Key Points
Nonacog beta pegol, a recombinant glycoPEGylated FIX with extended half-life, was developed to improve care for patients with hemophilia B.
Weekly prophylaxis with nonacog beta pegol was well tolerated and was associated with low bleeding rates and an improved quality of life.
Abstract
This multinational, randomized, single-blind trial investigated the safety and efficacy of nonacog beta pegol, a recombinant glycoPEGylated factor IX (FIX) with extended half-life, in 74 previously treated patients with hemophilia B (FIX activity ≤2 IU/dL). Patients received prophylaxis for 52 weeks, randomized to either 10 IU/kg or 40 IU/kg once weekly or to on-demand treatment of 28 weeks. No patients developed inhibitors, and no safety concerns were identified. Three hundred forty-five bleeding episodes were treated, with an estimated success rate of 92.2%. The median annualized bleeding rates (ABRs) were 1.04 in the 40 IU/kg prophylaxis group, 2.93 in the 10 IU/kg prophylaxis group, and 15.58 in the on-demand treatment group. In the 40 IU/kg group, 10 (66.7%) of 15 patients experienced no bleeding episodes into target joints compared with 1 (7.7%) of 13 patients in the 10 IU/kg group. Health-related quality of life (HR-QoL) assessed with the EuroQoL-5 Dimensions visual analog scale score improved from a median of 75 to 90 in the 40 IU/kg prophylaxis group. Nonacog beta pegol was well tolerated and efficacious for the treatment of bleeding episodes and was associated with low ABRs in patients receiving prophylaxis. Once-weekly prophylaxis with 40 IU/kg resolved target joint bleeds in 66.7% of the affected patients and improved HR-QoL. This trial was registered at www.clinicaltrials.gov as #NCT01333111.
Introduction
Hemophilia B is an X-linked recessive congenital bleeding disorder caused by a deficiency of factor IX (FIX), resulting in impaired blood coagulation. The severe and moderate forms with 5 IU/dL or lower FIX activity manifest with increased frequency of bleeding episodes, often into joints and muscles.1 Recurrent bleeding into the same joints, often referred to as target joints, may lead to hemophilic arthropathy, which, over time, results in significant morbidity and disability in a large fraction of patients with hemophilia.2 The primary aim of hemophilia care is to prevent bleeding by regular intravenous injections (2-3 times per week) with concentrates of the deficient coagulation factor.1 The most serious complication of replacement therapy today is the development of neutralizing FIX antibodies (FIX inhibitors).3-6 Nonacog beta pegol is a recombinant FIX derivative produced without human- or animal-derived materials. A 40-kDa polyethylene glycol (PEG) moiety is attached to the FIX activation peptide by site-directed glycoPEGylation. The activation peptide with the PEG is cleaved off during the coagulation process to leave native activated FIX.7,8 Pharmacokinetic data from the first human dose trial demonstrated a 5-fold increase in terminal half-life compared with commercially available standard FIX products, offering the possibility of once-weekly prophylaxis.9
The aim of this prospective, multinational, randomized, single-blind, phase 3 clinical trial was to evaluate the safety (including immunogenicity), efficacy, and pharmacokinetics of nonacog beta pegol in previously treated patients with hemophilia B.
Methods
Study conduct
Patients
Male patients aged 13 to 70 years with hemophilia B (FIX activity ≤2 IU/dL), with no history of inhibitors to FIX, and with at least 150 exposure days to any FIX product were included in the trial. Patients with a history of thromboembolic events (such as myocardial infarction or deep vein thrombosis) and immune-deficient patients with a CD4 lymphocyte count lower than 200 cells/µL were excluded. The complete eligibility criteria are provided in the supplemental Material, Sections 2-4, available on the Blood Web site.
Trial design
This was a multinational, randomized (prophylaxis groups only), single-blind trial with 2 nonacog beta pegol prophylaxis groups (10 and 40 IU/kg once weekly) and a single on-demand group. Single-blind meant patients and investigators were blinded to the prophylaxis dose, but the investigator could become unblinded if the FIX activity needed to be measured. The 10 IU/kg and 40 IU/kg once-weekly prophylactic treatment regimens were based on the modeling of pharmacokinetic data from the first human dose trial with nonacog beta pegol in patients with hemophilia B.8,9 At screening, the patient and investigator decided whether prophylaxis or an on-demand treatment regimen would be used. Patients who chose prophylaxis were randomly assigned 1:1 in a blinded fashion to 1 of the 2 prophylaxis groups. Assessments for safety and efficacy were performed during 10 visits at 4- to 8-week intervals throughout the trial. The duration of the trial was 52 weeks for prophylaxis patients and 28 weeks for on-demand patients. Bleeding episodes were treated with a single dose of 40 IU/kg nonacog beta pegol. If a severe bleeding episode (intracranial, retroperitoneal, iliopsoas, and neck) occurred, it was to be treated with a single dose of 80 IU/kg. The trial product was administered as intravenous bolus injections. A subset of prophylaxis patients was included in the pharmacokinetic assessments with 10 IU/kg and 40 IU/kg nonacog beta pegol.
Outcome measures
The primary safety end point was development of FIX inhibitors analyzed with a Nijmegen modified Bethesda assay during the trial.12 A patient was considered positive for FIX inhibitors if 2 consecutive samples had a titer of 0.6 Bethesda Units or higher. In addition, all samples were analyzed at the end of the trial in a heat/cold Nijmegen modified FIX Bethesda assay, where the effect of high residual FIX activity was minimized and the sensitivity of the assay increased to low titers of inhibitors (see supplemental Material, Section 6 for details). Other safety assessments included adverse events, assessment of noninhibitory binding antibodies against nonacog beta pegol and antibodies against host cell proteins, and clinical laboratory tests (see supplemental Material, Section 12 for details).
