Key Points
The spleen harbors ADAMTS13-specific memory B cells following acute acquired TTP.
The splenic anti-ADAMTS13 antibody repertoire is characterized by a set of unique and novel CDR3 motifs, 4 shared by 2 patients.
Abstract
Acquired thrombotic thrombocytopenic purpura (TTP) is the consequence of a severe ADAMTS13 deficiency resulting from autoantibodies inhibiting ADAMTS13 or accelerating its clearance. Despite the success of plasma exchange the risk of relapse is high. From 2 patients (A and B), splenectomized for recurrent episodes of acquired TTP, the splenic B-cell response against ADAMTS13 was characterized through generation of human monoclonal anti-ADAMTS13 autoantibodies (mAbs) by cloning an immunoglobulin G (IgG)4κ- and IgG4λ-Fab library using phage display technology and by Epstein-Barr virus transformation of switched memory B cells (CD19+/CD27+/IgG+). Sequence analysis of the anti-ADAMTS13 IgGs of both patients revealed that the VH gene use was limited in our patients to VH1-3 (55%), VH1-69 (17%), VH3-30 (7%), and VH4-28 (21%) and contained 8 unique and thus far not reported heavy-chain complementarity determining region 3 motifs, of which 4 were shared by the 2 patients. The discovery of several highly similar anti-ADAMTS13 autoantibodies in 2 unrelated TTP patients suggests that the autoimmune response is antigen driven, because the probability that such similar immunoglobulin rearrangements happen by chance is very low (<10−9).
Introduction
The hallmark of thrombotic thrombocytopenic purpura (TTP) is a severe ADAMTS13 (a disintegrin and metalloprotease with thrombo-spondin type 1 motifs, member 13) deficiency,1,2 which leads to the persistence of sticky, unusually large von Willebrand factor (VWF) multimers causing platelet clumping and microvascular occlusion, and organ ischemia and dysfunction, particularly in the brain and kidneys. In acquired TTP, severe ADAMTS13 deficiency (<5% of the normal) is caused by circulating anti-ADAMTS13 autoantibodies, inhibiting ADAMTS13 activity or accelerating its clearance. Acute TTP remains a life-threatening disease, leaving survivors of an acute bout at a high risk of relapse.3 Treatment of frequently relapsing or refractory acquired TTP has remained challenging and consists of supplementing plasma exchange (PEX) with adjuvant immunosuppressive agents including in more and more cases the chimeric anti-CD20 monoclonal antibody rituximab,4-10 known to efficiently deplete B cells in the circulation. Plasma cells, however, are not eliminated due to their low or lacking CD20 expression,11,12 and long-lived memory B cells (CD20+) have been postulated to hide in lymph organs, because antibody titers specific for tetanus, pneumococcal polysaccharides, and measles have remained generally unchanged after rituximab treatment.13,14
Evidence for the spleen as a reservoir for long-lived memory B cells is supported by the finding that the splenic marginal zone harbors a specific set of memory B cells with rearranged, somatically hypermutated immunoglobulin genes typically found in antigen-driven immune responses.15 Moreover, anti-smallpox–specific long-lived memory B cells were found to be enriched in the spleen withstanding rituximab treatment.16 In acquired TTP, clinical evidence hints at a role of the spleen in the pathogenic anti-ADAMTS13 immune response as splenectomy efficiently reduces the relapse rate and is associated with long-term remission in many patients.17,18
In B cells, VH (variable), DH (diversity), and JH (joining) of the heavy chain (H-chain) and VL and JL gene segments of the light chain (L-chain) rearrange to produce an individual, extremely variable immunoglobulin repertoire,19 which further undergoes affinity maturation by somatic hypermutation,20 most prevalent in the complementarity determining regions (CDR)1 to 3, of which CDR3 is the major antigen binding site.21-23 The analysis of the functional impact of the immunoglobulin VH gene polymorphisms for pathogenicity has been facilitated by techniques allowing the generation of monoclonal antibodies, such as phage display,24 hybridoma technology,25 and Epstein-Barr virus (EBV) transformation of B cells,26 enabling establishment of the link between the antibody genotype and phenotype.
Limited VH gene use with an overrepresentation of the VH3, VH4-21, and VH5 gene family was shown in systemic lupus erythematosus27-29 and rheumatoid arthritis,27,30 hinting at an antigen-driven clonal expansion of pathogenically relevant autoimmune B cells in specialized compartments (spleen and lymph nodes) even after rituximab treatment.28
In acquired TTP, circulating anti-ADAMTS13 antibodies are found in plasma of nearly all patients (94-97%) and are mainly of the immunoglobulin G (IgG) type, the dominating IgG subclass being IgG4, followed by IgG1, IgG2, and IgG3.31 Interestingly, the presence of IgG4 was associated with an increased risk of relapse. The peripheral blood IgG1 B-cell autoantibody repertoire of 5 survivors of an acute TTP bout was characterized using single chain phage display technology.32-34 The generated anti-ADAMTS13 monoclonal antibodies (mAbs) showed a moderate inhibitory potential (reduction of ADAMTS13 activity by 15-40%),32-34 with a prevailing use of VH1-69,33,34 mostly in combination with L-chain Vλ-3 family.34 These mAbs recognized a single ADAMTS13 epitope in the spacer domain32 reported to be the main epitope as plasma-derived anti-ADAMTS13 autoantibodies of virtually all patients with acute acquired TTP bound to this domain.35-37 Whether the random in vitro pairing of H-chain and L-chain inherent to phage display technology leads to the observed moderate inhibitory potential of these anti-ADAMTS13 mAbs remains to be determined.
We evaluated the splenic anti-ADAMTS13 B-cell response in 2 relapsing acquired TTP patients during (pat A) or 6 months after an acute episode and treatment with rituximab (pat B), hypothesizing that an important reservoir of pathogenically relevant B cells lies within their spleens; as of now, both patients have remained in remission following splenectomy for 11 and 8 years, respectively.
Materials and methods
Patients
Patient A was diagnosed with acquired TTP at the age of 40 years with typical TTP symptoms and an ADAMTS13 activity <5% in the presence of a strong inhibitor. After 8 weeks of daily PEX and replacement with fresh frozen plasma, she went into remission. One year later, she had her first of 3 relapses. In 2003, during her third relapse, she was splenectomized. In all relapses, a severe ADAMTS13 deficiency with a strong inhibitor was present.
