | Cause for treatment interruption or lack of completion . | Overall response . | Length of response (months) . | Cause of death . |
|---|---|---|---|---|---|
| 1 | Five consecutive daily doses | CR | 5 | See 1B | |
| 1B | Five consecutive daily doses | PD | — | Progressive disease | |
| 2 | Three consecutive daily doses | Low albumin + DVT | CR | 20+ | Alive in remission |
| 3 | Two consecutive daily doses; then 3 consecutive daily doses 1 week later | ALT elevation | PD | — | Alive with progressive disease |
| 4 | Five consecutive daily doses | PD | — | Progressive disease | |
| 5 | Five consecutive daily doses | CR | 3 | See 5B | |
| 5B | Five consecutive daily doses | PD | — | Progressive disease + advanced RCC | |
| 6 | Five consecutive daily doses | CR | 3+ | Alive in remission | |
| 7 | Five doses over 6 days | ALT elevation | CR | 7+ | Alive in remission |
| 8 | One dose | Creatinine elevation | PR | 1 | See 8B |
| 8B | Two consecutive daily doses | Creatinine elevation | PD | — | Alive with progressive disease |
| 9 | Two consecutive daily doses | ALT elevation | NE | — | Alive with progressive disease |
| 10 | Five consecutive daily doses | PR | 4+ | Alive with partial remission | |
| 11 | One dose | Clinical deterioration possibly due to BPDCN | NE | — | Presumed progressive disease |
B, second course; NE, not evaluable as withdrew consent; DVT, deep vein thrombosis.
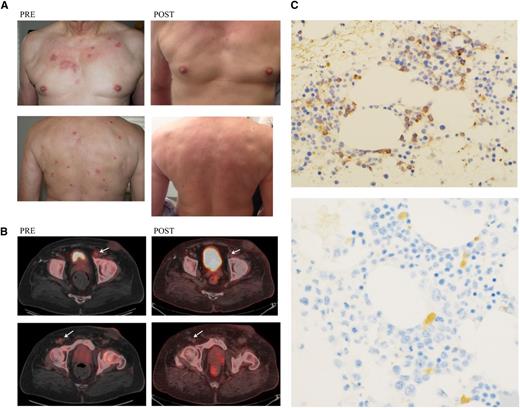
Photographs of skin, PET/CT scan, and bone marrow biopsy, pre– and post–SL-401. (A) Involved skin of patient 7 photographed before treatment and 4 months after treatment. (B) PET/CT images for patient 6 scanned before treatment and 1 month after treatment, demonstrating enlarged and PET-avid left inguinal node (1.2 × 1.6 cm; standardized uptake value Max 3.5) and right inguinal node (0.7 × 1 cm; standardized uptake value 2.3) before treatment. These inguinal nodes regressed and were no longer PET-avid at 1 month after treatment. (C) Immunostaining for CD123 on a bone marrow aspirated from patient 1 before treatment and at 1 month after treatment. Pretreatment showed 10% positive cells and after therapy showed <1% to 2% positive cells. Magnification pretreatment microphotograph is ×20; magnification post-treatment microphotograph is ×40.
Photographs of skin, PET/CT scan, and bone marrow biopsy, pre– and post–SL-401. (A) Involved skin of patient 7 photographed before treatment and 4 months after treatment. (B) PET/CT images for patient 6 scanned before treatment and 1 month after treatment, demonstrating enlarged and PET-avid left inguinal node (1.2 × 1.6 cm; standardized uptake value Max 3.5) and right inguinal node (0.7 × 1 cm; standardized uptake value 2.3) before treatment. These inguinal nodes regressed and were no longer PET-avid at 1 month after treatment. (C) Immunostaining for CD123 on a bone marrow aspirated from patient 1 before treatment and at 1 month after treatment. Pretreatment showed 10% positive cells and after therapy showed <1% to 2% positive cells. Magnification pretreatment microphotograph is ×20; magnification post-treatment microphotograph is ×40.