A main efficacy end point was the hemostatic effect of nonacog beta pegol when treating a bleeding episode. Patients classified the bleeds as spontaneous or traumatic and reported the duration of the bleed, the number of doses, and the amount of nonacog beta pegol used to treat the bleed. Patients also rated the hemostatic response from the time of treatment until 8 hours after treatment on a 4-point scale as excellent, good, moderate, or poor (see Table 1 for definitions of hemostatic responses). Bleeding episodes with an excellent or good hemostatic response were considered successful, whereas bleeding episodes with a moderate or poor hemostatic response were considered failures.
Details of bleeding episodes and hemostatic response
| Details . | 10 IU/kg . | 40 IU/kg . | On demand . | All . |
|---|---|---|---|---|
| Number of patients | 30 | 29 | 15 | 74 |
| Number of patients with bleeding episodes | 25 | 16 | 14 | 55 |
| Number of bleeding episodes | 132 | 70 | 143 | 345 |
| Type of bleeding episode, n (%) | ||||
| Spontaneous | 91 (68.9) | 34 (48.6) | 102 (71.3) | 227 (65.8) |
| Traumatic | 39 (29.5) | 36 (51.4) | 41 (28.7) | 116 (33.6) |
| After minor surgery | 1 (0.8) | — | — | 1 (0.3) |
| Other | 1 (0.8) | — | — | 1 (0.3) |
| Injections needed to treat bleeding episode (from start to stop of bleeding episode), n (%) | ||||
| 1 injection | 111 (84.1) | 69 (98.6) | 120 (83.9) | 300 (87.0) |
| 2 injections | 16 (12.1) | — | 20 (14.0) | 36 (10.4) |
| 3 injections | 2 (1.5) | 1 (1.4) | — | 3 (0.9) |
| ≥4 injections | 3 (2.3) | — | 3 (2.1) | 6 (1.7) |
| Hemostatic response, n (%)* | ||||
| Excellent | 41 (31.1) | 35 (50.0) | 43 (30.1) | 119 (34.5) |
| Good | 72 (54.5) | 32 (45.7) | 92 (64.3) | 196 (56.8) |
| Moderate | 13 (9.8) | 2 (2.9) | 7 (4.9) | 22 (6.4) |
| Poor | 4 (3.0) | — | — | 4 (1.2) |
| Missing | 2 (1.5) | 1 (1.4) | 1 (0.7) | 4 (1.2) |
| Successful hemostatic responses, n (%)†,¶ | 113 (86.9) | 67 (97.1) | 135 (95.1) | 315 (92.4) |
| Failure hemostatic responses, n (%)‡,¶ | 17 (13.1) | 2 (2.9) | 7 (4.9) | 26 (7.6) |
| Details . | 10 IU/kg . | 40 IU/kg . | On demand . | All . |
|---|---|---|---|---|
| Number of patients | 30 | 29 | 15 | 74 |
| Number of patients with bleeding episodes | 25 | 16 | 14 | 55 |
| Number of bleeding episodes | 132 | 70 | 143 | 345 |
| Type of bleeding episode, n (%) | ||||
| Spontaneous | 91 (68.9) | 34 (48.6) | 102 (71.3) | 227 (65.8) |
| Traumatic | 39 (29.5) | 36 (51.4) | 41 (28.7) | 116 (33.6) |
| After minor surgery | 1 (0.8) | — | — | 1 (0.3) |
| Other | 1 (0.8) | — | — | 1 (0.3) |
| Injections needed to treat bleeding episode (from start to stop of bleeding episode), n (%) | ||||
| 1 injection | 111 (84.1) | 69 (98.6) | 120 (83.9) | 300 (87.0) |
| 2 injections | 16 (12.1) | — | 20 (14.0) | 36 (10.4) |
| 3 injections | 2 (1.5) | 1 (1.4) | — | 3 (0.9) |
| ≥4 injections | 3 (2.3) | — | 3 (2.1) | 6 (1.7) |
| Hemostatic response, n (%)* | ||||
| Excellent | 41 (31.1) | 35 (50.0) | 43 (30.1) | 119 (34.5) |
| Good | 72 (54.5) | 32 (45.7) | 92 (64.3) | 196 (56.8) |
| Moderate | 13 (9.8) | 2 (2.9) | 7 (4.9) | 22 (6.4) |
| Poor | 4 (3.0) | — | — | 4 (1.2) |
| Missing | 2 (1.5) | 1 (1.4) | 1 (0.7) | 4 (1.2) |
| Successful hemostatic responses, n (%)†,¶ | 113 (86.9) | 67 (97.1) | 135 (95.1) | 315 (92.4) |
| Failure hemostatic responses, n (%)‡,¶ | 17 (13.1) | 2 (2.9) | 7 (4.9) | 26 (7.6) |
Definitions of hemostatic response: excellent = abrupt pain relief and/or clear improvement in objective signs of bleeding within 8 hours after a single injection; good = noticeable pain relief and/or improvement in signs of bleeding within 8 hours after a single injection; moderate = probable or slight beneficial effect within the first 8 hours after the first injection but requiring more than a single injection within 8 hours; poor = either no improvement or worsening of symptoms within 8 hours after 2 injections.