Patient B had her first TTP episode with severe ADAMTS13 deficiency with inhibitor at the age of 9 years. After daily PEX and immunosuppression, she went into remission but relapsed 3 years later while tapering immunosuppression. A second remission was achieved by PEX and increased doses of immunosuppressants lasting 15 months. She relapsed when steroids were withdrawn. PEX therapy with rituximab treatment (4 weekly doses of 375 mg/m2) was started, but only 17 months later, she had a third relapse, which was treated identically. After her third relapse, she was splenectomized in 2006, 6 months after receiving her last dose of rituximab.
As of now, both patients remain in remission following splenectomy for 11 (pat A) and 8 years (pat B), with normal ADAMTS13 activity in the absence of inhibitors. Both patients gave written informed consent to donate their spleen to our laboratory for research purposes.
ADAMTS13-related assays
ADAMTS13 activity (normal range 51% to >100%) was measured by the slightly modified fluorescence resonance energy transfer system (FRETS)-VWF73 assay as previously reported.38,39
ADAMTS13 functional inhibitor (normal range < 0.4 Bethesda units/mL) was assessed by preincubation of heat-inactivated patient plasma with normal human plasma 1:1 (volume:volume) for 2 hours at 37°C before analyzing residual ADAMTS13 activity in the mixture by the modified FRETS-VWF73 assay as previously described.3,39
Splenic mononuclear cell isolation
After splenectomy, the spleen was immediately placed in 0.9% sodium chloride, shipped overnight to our laboratory, and processed on arrival. Splenic mononuclear cells (SMCs) were isolated by size gradient separation, and SMCs were resuspended in freezing media, stepwise frozen, and stored in liquid nitrogen until further use (supplemental Materials, available on the Blood Web site).
Library construction, phage selection
From a total of 2 × 108 SMCs per patient, mRNA was extracted to construct 1 IgG4-Fab-κ and 1 IgG4-Fab-λ phage library using the pComb3H surface display system as reported previously.24,40-42 Briefly, enrichment of anti-ADAMTS13–bearing surface Fabs of both IgG4-κ/λ-phage libraries and an IgM Fab-κ/λ-phage library generated from cord blood, representing the naïve immune repertoire,42 was achieved by 5 selection rounds on full-length recombinant ADAMTS13 (rADAMTS13), expressed in human HEK293 cells (>99% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; gift from Fritz Scheiflinger, Baxter), and coated at a concentration of 1 µg/mL onto a microtiter plate. Phagemid DNA was prepared from the last panning round by removing the gene III fragment by restriction digestion with NheI and SpeI, followed by religation, and were subsequently used to transform XL1-blue cells to produce single clones secreting soluble anti-ADAMTS13 Fab fragments. As a preventive measure to exclude cross-contamination, the patient libraries were constructed sequentially.
Production and purification of anti-ADAMTS13 Fabs
A total of 34 single Escherichia coli colonies were picked and individually inoculated in superbroth media containing ampicillin (50 µg/mL) and tetracycline (10 µg/mL) overnight at 37°C prior to protein induction by addition of isopropyl-β-d-thiogalactopyranoside (1 mM) and cyclic AMP (0.4 mM). Anti-ADAMTS13 Fabs were purified from bacterial pellets by chromatography on goat-anti-human IgG F(ab)2 covalently coupled to protein A-Sepharose (Amersham Biosciences; supplemental Materials).
EBV transformation and single clone selection
Frozen SMCs were thawed and switched CD19+/CD27+/IgG+ memory B cells enriched from 2 × 108 SMCs by depletion of all non-B cells, as well as IgM-and IgD-bearing mature B cells using magnetic microbeads (MACS Technology, Miltenyi Biotec) according to the manufacturer’ s instructions and as previously described.26 After EBV transformation, IgG+ cultures were cloned by limiting dilution. After 4 to 6 weeks, culture supernatants of single clones were screened for IgG+/anti-ADAMTS13+ mAbs and only double-positive clones were expanded and purified by affinity chromatography on Protein G CL-4B Sepharose (Amersham Biosciences; supplemental Materials).
Specificity enzyme-linked immunosorbent assay of anti-ADAMTS13 Fabs/IgGs
Human rADAMTS13, human serum albumin, human factors VII (factor VII NF; Baxter), VIII (Advate; Baxter), and IX (BeneFIX 1000; Pfizer), and tetanus toxoid (Sigma-Aldrich) were coated on a microtiter plate in an equimolar concentration of 10 nM at 4°C overnight. After blocking, 100 nM of each anti-ADAMTS13 mAb was incubated for 3 hours at 37°C. Bound monoclonal Fabs and IgGs, as well as a control anti-ADAMTS13 mAb (I-9; gift of Jan Voorberg, Amsterdam, The Netherlands), were detected with horseradish peroxidase (HRP)-labeled goat anti-human IgG F(ab)2 Ab (Jackson Immunofluorescence), which was diluted to a final concentration of 1 μg/mL phosphate-buffered saline containing 1% bovine serum albumin and read at 450 nm after visualization with 3,3′,5,5′-tetramethylbenzidine substrate.
DNA purification, polymerase chain reaction amplification, and nucleic acid sequencing
Phagemid DNA of the Fab fragments and genomic DNA of EBV-transformed memory B cells were extracted using a DNA extraction kit (Qiagen). IgG subclass of the memory B cells was assessed by polymerase chain reaction using subclass specific primers (supplemental Materials). Purified polymerase chain reaction products were sequenced (Microsynth), and obtained sequences were aligned to the most homologous germ-line genes using the National Center for Biotechnology Information IgBlast GeneBank program (htpp://www.ncbi.nlm.nih.gov/igblast/) and International ImMunoGeneTics information system (IMGT) database (http://www.imgt.org/IMGT_vquest/share/textes/).21,22
Affinity measurement by surface plasmon resonance
Binding toward rADAMTS13, immobilized at 4000 response units on a CM5 sensor chip (Biacore) using 50 mM maleate buffer (pH 5.0), was assessed for all 29 anti-ADAMTS13 mAbs (16 phage-derived Fabs and 13 EBV-transformed whole IgGs) at 4 different concentrations (7.5-160 nM) on a Biacore-X-instrument (Biacore). Association and dissociation rates were measured for 3 minutes at a flow rate of 10 μL/min. A control buffer (HBS-EP; Biacore) was measured for each sample and subtracted from the sample sensorgrams. Kinetics were determined using BIAevaluation software (Biacore).