Discussion
This study demonstrated that SL-401, a fusion protein targeting the IL3R, has robust activity in patients with BPDCN, an aggressive malignancy that typically presents with insidious skin manifestations, but rapidly progresses to a terminal leukemic phase. The study, albeit small, was apparently the first and only prospective therapeutic trial in BPDCN. Currently, there is no consensus regarding the optimal treatment of BPDCN, and a panoply of treatments, including multiagent chemotherapy regimens designed for AML or aggressive lymphoma, symptomatic approaches, and ASCT, is offered to patients.1-3 Although objective responses to initial treatment are common, most responders develop recurrent disease and rapidly succumb. Median survival from diagnosis is ∼9 to 13 months, irrespective of the initial presentation of the disease.4 Protracted survival has been reported with ASCT, but BPDCN occurs predominantly in older patients who are not ideal candidates for ASCT, and most patients who undergo ASCT relapse.3
IL3R is expressed on BPDCN blasts and a wide variety of hematologic malignancies, but its expression is negligible on normal hematopoietic stem cells and mature hematopoietic cells except basophils and esoinophils.23 This differential expression indicates that targeting IL3R may confer a clinically relevant therapeutic index with minimal and reversible myelosuppression in patients with IL3R-expressing malignancies, as confirmed in the present study. In addition, the exquisite sensitivity of BPDCN as manifested by IC50 values in the femtomolar range and the robust clinical activity in the present study, may, in part, be explained by the ubiquitous expression and high IL3R density on BPDCN blasts.14,24,25 In addition to IL3R density and heterogeneity, however, there may be other critical determinants of SL-401 response including PK parameters, schedule, kinetics of drug-receptor internalization, and endosomal translocation, as well as other downstream cellular factors such as expression of antiapoptotic genes.26 Last, the small number of patients in this pilot study precludes the identification of patient demographic and/or biological characteristics that may be prognostic and predictive of responsiveness to SL-401 and overall outcome. Disease responsiveness occurred independent of age, site of disease, prior treatment, pretreatment antibody titer, or pharmacologic behavior as assessed by Cmax and t1/2 values.
The treatment-related AEs seen with SL-401 in BPDCN patients are similar to those reported previously in AML and myelodysplasia patients.16,17 All AEs were transient. The putative mechanism for several of the AEs noted in the present study may be a direct injury by SL-401 to reticuloendothelial cells, vascular endothelium, hepatocytes, and/or megakarocytes. With regard to AEs that were clearly infusion related, namely fever and chills, cytokine release may be triggered by macrophage pyroptosis in response to damage-associated molecular patterns released from necrotic cells and in response to pathogen-associated molecular patterns.27 Additionally, cytokines released from damaged BPDCN blasts may be, in part, responsible for the acute infusion-related AEs.28
Most patients had ≥1 AE such as hypoalbuminemia, edema, hyponatremia, hypocalcemia, uremia/elevated creatinine, fatigue, and headache reminiscent of vascular leak syndrome (VLS). One or more of these AEs was observed after several days of treatment, was generally mild to moderate, and completely resolved within days to weeks. The most consistent and early manifestation of VLS appeared to be hypoalbuminemia, and the severity of VLS-related AEs was reduced by administration of parenteral albumin and diuretics (eg, furosemide). Clinical VLS has been reported with other fusion proteins incorporating DT or Pseudomonas exotoxin fragments.29,30 The results of studies conducted in both tissue culture and animals implicated nonspecific update of these protein drugs by vascular endothelium as a putative mechanism for VLS.31 Once internalized, the catalytic proteins induce endothelial cell apoptosis and vessel wall leakage.32
Elevations of hepatic transaminase enzymes were noted in most patients. Transaminasemia resulted in treatment interruption or discontinuation prior to completion of 5 scheduled treatments in 1 and 2 patients, respectively. The transaminasemia resolved over several weeks and was not associated with hyperbilirubinemia, elevated alkaline phosphatase, or markedly elevated prothrombin time. Nonspecific hepatic SL-401 uptake and subsequent direct damage may be responsible for the transaminasemia. We established a rodent model for liver injury using DT fused to murine IL3 and observed only mild transient transaminasemia in treated animals without significant Kupffer cell or hepatocyte apoptosis.33 Immunohistochemical studies showed liver cells lacked IL3R.19
Myelosuppression was modest and reversible, perhaps reflecting the paucity of IL3R on normal myeloid progenitors.10,19 Thrombocytopenia was observed in several patients in the study, but resolution was rapid and complete. There were no signs of disseminated intravascular coagulation, microangiopathic anemia, or elevated mean platelet volume. Megakaryocyte injury due to the presence of IL3R on megakaryocytes is a possible mechanism.34 There was also no evidence of immunosuppression that is consistent with the absence of IL3R on normal lymphoid progenitors, as well as the relatively short duration of treatment.19 Additionally, the clinical relevance of targeting normal plasmacytoid dendritic cells is not known, but no evidence of AEs have been noted in plasmacytoid dendritic cell-deficient, interferon regulatory factor 8–deficient, or SLC15A4 mutant mice.35
SL-401’s PK and immunologic characteristics in BPDCN patients were similar to that previously reported in patients with other advanced hematologic malignancies.16,17 Cmax and t1/2 values for SL-401 were also comparable to those reported with other fusion proteins containing DT.36 The lack of correlation of PK parameters with response may be due to the extreme potency of SL-401, which is cytotoxic at picomolar concentrations. Similar findings were reported with denileukin diftitox and DT388 granulocyte macrophage–colony-stimulating factor.16,36,37 All study patients had pretreatment antibodies to SL-401 (antibody titers > 0.2 μg/mL), most likely due to prior immunization with diphtheria toxoid. Further, these results were similar to the finding of pretreatment anti-DT antibodies in 89% of other advanced hematologic malignancy patients.16,17 Low (0.2-2.4 μg/mL) and intermediate (2.5-50 μg/mL) antibody titers were present in 55% and 45% of pretreatment BPDCN patients, respectively, vs 67% and 22% of patients with other advanced hematologic malignancies.16,17 Neutralizing antibodies were not measured. DT antibody titers may reflect both childhood and adult tetanus/DT booster vaccinations. SL-401 antibody titers did not correlate with PK behavior, antitumor activity, or toxicity.