A successful hemostatic response was defined as an excellent or good hemostatic response. Note that this observed rate is slightly different from the model-based estimate presented in Results, which was 92.2%.
A failure hemostatic response was defined as a moderate or poor hemostatic response.
Bleeding episodes with a missing hemostatic response were not included in the calculation of successful and failure hemostatic responses.
Another main efficacy end point was the prophylactic effect of nonacog beta pegol, assessed by estimating the annualized bleeding rates (ABRs) in the trial. An ABR during the last 12 months before the trial was calculated on the basis of the patient-reported number of bleeding episodes during this time.
Predose FIX trough activities were estimated and compared statistically with a trough activity of 1 IU/dL. Emphasis was on the resolution of target joints (a target joint was defined as 3 or more bleeding episodes in a particular joint within a period of 6 months before trial). For F9 genotyping, null mutations included large deletions/insertions, inversions, and nonsense mutations. Nonnull mutations included small deletions/insertions, splice site and missense mutations, and substitutions.13 The presence and extent of arthropathy in joints at baseline was recorded at the discretion of the investigator and was based on descriptions by the patients.
Pharmacokinetic assessments were performed at trial initiation (single dose assessments) and after 12 to 44 weeks of prophylaxis (steady state assessments) with 10 IU/kg and 40 IU/kg. The assessments included 7 sampling points up to 168 hours postinjection. Pharmacokinetic assessments of FIX activity were based on a 1-stage clotting assay performed at a central laboratory and using a product-specific reference standard for calculation of the FIX activity (see supplemental Material, Section 9 for details).
Patient-reported outcomes were collected from all patients using the EuroQoL-5 Dimensions (EQ-5D) visual analog scale (VAS).
Statistical methods
The analyses are based on all patients exposed to nonacog beta pegol. With respect to the primary outcome measure (incidence of patients with FIX inhibitor), no formal sample size calculation was performed because of the rarity of the disease. A sample size of 50 patients was considered sufficient for a reasonable evaluation of FIX inhibitor formation. The incidence rate of inhibitors is reported with a 1-sided 97.5% upper confidence limit, based on an exact calculation for a binomial distribution. Adequate safety with regard to FIX inhibitors is concluded if the observed rate is lower than or equal to 2% and the upper 1-sided 97.5% confidence limit is below or equal to 10.7%.
Secondary outcome measures included the hemostatic effect, the ABR, FIX trough activity levels, pharmacokinetic analyses, assessment of target joints, and health-related quality-of-life (HR-QoL) measures.
With respect to estimating the success rate when treating bleeding episodes, a logistic regression with a repeated statement to account for correlation within patients was used, applying an exchangeable working correlation matrix. This model specified that multiple bleeding episodes for a particular patient were not treated as independent events and assumed the same within-patient correlation for all bleeding episodes. A hemostatic effect of nonacog beta pegol was concluded if the lower 95% confidence limit for the success rate (success defined as excellent or good hemostatic response) was above 65%. The observed success rate was calculated as the number of successfully treated bleeds divided by the total number of treated bleeds.
Key measures of the effect of prophylaxis were the median of the individual ABRs and the estimated mean ABR. These were estimated from a Poisson regression model with dose as factor, allowing for overdispersion and using treatment duration as offset. This allowed for ABRs to be estimated for each treatment regimen group while accounting for various durations of treatment. Individual ABRs were calculated as the number of bleeding episodes per patient scaled to a treatment duration of 1 year. Prophylactic effect of nonacog beta pegol was concluded if the upper 95% confidence limit for the ABR was below 4.8, which corresponds to a more than 60% reduction of a literature-based expected ABR for on-demand patients of 12 bleeding episodes/patient/year.14-17 This requirement for claiming prophylactic effect proved to be a conservative measure compared with the actual on-demand ABRs in the trial.
Efficacy of nonacog beta pegol was also assessed by FIX trough activity as a surrogate marker. Demonstration of an estimated mean FIX trough level significantly above 1 IU/dL was performed using a mixed model analysis of the log-transformed FIX activity, with dose as a factor and patient as a random effect. Only samples collected between 5 and 10 days after last dose and at least 14 days after last bleeding episode were included.
The number of target joints per patient was collected at baseline, and the number of bleeding episodes in target joints during the trial was summarized by treatment group.
Pharmacokinetic parameters were calculated using standard noncompartmental methods (see supplemental Material, Section 11 for definition and calculation of pharmacokinetic parameters).
HR-QoL was assessed using the EQ-5D VAS instrument and presented descriptively.
Drug product
Nonacog beta pegol was produced by expression in Chinese hamster ovary cells and was supplied as freeze-dried powder in single-use vials with a nominal content of 500 IU/vial or 2000 IU/vial. Both strengths were reconstituted with 4.2 mL histidine solvent for intravenous injection.
Results
Patients
From April 2011 through April 2013, 74 patients with hemophilia B (FIX ≤2 IU/dL) were enrolled and dosed at 39 sites in 13 countries (supplemental Material, Section 13). Of these, 67 patients (17 adolescents [13-17 years] and 50 adults [18-65 years]) completed the trial (Figure 1). None of the 7 withdrawals (2 patients in the 10 IU/kg prophylaxis group, 3 patients in the 40 IU/kg prophylaxis group, and 2 patients in the on-demand treatment group) were a result of adverse events (see supplemental Material, Section 5 for details on withdrawals). Patient characteristics are presented in Table 2.