Epitope mapping
A total of 910 chemically synthesized overlapping peptides covering either the entire ADAMTS13 protein or the spacer domain only (residues 556-685) were synthesized using chemically linked peptides on scaffolds (CLIPS) technology (Pepscan Presto; Lelystad43 ). Three different peptide libraries were generated for the peptide microarray: (1) 141 linear 20-mer peptides with an overlap of 10 amino acids covering the entire ADAMTS13; (2) 192 conformational CLIPS peptides (variable length of 9, 12, 15, and 18 residues); and (3) 577 different combinatorial sets of 24 pairwise double-looped structure constrained 11-mer peptides covering ADAMTS13 spacer domain to assess the potential discontinuous epitopes. Binding of all 29 mAbs was analyzed by PepScan enzyme-linked immunosorbent assay (ELISA).44
Results
Cloning of ADAMTS13-specific mAbs
Mononuclear cells isolated from the spleen of 2 patients with relapsing acquired TTP with severe ADAMTS13 deficiency due to strong functional ADAMTS13 inhibitors were used to construct individual κ- and λ-IgG4 Fab phage libraries. The library sizes were similar in both patients: 6.0 × 1010 (A) and 2.4 × 1010 (B) for IgGκ and 7.2 × 1010 (A) and 8.4 × 1010 (B) for IgGλ. After 5 rounds of selection, a 105-fold amplification of the eluted phages for the IgGκ library (A and B) and a 107-fold (A) but only a 1000-fold amplification (B) for IgGλ was reached.
No enrichment after the third panning round in eluted anti-ADAMTS13 phages was observed for the naïve control IgM library (size, 2 × 108) generated from 7 cord blood donors as described previously.42
Unique IgVHDHJH and IgVLJL gene rearrangements characterize the anti-ADAMTS13–specific splenic autoimmune response
Screening by ELISA revealed that 16/34 phage display-derived Fabs and 13 out of 145 EBV-transformed memory B-cell IgG+ clones showed specific binding towards rADAMTS13 with an absorption signal 3-times higher than observed for the control antibody b12, a human anti-HIV-1 monoclonal Fab (gift of Dennis Burton, The Scripps Research Institute, La Jolla, CA; supplemental Figure 1).
All anti-ADAMTS13 mAbs (n = 29) were sequenced and aligned to their most homologous germ-line genes. mAbs generated by phage display were of the IgG4 subclass and used κ-VL (pat A, 6/11 mAbs; pat B, 4/5 mAbs) or λ-VL, whereas whole IgG obtained by EBV transformation of switched memory B cells were of subclasses IgG1 (pat A, 2/5 mAbs; pat B, 6/8 mAbs) or IgG4 (pat A, 3/5 mAbs; pat B, 2/8 mAbs) and exclusively used λ-VL (Table 1; Figure 1A-B).
DNA sequence analysis and Igblast alignments of all 29 anti-ADAMTS13–specific mAbs grouped according to their consensus CDR3 motifs
| Patient . | Clones . | Heavy chain . | Light chain . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclass . | V-gene . | D-gene . | J-gene . | CDR3 . | R:S ratio . | Homology . | κ/λ . | V-gene . | J-gene . | CDR3 . | R:S ratio . | Homology . | ||||
| FR . | CDR . | FR . | CDR . | |||||||||||||
| A | 1a | IgG4 | IGVH1-3*01 | IGHD2-15*01 | IGHJ6*04 | 17 | 1.2 | 2.2 | 86.5 | κ | IGKV4-1*01 | IGKJ4*01 | 12 | 3.2 | 3.0 | 89.5 |
| A | 1e | IgG4 | IGHD3-16*02 | IGHJ6*04 | 17 | 2.0 | 5.0 | 84.7 | κ | IGKV1-5*02 | IGKJ5*01 | 10 | 1.0 | 1.8 | 88.4 | |
| A | 1f | IgG4 | IGHD2-15*01 | IGHJ6*04 | 18 | 1.9 | 2.4 | 81.6 | κ | IGKV3-20*02 | IGKJ4*01 | 10 | 9.0 | 8.0 | 89.5 | |
| A | 1g | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 1.4 | 1.2 | 81.6 | κ | IGKV1-5*02 | IGKJ5*01 | 10 | 2.1 | 2.6 | 87.5 | |
| A | 2c | IgG4 | IGHD4-17*01 | IGHJ1*01 | 18 | 1.8 | 2.4 | 86.1 | λ | IGLV3-21*02 | IGLJ6*01 | 12 | 1.5 | 2.6 | 90.0 | |
| A | A-3 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 18 | 1.1 | 2.4 | 84.7 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 2.1 | 2.5 | 91.1 | |
| A | A-6 | IgG1 | IGHD5-12*01 | IGHJ6*04 | 18 | 4.0 | 2.2 | 85.7 | λ | IGLV3-1*01 | IGLJ1*01 | 14 | 2.0 | 2.5 | 91.1 | |
| B | B-7 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 17 | 3.4 | 7.0 | 84.6 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.8 | 4.0 | 89.1 | |
| A | 2a | IgG4 | IGVH1-3*01 | IGHD4-17*01 | IGHJ6*04 | 17 | 0.9 | 1.2 | 85.7 | λ | IGLV5-52*01 | IGLJ5*02 | 12 | 4.3 | 3.0 | 88.6 |
| A | 2j | IgG4 | IGHD5-12*01 | IGHJ1*01 | 17 | 0.9 | 1.3 | 85.7 | λ | IGLV3-21*02 | IGLJ6*01 | 11 | 3.8 | 4.0 | 87.5 | |
| A | A-1 | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 0.9 | 2.2 | 86.4 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 2.4 | 4.0 | 90.5 | |
| A | A-2 | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 2.0 | 2.2 | 85.7 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.1 | 4.0 | 89.2 | |
