In this issue of Blood, Collins et al provide the results of a prospective, randomized, single-blind, phase 3 trial on the use of nonacog beta pegol, a new long-acting glycoPEGylated factor IX (FIX) molecule for the treatment and prevention of bleeding episodes in 74 patients with hemophilia B.1
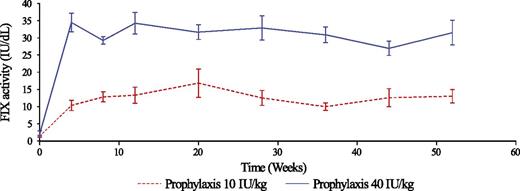
FIX trough levels attained and maintained with nonacog beta pegol used on prophylaxis at 10 IU/kg/week (broken line) and 40 IU/kg/week (continuous line). See Figure 2 in the article by Collins et al that begins on page 3880.
FIX trough levels attained and maintained with nonacog beta pegol used on prophylaxis at 10 IU/kg/week (broken line) and 40 IU/kg/week (continuous line). See Figure 2 in the article by Collins et al that begins on page 3880.
It was 50 years ago that Dr Judith Pool published a paper about cryoprecipitate, the first form of replacement therapy for patients with hemophilia.2 There have been many steps forward in hemophilia therapy since that seminal discovery. Clotting factor VIII (FVIII) and FIX concentrates were first derived from human plasma, and in the 1990s, they were manufactured using the recombinant technology.3,4
During the last 2 decades, the availability of safe and effective replacement therapy has changed the natural history of the disease, thanks to rapid bleeding control and the widespread use of prophylaxis, which is the standard of care aimed at avoiding crippling joint damage.
In addition to its undeniable benefits, replacement therapy still has drawbacks mainly related to the intravenous route of administration and the relatively short half-life of clotting factors. Recently, bioengineered molecules have been developed to overcome some of these limits. In particular, long-acting FIX molecules, although still delivered intravenously, will have a profound effect on prophylaxis feasibility and adherence to treatment in patients with hemophilia B.
Until now, 3 long-acting FIX molecules have been studied for weekly prophylaxis, but results from phase 3 trials are available only for 2 of them: nonacog beta pegol and rFIXFc.1,5
In the phase 3 trial reported by Collins et al, weekly prophylaxis with 10 and 40 IU/kg and on-demand treatment with nonacog beta pegol were assessed in patients with severe hemophilia B.1
Many aspects of this new long-acting FIX concentrate deserve consideration:
The terminal half-life of 96 to 110 hours, which is fivefold longer than that of unmodified FIX, allowed the performance of successful prophylaxis with no more than 1 injection per week. This implies at least a 50% reduction in the number of total injections per year, with significant advantages in terms of quality of life, adherence to prescribed treatment, and less need of central venous lines insertions in the pediatric population.
The in vivo recovery of nonacog beta pegol was twofold higher compared with standard recombinant FIX, resulting in higher plasmatic FIX levels while using lower doses during weekly prophylaxis.1
As shown in the figure, patients treated with 40 IU/kg/week maintained FIX trough activity well above 25 IU/dL, ensuring good protection from breakthrough bleeds and thus allowing a normal active life. These results support increasing the interval between injections and may allow prophylaxis with 1 injection every 2 to 3 weeks on the basis of individual pharmacokinetics.
The high efficacy rate by means of a single injection and the successful protection from bleeding into target joints is reassuring. In fact, up to 99% of bleeds were resolved with a single injection, and up to 70% of patients with established target joints at study entry did not bleed in their target joints during the trial.
A good safety profile was confirmed because no patient developed neutralizing anti-FIX inhibitors.
Finally, at variance with rFIXFc,5 annualized bleeding rates were twofold lower and the success rate of bleeding control with a single injection was slightly higher with nonacog beta pegol, suggesting that this molecule may convey superior efficacy than rFIXFc, although a head-to-head comparison would be needed to confirm this.
In this light, long-acting FIX molecules represent a terrific advance in hemophilia care, and the results presented in the paper by Collins et al1 confirm this. However, in addition to all the tangible advantages, several aspects still need to be further investigated:
The long-term safety related to the chronic exposure to the polyethylene glycol moiety;
The meaning and relevance, if any, of the development of noninhibitory antibodies already reported in the phase 3 trial,1 as well as the immunogenicity of the molecule in previously untreated patients;
The adequacy and reliability of current clotting factor laboratory assays to predict and monitor treatment efficacy; and
The cost-effectiveness of the new drug compared with standard products, taking into account the possibility of treatment optimization and individualization.
All in all, with this new molecule, which will hopefully soon be available on the market, the progress of hemophilia replacement therapy has taken an additional step forward to ameliorate treatment feasibility and patients’ quality of life.
Conflict-of-interest disclosure: M.E.M. has acted as a consultant and/or speaker for Bayer Healthcare, Novo Nordisk, CSL Behring, Baxter Healthcare, Pfizer, and SOBI.