Patient enrolment and outcomes. A total of 86 patients were screened, of whom 12 were screening failures, leaving 74 patients who were exposed to nonacog beta pegol. At the screening visit, the patient and the investigator decided together whether the patient should be allocated to prophylaxis (59 patients) or on-demand treatment (15 patients). Patients allocated to prophylaxis were randomly assigned to once-weekly dosing of either 10 or 40 IU/kg. A total of 7 patients were withdrawn during the trial, distributed evenly between the treatment groups. None of the withdrawals were a result of adverse events. Screening failures and withdrawals together constituted 19 (22%) of the 86 screened patients. A total of 17 patients participated in a pharmacokinetic session at trial initiation, and all but 1 had a second pharmacokinetic session after 12 to 44 weeks of prophylaxis, leaving 16 patients (7 in the 10 IU/kg group and 9 in the 40 IU/kg group) with complete pharmacokinetic assessments.
Patient enrolment and outcomes. A total of 86 patients were screened, of whom 12 were screening failures, leaving 74 patients who were exposed to nonacog beta pegol. At the screening visit, the patient and the investigator decided together whether the patient should be allocated to prophylaxis (59 patients) or on-demand treatment (15 patients). Patients allocated to prophylaxis were randomly assigned to once-weekly dosing of either 10 or 40 IU/kg. A total of 7 patients were withdrawn during the trial, distributed evenly between the treatment groups. None of the withdrawals were a result of adverse events. Screening failures and withdrawals together constituted 19 (22%) of the 86 screened patients. A total of 17 patients participated in a pharmacokinetic session at trial initiation, and all but 1 had a second pharmacokinetic session after 12 to 44 weeks of prophylaxis, leaving 16 patients (7 in the 10 IU/kg group and 9 in the 40 IU/kg group) with complete pharmacokinetic assessments.
Baseline demographics and patient characteristics
| Characteristics . | 10 IU/kg . | 40 IU/kg . | On demand . | All . |
|---|---|---|---|---|
| Number of patients | 30 | 29 | 15 | 74 |
| Age, years | ||||
| Mean (SD) | 32.4 (13.9) | 30.0 (15.8) | 32.4 (12.0) | 31.4 (14.2) |
| Weight, kg | ||||
| Mean (SD) | 75.6 (13.0) | 70.4 (15.1) | 76.1 (16.6) | 73.7 (14.7) |
| Race, n (%) | ||||
| White | 16 (53.3) | 21 (72.4) | 11 (73.3) | 48 (64.9) |
| Asian | 8 (26.7) | 5 (17.2) | 3 (20.0) | 16 (21.6) |
| Black or African American | 2 (6.7) | 3 (10.3) | — | 5 (6.8) |
| Other | 4 (13.3) | — | 1 (6.7) | 5 (6.8) |
| Previous treatment regimen,*n (%) | ||||
| Prophylaxis | 20 (66.7) | 17 (58.6) | 2 (13.3) | 39 (52.7) |
| On demand | 10 (33.3) | 12 (41.4) | 13 (86.7) | 35 (47.3) |
| Previous prophylaxis patients, n (%) | ||||
| Recombinant FIX | 11 (55.0) | 10 (58.8) | — | 21 (53.8) |
| Plasma FIX product | 9 (45.0) | 7 (41.2) | 2 (100.0) | 18 (46.2) |
| Classification of hemophilia,†n (%) | ||||
| Moderate (1-2 IU/dL) | 7 (23.3) | 5 (17.2) | 2 (13.3) | 14 (18.9) |
| Severe (<1 IU/dL) | 23 (76.7) | 24 (82.8) | 13 (86.7) | 60 (81.1) |
| F9 genotype,‡n (%) | ||||
| N | 27 (100.0) | 24 (100.0) | 15 (100.0) | 66 (100.0) |
| Null mutations | 7 (25.9) | 1 (4.2) | 5 (33.3) | 13 (19.7) |
| Nonnull mutations | 20 (74.1) | 23 (95.8) | 10 (66.7) | 53 (80.3) |
| Arthropathy at baseline,¶n (%) | ||||
| Yes | 20 (66.7) | 18 (62.1) | 10 (66.7) | 48 (64.9) |
| No | 10 (33.3) | 11 (37.9) | 5 (33.3) | 26 (35.1) |
| Target joints at baseline,§ n (%) | ||||
| Yes | 13 (43.3) | 15 (51.7) | 12 (80.0) | 40 (54.1) |
| No | 17 (56.7) | 14 (48.3) | 3 (20.0) | 34 (45.9) |
| Characteristics . | 10 IU/kg . | 40 IU/kg . | On demand . | All . |
|---|---|---|---|---|
| Number of patients | 30 | 29 | 15 | 74 |
| Age, years | ||||
| Mean (SD) | 32.4 (13.9) | 30.0 (15.8) | 32.4 (12.0) | 31.4 (14.2) |
| Weight, kg | ||||
| Mean (SD) | 75.6 (13.0) | 70.4 (15.1) | 76.1 (16.6) | 73.7 (14.7) |
| Race, n (%) | ||||
| White | 16 (53.3) | 21 (72.4) | 11 (73.3) | 48 (64.9) |
| Asian | 8 (26.7) | 5 (17.2) | 3 (20.0) | 16 (21.6) |
| Black or African American | 2 (6.7) | 3 (10.3) | — | 5 (6.8) |
| Other | 4 (13.3) | — | 1 (6.7) | 5 (6.8) |
| Previous treatment regimen,*n (%) | ||||
| Prophylaxis | 20 (66.7) | 17 (58.6) | 2 (13.3) | 39 (52.7) |
| On demand | 10 (33.3) | 12 (41.4) | 13 (86.7) | 35 (47.3) |
| Previous prophylaxis patients, n (%) | ||||
| Recombinant FIX | 11 (55.0) | 10 (58.8) | — | 21 (53.8) |