There are additional opportunities to target IL3R that are worthy of clinical exploration. We genetically modified SL-401 by preparing the variant SL-501 containing IL3 K116W that resulted in 15-fold greater potency and selectivity in vitro and in vivo.38 Because SL-401’s principal AEs, VLS and transaminasemia, likely relate to total drug protein concentration and/or drug exposure, the use of SL-501, a more potent and selective IL3R-targeted therapeutic, may confer a similar or greater level of antitumor efficacy with fewer AEs at much lower doses. Other methods aimed at targeting IL3R that have advanced to preclinical studies include a humanized anti-CD123 antibody,39 anti-CD123 chimeric antigen receptor T cells and cytokine-induced killer cells,40,41 anti-CD123 Fv-Pseudomonas exotoxin immunotoxins,42,43 bispecific conjugates that react with CD123 and CD16,43 and anti-CD123 radioimmunoconjugates.44
This prospective pilot study, albeit small, demonstrated a definite positive signal for SL-401 in BPDCN and provides support for additional evaluations of SL-401 in BPDCN and other IL3R-expressing malignancies. The therapeutic index was large based on the high proportion of CRs and the prolife of transient and fully reversible AEs. Although objective responses occurred in 78% of BPDCN patients, the antitumor activity and response duration of SL-401 in BPDCN cannot be quantified based on the results of the present study that only evaluated a single course of treatment. Instead, a larger study with BPDCN patients treated until disease progression with multiple sequential courses of treatment is currently being planned to provide an accurate estimate of the true response rate and response duration. Applications in minimal residual disease, particularly as a consolidation treatment in patients with high-risk AML and other IL3R-expressing malignancies who achieve a CR with induction treatment or early chemotherapy relapse with lower tumor burdens, may be valuable. Patient stratification based on blast IL3R density by flow cytometry may yield an enrichment biomarker as was seen in our in vitro work.24,25 Additionally, evaluations of treatment with SL-401 as induction therapy or consolidation treatment prior to or following ASCT or as a component of rationally designed combination regimens may also be warranted for patients with BPDCN and other IL3R expressing malignancies, because SL-401 has distinct and nonoverlapping toxicities with many conventional agents. In support of combining SL-401 with other anticancer therapeutics, synergy between SL-401 and cytarabine in leukemia xenograft models was seen.45 Other IL3R-expressing malignancies may also benefit from SL-401 based on clinical and preclinical observations in AML, myelodysplasia, acute lymphoblastic leukemia, chronic myeloid leukemia, hairy cell leukemia, chronic eosinophilic leukemia, mastocytosis, and multiple myeloma.16,17,46-48
In summary, this pilot study supports the advancement of SL-401 into pivotal phase 2 and 3 trials in BPDCN to firmly establish its role in the management of this rare and lethal disease, as well as in other relevant hematologic malignancies.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.E.F. contributed the reagents, designed and performed the research, and analyzed data; J.H.W. performed research; N.P., B.C.M., H.E.C., O.F., S.J.F., and X.A.Y. performed research; and C.A., M.K., F.G.-O., F.A.-D., C.B., M.S., and E.R. analyzed data.
Conflict-of-interest disclosure: A.E.F. holds a patent and receives research funding from Stemline Therapeutics. J.H.W., M.K., F.G.-O., and F.A.-D. receive research funding from Stemline Therapeutics. C.B., M.S., and E.R. are employees of Stemline Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Arthur E. Frankel, NB2.402A, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; e-mail: arthur.frankel@utsouthwestern.edu.