| B | 4a | IgG4 | IGHD2-15*01 | IGHJ6*04 | 18 | 1.3 | 2.0 | 86.4 | λ | IGLV5-52*01 | IGLJ7*01 | 9 | 4.2 | 4.3 | 87.6 | |
| B | B-1 | IgG4 | IGHD5-12*01 | IGHJ6*03 | 18 | 2.3 | 2.5 | 84.7 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 2.1 | 4.0 | 87.1 | |
| B | B-3 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 19 | 3.2 | 4.3 | 84.5 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 3.0 | 3.0 | 89.2 | |
| B | B-4 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 19 | 4.5 | 4.3 | 84.7 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 2.7 | 3.0 | 88.4 | |
| A | 1d | IgG4 | IGVH4-28*01 | IGHD4-17*01 | IGHJ4*02 | 11 | 0.6 | 4.0 | 90.9 | κ | IGKV4-1*01 | IGKJ2*04 | 12 | 1.5 | 2.3 | 87.9 |
| A | 1h | IgG4 | IGHD4-17*01 | IGHJ6*02 | 11 | 1.2 | 5.0 | 89.5 | κ | IGKV4-1*01 | IGKJ5*01 | 10 | 1.7 | 1.5 | 84.9 | |
| A | 2h | IgG4 | IGHD4-17*01 | IGHJ6*04 | 11 | 1.1 | 5.0 | 90.9 | λ | IGLV3-21*02 | IGLJ5*01 | 10 | 2.2 | 2.5 | 84.7 | |
| A | 2i | IgG4 | IGHD4-17*01 | IGHJ4*02 | 11 | 1.0 | 5.0 | 91.1 | λ | IGLV5-52*01 | IGLJ5*02 | 11 | 2.1 | 2.5 | 89.7 | |
| B | 3c | IgG4 | IGHD5-12*01 | IGHJ1*01 | 10 | 1.2 | 7.0 | 89.9 | κ | IGKV4-1*01 | IGKJ3*01 | 8 | 2.6 | 2.0 | 90.5 | |
| B | 3h | IgG4 | IGHD4-17*01 | IGHJ1*01 | 10 | 0.8 | 0.5 | 90.1 | κ | IGKV1-5*03 | IGKJ4*02 | 8 | 3.3 | 3.0 | 92.1 | |
| A | A-5 | IgG4 | IGVH3-30*01 | IGHD5-12*01 | IGHJ6*02 | 17 | 2.8 | 3.0 | 91.7 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.0 | 3.0 | 91.6 |
| B | B-8 | IgG1 | IGHD5-12*01 | IGHJ6*02 | 17 | 1.4 | 3.0 | 86.7 | λ | IGLV3-1*01 | IGLJ1*01 | 10 | 2.2 | 3.0 | 91.5 | |
| B | B-2 | IgG4 | IGVH1-69*01 | IGHD6-6*01 | IGHJ4*02 | 12 | 3.6 | 2.0 | 93.2 | λ | IGLV3-1*01 | IGLJ6*01 | 13 | 1.2 | 1.5 | 86.3 |
| B | B-6 | IgG1 | IGHD5-12*01 | IGHJ6*02 | 12 | 0.7 | 1.5 | 93.6 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 1.7 | 3.0 | 90.3 | |
| B | B-5 | IgG1 | IGVH1-69*12 | IGHD2-21*02 | IGHJ6*01 | 13 | 2.5 | 3.0 | 95.8 | λ | IGLV3-1*01 | IGLJ1*01 | 12 | 2.4 | 4.0 | 91 |
| B | 3b | IgG4 | IGVH1-69*03 | IGHD3-16*02 | IGHJ6*02 | 17 | 4.0 | 3.0 | 97.1 | κ | IGKV1-5*03 | IGKJ4*02 | 9 | 5.0 | 3.0 | 95.1 |
| B | 3j | IgG4 | IGVH1-69*06 | IGHD2-21*02 | IGHJ4*02 | 8 | 2.0 | 3.0 | 93.2 | κ | IGKV4-1*01 | IGKJ5*01 | 10 | 1.8 | 2.0 | 90.1 |
| Patient . | Clones . | Heavy chain . | Light chain . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclass . | V-gene . | D-gene . | J-gene . | CDR3 . | R:S ratio . | Homology . | κ/λ . | V-gene . | J-gene . | CDR3 . | R:S ratio . | Homology . | ||||
| FR . | CDR . | FR . | CDR . | |||||||||||||
| A | 1a | IgG4 | IGVH1-3*01 | IGHD2-15*01 | IGHJ6*04 | 17 | 1.2 | 2.2 | 86.5 | κ | IGKV4-1*01 | IGKJ4*01 | 12 | 3.2 | 3.0 | 89.5 |
| A | 1e | IgG4 | IGHD3-16*02 | IGHJ6*04 | 17 | 2.0 | 5.0 | 84.7 | κ | IGKV1-5*02 | IGKJ5*01 | 10 | 1.0 | 1.8 | 88.4 | |
| A | 1f | IgG4 | IGHD2-15*01 | IGHJ6*04 | 18 | 1.9 | 2.4 | 81.6 | κ | IGKV3-20*02 | IGKJ4*01 | 10 | 9.0 | 8.0 | 89.5 | |
| A | 1g | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 1.4 | 1.2 | 81.6 | κ | IGKV1-5*02 | IGKJ5*01 | 10 | 2.1 | 2.6 | 87.5 | |
| A | 2c | IgG4 | IGHD4-17*01 | IGHJ1*01 | 18 | 1.8 | 2.4 | 86.1 | λ | IGLV3-21*02 | IGLJ6*01 | 12 | 1.5 | 2.6 | 90.0 | |
| A | A-3 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 18 | 1.1 | 2.4 | 84.7 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 2.1 | 2.5 | 91.1 | |
| A | A-6 | IgG1 | IGHD5-12*01 | IGHJ6*04 | 18 | 4.0 | 2.2 | 85.7 | λ | IGLV3-1*01 | IGLJ1*01 | 14 | 2.0 | 2.5 | 91.1 | |
| B | B-7 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 17 | 3.4 | 7.0 | 84.6 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.8 | 4.0 | 89.1 | |
| A | 2a | IgG4 | IGVH1-3*01 | IGHD4-17*01 | IGHJ6*04 | 17 | 0.9 | 1.2 | 85.7 | λ | IGLV5-52*01 | IGLJ5*02 | 12 | 4.3 | 3.0 | 88.6 |
| A | 2j | IgG4 | IGHD5-12*01 | IGHJ1*01 | 17 | 0.9 | 1.3 | 85.7 | λ | IGLV3-21*02 | IGLJ6*01 | 11 | 3.8 | 4.0 | 87.5 | |
| A | A-1 | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 0.9 | 2.2 | 86.4 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 2.4 | 4.0 | 90.5 | |
| A | A-2 | IgG4 | IGHD5-12*01 | IGHJ6*04 | 18 | 2.0 | 2.2 | 85.7 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.1 | 4.0 | 89.2 | |
| B | 4a | IgG4 | IGHD2-15*01 | IGHJ6*04 | 18 | 1.3 | 2.0 | 86.4 | λ | IGLV5-52*01 | IGLJ7*01 | 9 | 4.2 | 4.3 | 87.6 | |
| B | B-1 | IgG4 | IGHD5-12*01 | IGHJ6*03 | 18 | 2.3 | 2.5 | 84.7 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 2.1 | 4.0 | 87.1 | |
| B | B-3 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 19 | 3.2 | 4.3 | 84.5 | λ | IGLV1-51*02 | IGLJ6*01 | 12 | 3.0 | 3.0 | 89.2 | |