| Plasma FIX product | 9 (45.0) | 7 (41.2) | 2 (100.0) | 18 (46.2) |
| Classification of hemophilia,†n (%) | ||||
| Moderate (1-2 IU/dL) | 7 (23.3) | 5 (17.2) | 2 (13.3) | 14 (18.9) |
| Severe (<1 IU/dL) | 23 (76.7) | 24 (82.8) | 13 (86.7) | 60 (81.1) |
| F9 genotype,‡n (%) | ||||
| N | 27 (100.0) | 24 (100.0) | 15 (100.0) | 66 (100.0) |
| Null mutations | 7 (25.9) | 1 (4.2) | 5 (33.3) | 13 (19.7) |
| Nonnull mutations | 20 (74.1) | 23 (95.8) | 10 (66.7) | 53 (80.3) |
| Arthropathy at baseline,¶n (%) | ||||
| Yes | 20 (66.7) | 18 (62.1) | 10 (66.7) | 48 (64.9) |
| No | 10 (33.3) | 11 (37.9) | 5 (33.3) | 26 (35.1) |
| Target joints at baseline,§ n (%) | ||||
| Yes | 13 (43.3) | 15 (51.7) | 12 (80.0) | 40 (54.1) |
| No | 17 (56.7) | 14 (48.3) | 3 (20.0) | 34 (45.9) |
FIX, coagulation factor IX; SD, standard deviation.
Two patients receiving previous prophylaxis chose to enroll into the on-demand treatment group. These 2 patients entered the trial at a time at which the prophylaxis groups were not yet opened for enrollment because of regulatory authority requirements in the patients’ country of origin.
Classification as defined in medical records. Only patients with FIX activity 2 IU/dL or less were eligible.
Null mutation genotypes included large deletions/insertions, inversions, and nonsense mutations. Nonnull mutations included small deletions/insertions, splice site and missense mutations, and substitutions.13 Mutations in the F9 gene were determined by either laboratory analysis carried out in the trial or, alternatively, by post hoc classification of gene defects reported in patients’ medical records where possible.
The presence and extent of arthropathy in joints at baseline was recorded at the discretion of the investigator and was based on descriptions by the patients.
A target joint was defined as 3 or more bleeding episodes in a particular joint within a period of 6 months before trial.
Safety
Mean number of exposure days to nonacog beta pegol was 54 for patients receiving prophylaxis and 14 for patients receiving on-demand treatment. No patients developed FIX inhibitors, and no deaths, thromboembolic events, or allergic reactions related to nonacog beta pegol occurred. One patient was positive for host cell protein antibodies before and after exposure. Two patients in the 10 IU/kg group and 1 patient in the 40 IU/kg group were transiently positive for noninhibitory FIX-binding antibodies (2 were positive before exposure). The highest antibody titer for these 3 patients was 4; it did not affect drug recovery and had no effect on bleeding patterns. None of the antibodies were present at the end of trial (see supplemental Material, Section 10 for details).
A total of 215 adverse events (7 severe, 25 moderate, and 183 mild) in 60 (81%) patients were reported, corresponding to 3.33 adverse events per patient year of exposure. The most commonly reported adverse events were nasopharyngitis (13 events in 10 patients [13.5%]), influenza (10 events in 8 patients [10.8%]), and upper respiratory tract infection (10 events in 8 patients [10.8%]), which are not unusual observations in clinical trials. There were 4 serious adverse events (hip fracture, worsening of skin ulcer, retroperitoneal hematoma, and abdominal pain) in 4 patients (5.4%). These serious adverse events were reported by the investigator as unlikely to be related to nonacog beta pegol. No safety concerns were identified from physical examinations or clinical laboratory tests.
Efficacy
Treatment of bleeding episodes.
A total of 345 bleeding episodes in 55 (74%) patients were treated with nonacog beta pegol, of which 202 bleeding episodes were in prophylaxis patients and 143 bleeding episodes were in on-demand patients. Approximately two-thirds were reported as spontaneous and one-third as traumatic (Table 1). The majority of bleeding episodes (78.5%) were in joints (see supplemental Material, Section 15). The overall success rate for treatment of all bleeding episodes was estimated to be 92.2% (95% confidence interval [CI], 86.9-95.4). In the 40 IU/kg group, 99% of the bleeding episodes were resolved with 1 injection of nonacog beta pegol compared with 84% in both the 10 IU/kg and on-demand groups (Table 1). One severe bleeding episode was reported in the trial: a bleeding episode in the knee joint in a patient in the 10 IU/kg prophylaxis group. The bleeding episode was treated with 80 IU/kg nonacog beta pegol, and the hemostatic response was rated as excellent.
Prophylaxis.
The number of patients who did not have a treatment-requiring bleeding episode during the trial was 5 (17%) of 30 patients in the 10 IU/kg group, 13 (45%) of 29 patients in the 40 IU/kg group, and 1 (7%) of 15 patients in the on-demand group. The median ABR and the estimated mean ABR in the 10 IU/kg group were 2.93 (interquartile range [IQR], 0.99-6.02) and 4.56 (95% CI, 3.01-6.90), respectively. The median ABR and the estimated mean ABR in the 40 IU/kg group were 1.04 (IQR, 0.00-4.00) and 2.51 (95% CI, 1.42-4.43), respectively (Table 3).
ABRs
| Characteristics . | 10 IU/kg . | 40 IU/kg . | On demand . |
|---|---|---|---|
| All patients | |||
| N | 30 | 29 | 15 |
| Median (IQR) | 2.93 (0.99-6.02) | 1.04 (0.00-4.00) | 15.58 (9.56-26.47) |
| Estimated rate (95% CI)* | 4.56 (3.01-6.90) | 2.51 (1.42-4.43) | — |
| P value† | .40 | .01 | |
| Previous prophylaxis patients | |||
| N | 20 | 17 | 2 |
| Bleeding rate during the last 12 months before trial‡ | |||
| Median | 4.75 | 4.00 | 9.50 |
| Estimated rate | 5.13 | 7.49 | 9.50 |
| Bleeding rates during trial | |||
| Median | 2.99 | 1.93 | 25.69 |
| Estimated rate | 4.68 | 3.33 | 29.4 |
| Previous on-demand patients | |||
| N | 10 | 12 | 13 |
| Bleeding rate during the last 12 months before trial‡ | |||
| Median | 14.0 | 12.5 | 15.0 |
| Estimated rate | 17.9 | 21.2 | 22.7 |
| Bleeding rates during trial | |||
| Median | 2.06 | 0.52 | 13.0 |
| Estimated rate | 4.30 | 1.32 | 17.6 |
| All patients by type of bleed | |||
| Spontaneous bleeding episodes | |||
| Median (IQR) | 0.97 (0.00-4.01) | 0.00 (0.00-0.98) | 11.1 (7.16-15.8) |
| Estimated rate (95% CI)* | 3.14 (1.78-5.56) | 1.22 (0.48-3.10) | |
| Traumatic bleeding episodes | |||
| Median (IQR) | 0.98 (0.00-1.93) | 0.00 (0.00-2.04) | 1.73 (0.00-8.95) |
| Estimated rate (95% CI)* | 1.35 (0.81-2.24) | 1.29 (0.76-2.19) |
| Characteristics . | 10 IU/kg . | 40 IU/kg . | On demand . |
|---|---|---|---|
| All patients | |||
| N | 30 | 29 | 15 |
| Median (IQR) | 2.93 (0.99-6.02) | 1.04 (0.00-4.00) | 15.58 (9.56-26.47) |
| Estimated rate (95% CI)* | 4.56 (3.01-6.90) | 2.51 (1.42-4.43) | — |
| P value† | .40 | .01 | |
| Previous prophylaxis patients | |||
| N | 20 | 17 | 2 |
| Bleeding rate during the last 12 months before trial‡ | |||
| Median | 4.75 | 4.00 | 9.50 |
| Estimated rate | 5.13 | 7.49 | 9.50 |
| Bleeding rates during trial | |||
| Median | 2.99 | 1.93 | 25.69 |
| Estimated rate | 4.68 | 3.33 | 29.4 |
| Previous on-demand patients | |||
| N | 10 | 12 | 13 |
| Bleeding rate during the last 12 months before trial‡ | |||
| Median | 14.0 | 12.5 | 15.0 |
| Estimated rate | 17.9 | 21.2 | 22.7 |
| Bleeding rates during trial | |||
| Median | 2.06 | 0.52 | 13.0 |
| Estimated rate | 4.30 | 1.32 | 17.6 |
| All patients by type of bleed | |||
| Spontaneous bleeding episodes | |||
| Median (IQR) | 0.97 (0.00-4.01) | 0.00 (0.00-0.98) | 11.1 (7.16-15.8) |
| Estimated rate (95% CI)* | 3.14 (1.78-5.56) | 1.22 (0.48-3.10) | |
| Traumatic bleeding episodes | |||
| Median (IQR) | 0.98 (0.00-1.93) | 0.00 (0.00-2.04) | 1.73 (0.00-8.95) |
| Estimated rate (95% CI)* | 1.35 (0.81-2.24) | 1.29 (0.76-2.19) |
Estimated rates for prophylaxis patients are based on a Poisson regression model with dose as a factor, allowing for overdispersion and using treatment duration as an offset.
P values are from the 1-sided test of the null hypothesis that the estimated rate is at least 4.8, evaluated at the 2.5% level.
Bleeding rate during the last 12 months before trial on that particular treatment regimen.
Furthermore, 10 (66.7%) of 15 patients with target joints at trial entry had no treatment-requiring bleeding episodes in their target joints during prophylaxis with 40 IU/kg treatment compared with 1 (7.7%) of 13 patients in the 10 IU/kg group. In the on-demand group, 2 (16.7%) of 12 patients did not bleed into their target joints during the trial. Both of these on-demand patients entered the trial from a previous on-demand treatment regimen, whereas 1 of the patients withdrew for personal reasons after 2.5 months in the trial.