| B | B-4 | IgG1 | IGHD5-12*01 | IGHJ6*03 | 19 | 4.5 | 4.3 | 84.7 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 2.7 | 3.0 | 88.4 | |
| A | 1d | IgG4 | IGVH4-28*01 | IGHD4-17*01 | IGHJ4*02 | 11 | 0.6 | 4.0 | 90.9 | κ | IGKV4-1*01 | IGKJ2*04 | 12 | 1.5 | 2.3 | 87.9 |
| A | 1h | IgG4 | IGHD4-17*01 | IGHJ6*02 | 11 | 1.2 | 5.0 | 89.5 | κ | IGKV4-1*01 | IGKJ5*01 | 10 | 1.7 | 1.5 | 84.9 | |
| A | 2h | IgG4 | IGHD4-17*01 | IGHJ6*04 | 11 | 1.1 | 5.0 | 90.9 | λ | IGLV3-21*02 | IGLJ5*01 | 10 | 2.2 | 2.5 | 84.7 | |
| A | 2i | IgG4 | IGHD4-17*01 | IGHJ4*02 | 11 | 1.0 | 5.0 | 91.1 | λ | IGLV5-52*01 | IGLJ5*02 | 11 | 2.1 | 2.5 | 89.7 | |
| B | 3c | IgG4 | IGHD5-12*01 | IGHJ1*01 | 10 | 1.2 | 7.0 | 89.9 | κ | IGKV4-1*01 | IGKJ3*01 | 8 | 2.6 | 2.0 | 90.5 | |
| B | 3h | IgG4 | IGHD4-17*01 | IGHJ1*01 | 10 | 0.8 | 0.5 | 90.1 | κ | IGKV1-5*03 | IGKJ4*02 | 8 | 3.3 | 3.0 | 92.1 | |
| A | A-5 | IgG4 | IGVH3-30*01 | IGHD5-12*01 | IGHJ6*02 | 17 | 2.8 | 3.0 | 91.7 | λ | IGLV1-51*02 | IGLJ6*01 | 14 | 2.0 | 3.0 | 91.6 |
| B | B-8 | IgG1 | IGHD5-12*01 | IGHJ6*02 | 17 | 1.4 | 3.0 | 86.7 | λ | IGLV3-1*01 | IGLJ1*01 | 10 | 2.2 | 3.0 | 91.5 | |
| B | B-2 | IgG4 | IGVH1-69*01 | IGHD6-6*01 | IGHJ4*02 | 12 | 3.6 | 2.0 | 93.2 | λ | IGLV3-1*01 | IGLJ6*01 | 13 | 1.2 | 1.5 | 86.3 |
| B | B-6 | IgG1 | IGHD5-12*01 | IGHJ6*02 | 12 | 0.7 | 1.5 | 93.6 | λ | IGLV3-1*01 | IGLJ1*01 | 13 | 1.7 | 3.0 | 90.3 | |
| B | B-5 | IgG1 | IGVH1-69*12 | IGHD2-21*02 | IGHJ6*01 | 13 | 2.5 | 3.0 | 95.8 | λ | IGLV3-1*01 | IGLJ1*01 | 12 | 2.4 | 4.0 | 91 |
| B | 3b | IgG4 | IGVH1-69*03 | IGHD3-16*02 | IGHJ6*02 | 17 | 4.0 | 3.0 | 97.1 | κ | IGKV1-5*03 | IGKJ4*02 | 9 | 5.0 | 3.0 | 95.1 |
| B | 3j | IgG4 | IGVH1-69*06 | IGHD2-21*02 | IGHJ4*02 | 8 | 2.0 | 3.0 | 93.2 | κ | IGKV4-1*01 | IGKJ5*01 | 10 | 1.8 | 2.0 | 90.1 |
Listed are the genes used for the variable H- and L-chain region, showing the V-, D-, and J-genes (Fabs and whole IgGs), the IgG subclass, the percentage of homology to the closest germ-line genes, the length of the CDR3 regions, and the ratio of the replacement (R) to silent (S) somatic hypermutation in the framework regions (FR1-3) and the CDR1 and CDR2 region. Clone identity: phage display-derived Fabs are indicated by italic letters and numbered with either 1 (pat A) or 3 (pat B) when originating from the κ library and 2 (pat A) or 4 (pat B) when originating from the λ library; whole IgGs derived from EBV-transformed switched memory B cells are labeled with capital letters.
Alignment of the deduced amino acid sequence of all 29 mAbs to their most homologous germ-line genes. Amino acid sequence of (A) the variable H-chain segments and (B) L-chain segments of phage display-derived IgG Fabs (panning round 5) and whole IgG of EBV-transformed switched memory B cells directed against ADAMTS13 generated from the spleens of 2 patients with relapsing acquired TTP. Clone identity: phage display-derived Fabs are indicated by italic letters and numbered with either 1 or 3 when originating from the κ library and 2 or 4 when originating from the λ library. Whole IgGs derived from EBV-transformed switched memory B cells are labeled with capital letters. According to the H-chain CDR3 amino acid sequences, all 29 mAbs are grouped into 8 CDR3 motifs; clones belonging to the same CDR3 motif are colored identically. The differences within 1 CDR3 motif are highlighted in red, dashes indicate missing, and letters indicate the replaced amino acids compared with the first top clone in each motif.
Alignment of the deduced amino acid sequence of all 29 mAbs to their most homologous germ-line genes. Amino acid sequence of (A) the variable H-chain segments and (B) L-chain segments of phage display-derived IgG Fabs (panning round 5) and whole IgG of EBV-transformed switched memory B cells directed against ADAMTS13 generated from the spleens of 2 patients with relapsing acquired TTP. Clone identity: phage display-derived Fabs are indicated by italic letters and numbered with either 1 or 3 when originating from the κ library and 2 or 4 when originating from the λ library. Whole IgGs derived from EBV-transformed switched memory B cells are labeled with capital letters. According to the H-chain CDR3 amino acid sequences, all 29 mAbs are grouped into 8 CDR3 motifs; clones belonging to the same CDR3 motif are colored identically. The differences within 1 CDR3 motif are highlighted in red, dashes indicate missing, and letters indicate the replaced amino acids compared with the first top clone in each motif.