ABRs estimated from spontaneous bleeding episodes occurring up until and including 4 days from previous prophylactic treatment, or later than 4 days from previous prophylactic treatment, were 1.87 (95% CI, 1.08-3.23) and 2.16 (95% CI, 1.21-3.88), respectively. Thus, bleeding rates were only slightly higher toward the end of the dosing intervals, reflecting that patients had high FIX activity levels during the entire time between prophylactic dose administrations. Reduction in ABR for those patients who entered the trial from a prophylaxis treatment regimen was most pronounced in the 40 IU/kg group (from 7.49 to 3.33) compared with in the 10 IU/kg group (from 5.13 to 4.68) (Table 3). The frequencies of bleeding episodes in patients are presented in the supplemental Material, Section 16.
FIX trough activity
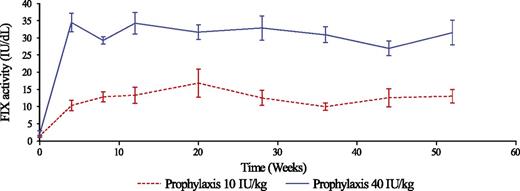
Throughout the trial, the mean predose FIX activities were statistically significantly above 1 IU/dL for both the 10 IU/kg and the 40 IU/kg prophylaxis groups (Figure 2). The estimated mean FIX trough activities were 8.5 IU/dL (95% CI, 7.7-9.3; P < .001) and 27.3 IU/dL (95% CI, 24.8-30.0; P < .001), respectively.
Mean predose FIX activity during trial. The graph shows the mean (± standard error of the mean) FIX activity in predose samples for the 10 IU/kg and 40 IU/kg prophylaxis groups. A 1-stage clotting assay was used to determine the FIX activity (IU/dL) in predose blood samples collected at clinical visits during the trial. Analysis was performed at a central laboratory, using a product-specific reference standard for calculation of the FIX activity.
Mean predose FIX activity during trial. The graph shows the mean (± standard error of the mean) FIX activity in predose samples for the 10 IU/kg and 40 IU/kg prophylaxis groups. A 1-stage clotting assay was used to determine the FIX activity (IU/dL) in predose blood samples collected at clinical visits during the trial. Analysis was performed at a central laboratory, using a product-specific reference standard for calculation of the FIX activity.
Pharmacokinetics
The single-dose and steady state geometric mean half-lives of nonacog beta pegol were 93 hours (coefficient of variation [CV%] 19.5) and 107 hours (CV% 21.8), respectively, in the 10 IU/kg group, and 85 hours (CV% 21.8) and 111 hours (CV% 11.8), respectively, in the 40 IU/kg group. Incremental recovery (between 0.02 and 0.03 [IU/mL]/[IU/kg]) and clearance (between 0.36 and 0.43 mL/h/kg) were similar between the 2 dose groups (see supplemental Material, Section 17).
Patient-reported outcomes
HR-QoL was measured on the EQ-5D VAS scale. In the 10 IU/kg group, the median score was 80 (range, 30-100) at trial entry and 85 (range, 0-100) at the end of the trial. In the 40 IU/kg group, the median score increased from 75 (range, 35-100) at trial entry to 90 (range, 60-100) at trial end (see supplemental Material, Section 14 for details).
Discussion
This prospective trial demonstrated that nonacog beta pegol was safe and effective for the treatment and prevention of bleeding episodes in patients with FIX deficiency. No clinically significant safety issues were identified in the trial. Specifically, no FIX inhibitors developed and no thrombotic or hypersensitivity events related to treatment with nonacog beta pegol were reported.
Once-weekly injections with nonacog beta pegol resulted in substantially higher FIX trough activities compared with currently available standard recombinant FIX products, reflecting that patients had high FIX activity levels during the entire time between the nonacog beta pegol prophylactic dose administrations.6,18-21 A recently approved long-acting FIX product, rFIXFc, demonstrated a terminal half-life of 82.1 hours when patients were dosed 50 IU/kg once weekly, whereas the steady state half-life of nonacog beta pegol was between 107 and 111 hours.21 However, key differences between the trials for rFIXFc and nonacog beta pegol, namely prophylaxis regimes, dose adjustments, and pharmacokinetic analysis, make direct comparisons between these products difficult.21 With the prolonged half-life and high incremental recovery of nonacog beta pegol, patients with severe or moderate hemophilia B should be able to exhibit a bleeding phenotype of mild hemophilia B with FIX activity well above 5 IU/dL.1
In the 40 IU/kg prophylaxis group, an improvement in patient-reported HR-QoL VAS score from a median of 75 at trial entry to 90 after 52 weeks of treatment was observed. This change during the trial, moving the patients in the 40 IU/kg group to a score similar to that of a general population,22 was likely related to fewer bleeding episodes, fewer injections, resolution of target joint bleeding, or a combination of these and other factors.
The success rate for treatment of all bleeding episodes was estimated to be 92.2% (95% CI, 86.9%-95.4%), demonstrating a hemostatic effect of nonacog beta pegol in line with currently available FIX products.6,18,20
The observed bleeding rates in both the 10 IU/kg and 40 IU/kg prophylaxis groups were similar to, or better than, reported bleeding rates for commercially available products.18,20,21,23 The predefined criteria for effective prophylaxis (estimated mean ABR significantly below 4.8) were met with the 40 IU/kg dose, but not the 10 IU/kg dose, despite a clinical effect with both doses. In the 40 IU/kg group, 45% of the patients did not bleed at all; this proportion was 17% in the 10 IU/kg group.