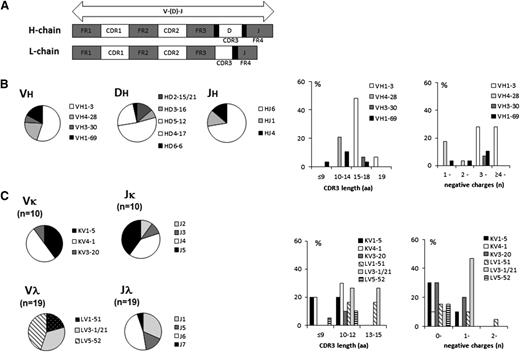
The anti-ADAMTS13 mAbs were derived from VH germ-line genes VH1-3 (16 mAbs; 55%), VH4-28 (6 mAbs; 21%), and VH3-30 (2 mAbs; 7%) in both patients (Figures 1A and 2). For patient B, where treatment had included rituximab, 5 mAbs (17%) were found to use VH1-69. The homology to the closest VH germ-line gene was 85% (81.6-86.5%) for mAbs using VH1-3, 90.4% (89.5-91.1%) for VH4-28, 89.2% for VH3-30, and 94.6% (93.2-97.1%) for VH1-69. The highest somatic hypermutation rate was thus observed in mAbs derived from germ-line gene VH1-3 and the lowest from those in VH1-69, but it was similar for both patients within the same VH genes, except for VH3-30 (homology 91.7% in pat A and 86.7% in pat B). The replacement (R) to silent (S) ratio (R:S) for the CDR1 and CDR2 region was higher than for the framework regions (FR1, FR2, and FR3) in 23/29 (79%) mAbs (Table 1).
The immunoglobulin gene features of the 29 anti-ADAMTS13 mAbs. (A) Schematic organization of the immunoglobulin V(D)J junction: framework regions FR1 to 4 and CDR1 to 3, with CDR3 being the main antigen-binding site. Sites of random nontemplated nucleotide insertions locus at the V-D and D-J junctions are indicated in black. (B) Pie charts of the composition of the H-chain gene use for the IgVHDHJH segments and the percentage for the CDR3 amino acid length and amount of negatively charged amino acids. (C) Pie charts of the composition of the L-chain gene use for the IgVLJL segments and the percentage for the L-chain CDR3 amino acid length and the amount of negatively charged amino acids.
The immunoglobulin gene features of the 29 anti-ADAMTS13 mAbs. (A) Schematic organization of the immunoglobulin V(D)J junction: framework regions FR1 to 4 and CDR1 to 3, with CDR3 being the main antigen-binding site. Sites of random nontemplated nucleotide insertions locus at the V-D and D-J junctions are indicated in black. (B) Pie charts of the composition of the H-chain gene use for the IgVHDHJH segments and the percentage for the CDR3 amino acid length and amount of negatively charged amino acids. (C) Pie charts of the composition of the L-chain gene use for the IgVLJL segments and the percentage for the L-chain CDR3 amino acid length and the amount of negatively charged amino acids.
The anti-ADAMTS13 mAbs were composed of 5 different DH genes (DH5, 14/29 mAbs; DH4, 7/29 mAbs; DH2, 5/29 mAbs; DH3, 2/29 mAbs; DH6, 1/29 mAb) and 3 JH genes (JH6, 21/29 [72%] mAbs; JH1 and JH4, 4/29 [14%] mAbs each). The V-D-J gene rearrangement VH1-3/DH5-12/JH6 was observed in 9/29 (31%) mAbs and VH1-3/DH2-15/JH6 in 3/29 (10%) mAbs, whereas the remaining 17 mAbs (59%) had individual V-D-J gene rearrangements (Table 1; Figure 2B).
The IgVH CDR3 regions, most relevant for antigen binding, were composed of 17 to 19 amino acids in VH1-3 and VH3-30 mAbs, of 10 to 11 amino acids in VH4-28 mAbs, and of variable length (8-17 amino acids) in VH1-69 mAbs (Figure 2B). Alignment of CDR3 amino acid sequences revealed 8 different CDR3 motifs (Figure 1A), of which 4 were observed in both patients (CDR3 motifs 1-4). Two of the 4 shared CDR3 motifs were observed in mAbs generated by phage display, as well as by EBV transformation of switched memory B cells and comprised Abs of both IgG1 and IgG4 subclasses. The 5 VH1-69 mAbs found in patient B only, generated by either phage display or EBV transformation, displayed 4 different CDR3 motifs (CDR3 motifs 5-8). Although CDR3 motif 3 mAbs were all generated by phage display, those of CDR3 motifs 4-6 originated from EBV-transformed switched memory B cells only. Within a CDR3 motif, amino acid sequences differed by a maximum of 4 amino acids, primarily through deletion/insertion of 1 or 2 amino acids (Figure 1A). No similar VHDHJH rearrangements with significant homologies to the consensus sequences of the 8 CDR3 motifs were found in the public domain (Figure 1A).
Alignment of IgVL genes revealed that 19/29 (65.5%) mAbs, among them all whole IgGs derived from EBV-transformed switched memory B cells, used λ L-chains, and the remaining 10 Abs were composed of κ L-chains (Table 1; Figure 1B). The latter were derived from 3 germ-line VL genes: KV1 (4/29 mAbs), KV4 (5/29 mAbs), and KV3 in a single clone. The lambda L-chains were derived from LV1 (6 mAbs, all whole IgGs), the LV3 superfamily (10 mAbs, 34.5%), and LV5 (3 mAbs). The average homology to the closest VL germ-line genes was 89.3% (range, 84.7-95.1%), and the CDR3 regions were 8-14 amino acids long (Figure 2C; Table 1).
In contrast to the IgVH situation, there were no VL germ-line genes shared by phage-display Fabs and whole IgGs derived from EBV-transformed switched memory B cells (Table 1; Figure 1B).
To investigate the presence of anti-ADADMTS13 antibodies in the natural antibody repertoire, IgM-κ and -λ phage libraries, generated from umbilical cord blood lymphocytes of 7 donors,42 were selected by panning on rADAMTS13. ADAMTS13-specific IgM (n = 9) were composed of κ L-chains in combination with VH genes from the IgVH1 superfamily (3 clones), IgVH3 superfamily (3 clones), and IgVH6 (3 clones). The IgVH CDR3 was distinct from the CDR3 motifs in the ADAMTS13 Ab repertoire of the 2 TTP patients.
Specificity of all human anti-ADAMTS13 mAbs
After purification, none of the 29 mAbs showed cross-reactivity with human serum albumin or human factors VII, VIII, and IX coated at equimolar concentration as rADAMTS13 when assessed by ELISA (Figure 3; Tables 2 and 3). The frequency of ADAMTS13-specific switched IgG+ memory B cells was 5% (5 out of 101 clones) in patient A and 18% (8 out of 44 clones) in patient B, and thus, there was a threefold increase in the rituximab-treated patient B (supplemental Table 1; supplemental Figure 1).
Specificity of all 29 anti-ADAMTS13 mAbs toward recombinant ADAMTS13 and control antigens at equimolar concentrations assessed by ELISA. Black bars represent absorbance at 450 nm as an average of the signal obtained for each mAb separately within the corresponding CDR3 motifs M-1 to M-8. Antigens (human rADAMTS13, human factors VII [hVII], VIII [hVIII], and IX [hIX]) and tetanus toxoid (TT) were coated at equimolar concentration on an ELISA plate. Anti-ADAMTS13 mAb I-9 (gift of Jan Voorberg, Amsterdam, The Netherlands) served as a positive control and the secondary antibody (II-Ab) as a negative control.
Specificity of all 29 anti-ADAMTS13 mAbs toward recombinant ADAMTS13 and control antigens at equimolar concentrations assessed by ELISA. Black bars represent absorbance at 450 nm as an average of the signal obtained for each mAb separately within the corresponding CDR3 motifs M-1 to M-8. Antigens (human rADAMTS13, human factors VII [hVII], VIII [hVIII], and IX [hIX]) and tetanus toxoid (TT) were coated at equimolar concentration on an ELISA plate. Anti-ADAMTS13 mAb I-9 (gift of Jan Voorberg, Amsterdam, The Netherlands) served as a positive control and the secondary antibody (II-Ab) as a negative control.
Functional characteristics and binding pattern toward rADAMTS13 of the 16 human anti-ADAMTS13 Fabs generated by phage display technology
| mAbs . | Inhibitory potential assessd by residual ADAMTS13 activity (%) . | Binding pattern toward rADAMTS13 as assesed by . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone . | IgG subclass . | CDR3 motif . | 10 nM . | 100 nM . | KD Biacore (M) . | ELISA 405 nm* . | CLIPS conformational epitope . | Dot-blot reactivity with† . | |
| Full-length . | MDTCS . | ||||||||
| 1a | IgG4 | 1 | 8.1 | <1 | 4.6E-09 | 2.611 | Group 1 | +++ | − |
| 1d | IgG4 | 3 | 29.3 | 1.6 | 4.7E-08 | 2.759 | Group 1 | +++ | − |
| 1e | IgG4 | 1 | 71.5 | <1 | 2.5E-08 | 1.460 | Group 1 | +++ | +++ |
| 1f | IgG4 | 1 | 69.2 | <1 | 1.5E-09 | 1.848 | ‡ | +++ | ++ |
| 1g | IgG4 | 1 | 124 | 100 | 1.0E-08 | 2.105 | ‡ | +++ | + |
| 1h | IgG4 | 3 | 100 | <1 | 7.2E-09 | 1.424 | Group 3 | +++ | ++ |
| 2a | IgG4 | 2 | 56.7 | 1.1 | 2.6E-08 | 1.210 | Group 3 | +++ | + |
| 2c | IgG4 | 1 | 92.5 | 5.4 | 7.9E-08 | 2.623 | Group 1 | +++ | + |
| 2h | IgG4 | 3 | 91.9 | 2.6 | 3.5E-08 | 1.859 | Group 3 | +++ | + |
| 2i | IgG4 | 3 | 72.5 | 1.3 | 9.2E-09 | 1.741 | Group 2 | +++ | + |
| 2j | IgG4 | 2 | 100 | <1 | 9.6E-08 | 1.063 | Group 2 | +++ | ++ |
| 3b | IgG4 | 7 | 100 | 3.5 | 9.0E-09 | 1.549 | Group 2 | +++ | +++ |
| 3c | IgG4 | 3 | 100 | <1 | 6.6E-08 | 2.818 | ‡ | +++ | +++ |
| 3h | IgG4 | 3 | 100 | <1 | 9.2E-08 | 1.373 | Group 1 | +++ | − |
| 3j | IgG4 | 8 | 100 | 39.2 | 9.2E-09 | 0.792 | Group 2 | +++ | +++ |
| 4a | IgG4 | 2 | 100 | 13.6 | 5.8E-08 | 1.401 | Group 2 | +++ | +++ |
| mAbs . | Inhibitory potential assessd by residual ADAMTS13 activity (%) . | Binding pattern toward rADAMTS13 as assesed by . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone . | IgG subclass . | CDR3 motif . | 10 nM . | 100 nM . | KD Biacore (M) . | ELISA 405 nm* . | CLIPS conformational epitope . | Dot-blot reactivity with† . | |
| Full-length . | MDTCS . | ||||||||
| 1a | IgG4 | 1 | 8.1 | <1 | 4.6E-09 | 2.611 | Group 1 | +++ | − |
| 1d | IgG4 | 3 | 29.3 | 1.6 | 4.7E-08 | 2.759 | Group 1 | +++ | − |
| 1e | IgG4 | 1 | 71.5 | <1 | 2.5E-08 | 1.460 | Group 1 | +++ | +++ |
| 1f | IgG4 | 1 | 69.2 | <1 | 1.5E-09 | 1.848 | ‡ | +++ | ++ |
| 1g | IgG4 | 1 | 124 | 100 | 1.0E-08 | 2.105 | ‡ | +++ | + |
| 1h | IgG4 | 3 | 100 | <1 | 7.2E-09 | 1.424 | Group 3 | +++ | ++ |
| 2a | IgG4 | 2 | 56.7 | 1.1 | 2.6E-08 | 1.210 | Group 3 | +++ | + |
| 2c | IgG4 | 1 | 92.5 | 5.4 | 7.9E-08 | 2.623 | Group 1 | +++ | + |
| 2h | IgG4 | 3 | 91.9 | 2.6 | 3.5E-08 | 1.859 | Group 3 | +++ | + |
| 2i | IgG4 | 3 | 72.5 | 1.3 | 9.2E-09 | 1.741 | Group 2 | +++ | + |
| 2j | IgG4 | 2 | 100 | <1 | 9.6E-08 | 1.063 | Group 2 | +++ | ++ |
| 3b | IgG4 | 7 | 100 | 3.5 | 9.0E-09 | 1.549 | Group 2 | +++ | +++ |
| 3c | IgG4 | 3 | 100 | <1 | 6.6E-08 | 2.818 | ‡ | +++ | +++ |
| 3h | IgG4 | 3 | 100 | <1 | 9.2E-08 | 1.373 | Group 1 | +++ | − |
| 3j | IgG4 | 8 | 100 | 39.2 | 9.2E-09 | 0.792 | Group 2 | +++ | +++ |
| 4a | IgG4 | 2 | 100 | 13.6 | 5.8E-08 | 1.401 | Group 2 | +++ | +++ |
Functional tests: inhibitory capacity against plasma-derived ADAMTS13 in a Bethesda-like assay determining the residual ADAMTS13 activity assessed by FRETS-VWF73 (in %). Binding pattern assays included affinity analysis by SPR (Biacore), specificity tests by ELISA (Figure 3), and epitope mapping performed by either conformational CLIPS technology and dot-blot technique on either recombinant full-length or on ADAMTS13 stop mutant (MDTCS-13, consisting of ADAMTS13–metalloprotease–disintegrin–thrombospondin type I repeat number 1–cys-rich and spacer domain MDTCS) (supplemental Materials).