Differences between the 2 prophylaxis groups are, however, noteworthy and could be attributed to the substantially higher FIX trough levels observed in the high-dose prophylaxis group. First, patients in the 40 IU/kg group had lower bleeding rates compared with those in the 10 IU/kg group and demonstrated the largest decrease compared with the patients’ own bleeding rate during the last 12 months before the trial. Second, when patients receiving prophylaxis bled, those in the higher-dose group were more likely to respond to 1 treatment dose compared with patients in the lower-dose group (99% vs 84%). Third, and perhaps most noteworthy from a clinical perspective, patients with target joints at trial entry had a substantially higher likelihood of resolving their target joint if they were in the higher-dose group, with a 67% resolution rate compared with 7.7% in the lower-dose group.
With the trough levels of FIX activity observed in the prophylaxis groups, spontaneous bleeding episodes may not have been expected, yet approximately 70% and 50% of the bleeding episodes in the 10 IU/kg and 40 IU/kg prophylaxis groups, respectively, were reported by the patients as spontaneous. One explanation could be that the majority of the patients had hemophilic arthropathy in 1 or more joints, such that even in the presence of the attained FIX activity, spontaneous bleeding episodes could occur. Another explanation could be that symptoms of hemophilic arthropathy, such as pain, swelling, and stiffness, may have been perceived as bleeding episodes, and therefore treated as such, even though no bleeding episode had actually occurred.24 The results presented here demonstrate wide interpatient variability, and this unexpected finding requires further investigation.
Limitations of this trial were the exclusion of patients older than 70 years and those with a history of thromboembolic events, such as myocardial infarction or deep vein thrombosis. The patients’ free choice between entering into prophylaxis or receiving on-demand treatment made direct comparisons between the prophylaxis groups and the on-demand group unfeasible. Trial product-related safety assessments in on-demand patients were limited by the few exposure days to nonacog beta pegol during the trial. However, 12 of the 13 on-demand patients who completed the trial chose to progress into the extension trial (ClinicalTrials.gov, #NCT01395810) and have therefore received subsequent doses of nonacog beta pegol beyond the limited exposure in this trial.
In conclusion, no clinically significant safety concerns were identified and no inhibitory antibodies were observed. Nonacog beta pegol was effective for the treatment of bleeding episodes in both prophylaxis and on-demand patients. Low bleeding rates were observed in patients receiving prophylaxis and are likely related to high trough levels resulting from the extended half-life of nonacog beta pegol. Clinical improvement of target joints and HR-QoL was observed in the 40 IU/kg group. These data suggest that once-weekly prophylaxis with nonacog beta pegol may provide a new and safe alternative for the prevention and treatment of bleeding episodes in patients with hemophilia B. Further evaluations in a subsequent extension trial (ClinicalTrials.gov, #NCT01395810) of these patients will hopefully confirm these findings, along with the investigation of nonacog beta pegol in previously treated and previously untreated pediatric patients with hemophilia B (ClinicalTrials.gov, #NCT01467427 and #NCT02141074, respectively). In addition, nonacog beta pegol was investigated in a recently completed clinical trial of patients with hemophilia B undergoing surgery (ClinicalTrials.gov, #NCT01386528).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the investigators, trial staff, and the patients and their families for participating in the trial. Ramin Tehranchi and Torben Colberg (Novo Nordisk A/S) are acknowledged for data acquisition and analysis, conduct of the trial, final reporting, and critical review of the manuscript. Paula Persson (Novo Nordisk A/S) performed the assessment of the pharmacokinetic data. Kamilla Begtrup (Novo Nordisk A/S) provided the statistical analyses. Rasmus Høigaard Nielsen, Merete Pedersen, and Erik Andersen (Novo Nordisk A/S) provided editorial support for manuscript preparation.
This trial was sponsored by Novo Nordisk A/S (Bagsværd, Denmark).
Authorship
Contribution: P.W.C., G.Y., F.A.K., P.A., T.G., J.M., T.M., E.P.M.-B., J.O., C.E.W., and C.N. were principal investigators and enrolled and cared for patients during the trial. K.K. and C.B. were involved in the design of the trial. The authors designed the trial protocol, directed the data analysis, and wrote the manuscript. The sponsor was responsible for trial operations, including data analysis. All authors had access to the primary clinical trial data. All authors were involved in interpretation of the trial results and preparation of the manuscript outline, provided input during the review stages, and approved the final manuscript. The principal investigator (P.W.C.) assumes full responsibility for the accuracy and completeness of the reported data.
Conflict-of-interest disclosure: P.W.C. has received research support from CSL Behring and has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Baxter, Novo Nordisk, CSL Behring, and Bayer. G.Y. received honoraria from Novo Nordisk for speaking engagements and advisory boards. K.K. and C.B. are employees of Novo Nordisk A/S. T.M. has acted as a paid consultant to Novo Nordisk. E.P.M.-B. has received a fee from Sanquin, Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, and Pfizer for research, presentations, and educational purposes. J.O. has received reimbursement for attending symposia/congresses, and/or honoraria for speaking, and/or honoraria for consulting, and/or funds for research from Baxter, Bayer, Biogen Idec, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Swedish Orphan Biovitrum, and Pfizer. C.N. has received honoraria for lectures, research support, and consultancy fees from Novo Nordisk, Baxter, Bayer, CSL Behring, LFB, and Pfizer. The remaining authors declare no competing financial interests.
A complete list of the principle paradigm 2 Investigators appears in the online data supplement.
Correspondence: Peter William Collins, Arthur Bloom Haemophilia Centre, Institute of Infection and Immunity, School of Medicine, Cardiff University, Heath Park, Cardiff CF14 4XN, United Kingdom; e-mail: peter.collins@wales.nhs.uk.