ELISA data in Figure 3.
Dot-blot technique described in supplemental Data.
No binding pattern found by CLIPS technology.
Functional characteristics and binding pattern toward rADAMTS13 of the 13 human anti-ADAMTS13 whole IgGs generated by EBV transformation of switched memory B cells
| mAbs . | Inhibitory potential assessed by residual ADAMTS13 activity (%) . | Binding pattern toward rADAMTS13 as assesed by . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone . | IgG subclass . | CDR3 motif . | 10 nM . | 100 nM . | KD Biacore (M) . | ELISA 405 nm* . | CLIPS conformational epitope . | Dot-blot reactivity with† . | |
| Full-length . | MDTCS . | ||||||||
| A-1 | IgG4 | 2 | 6.6 | 4.7 | 9.0E-09 | 2.186 | Group 1 | +++ | + |
| A-2 | IgG4 | 2 | 1 | 8.1 | 2.7E-10 | 1.485 | Group 1 | +++ | + |
| A-3 | IgG1 | 1 | 11.0 | 8.5 | 4.0E-09 | 1.304 | Group 1 | +++ | ++ |
| A-5 | IgG4 | 4 | <1 | <1 | 2.8E-09 | 2.817 | Group 1 | +++ | + |
| A-6 | IgG1 | 1 | 100 | 100 | 3.8E-08 | 2.278 | ‡ | − | − |
| B-1 | IgG4 | 2 | 100 | 16.0 | 2.1E-09 | 0.757 | Group 1 | +++ | + |
| B-2 | IgG4 | 5 | 27.4 | 13.7 | 2.8E-08 | 1.090 | Group 3 | ++ | ++ |
| B-3 | IgG1 | 2 | 100 | 20.4 | 2.0E-08 | 1.118 | Group 2 | ++ | + |
| B-4 | IgG1 | 2 | 1 | 4.2 | 4.6E-09 | 1.903 | Group 1 | +++ | ++ |
| B-5 | IgG1 | 6 | 2.4 | 4.4 | 2.9E-09 | 2.255 | ‡ | +++ | + |
| B-6 | IgG1 | 5 | 100 | 5.5 | 4.8E-10 | 0.802 | ‡ | +++ | ++ |
| B-7 | IgG1 | 1 | 22.2 | 5.4 | 4.3E-10 | 1.472 | Group 2 | +++ | +++ |
| B-8 | IgG1 | 4 | 21.5 | 3.2 | 9.5-09 | 1.488 | Group 1 | +++ | + |
| mAbs . | Inhibitory potential assessed by residual ADAMTS13 activity (%) . | Binding pattern toward rADAMTS13 as assesed by . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone . | IgG subclass . | CDR3 motif . | 10 nM . | 100 nM . | KD Biacore (M) . | ELISA 405 nm* . | CLIPS conformational epitope . | Dot-blot reactivity with† . | |
| Full-length . | MDTCS . | ||||||||
| A-1 | IgG4 | 2 | 6.6 | 4.7 | 9.0E-09 | 2.186 | Group 1 | +++ | + |
| A-2 | IgG4 | 2 | 1 | 8.1 | 2.7E-10 | 1.485 | Group 1 | +++ | + |
| A-3 | IgG1 | 1 | 11.0 | 8.5 | 4.0E-09 | 1.304 | Group 1 | +++ | ++ |
| A-5 | IgG4 | 4 | <1 | <1 | 2.8E-09 | 2.817 | Group 1 | +++ | + |
| A-6 | IgG1 | 1 | 100 | 100 | 3.8E-08 | 2.278 | ‡ | − | − |
| B-1 | IgG4 | 2 | 100 | 16.0 | 2.1E-09 | 0.757 | Group 1 | +++ | + |
| B-2 | IgG4 | 5 | 27.4 | 13.7 | 2.8E-08 | 1.090 | Group 3 | ++ | ++ |
| B-3 | IgG1 | 2 | 100 | 20.4 | 2.0E-08 | 1.118 | Group 2 | ++ | + |
| B-4 | IgG1 | 2 | 1 | 4.2 | 4.6E-09 | 1.903 | Group 1 | +++ | ++ |
| B-5 | IgG1 | 6 | 2.4 | 4.4 | 2.9E-09 | 2.255 | ‡ | +++ | + |
| B-6 | IgG1 | 5 | 100 | 5.5 | 4.8E-10 | 0.802 | ‡ | +++ | ++ |
| B-7 | IgG1 | 1 | 22.2 | 5.4 | 4.3E-10 | 1.472 | Group 2 | +++ | +++ |
| B-8 | IgG1 | 4 | 21.5 | 3.2 | 9.5-09 | 1.488 | Group 1 | +++ | + |
Functional tests: inhibitory capacity against plasma-derived ADAMTS13 in a Bethesda-like assay determining the residual ADAMTS13 activity assessed by FRETS-VWF73 (in %). Binding pattern assays included affinity analysis by SPR (Biacore), specificity tests by ELISA (Figure 3), and epitope mapping performed by either conformational CLIPS technology and dot-blot technique on either recombinant full-length or on ADAMTS13 stop mutant (MDTCS-13, consisting of ADAMTS13–metalloprotease–disintegrin–thrombospondin type I repeat number 1–cys-rich and spacer domain MDTCS) (supplemental Materials).
ELISA data in Figure 3.
Dot-blot technique described in supplemental Data.
No binding pattern found by CLIPS technology.
Potential of the anti-ADAMTS13 mAbs to recognize and inhibit ADAMTS13 activity
Affinity analysis of all purified 29 anti-ADAMTS13 mAbs by surface plasmon resonance (SPR) was in the following KD range: 1 × 10−8 to 5 × 10−10 M, and Fabs showed on average a 1 log higher KD due to the different avidities of 1 (Fab) vs 2 antigen-binding sites [F(ab)2] in IgG (Tables 2 and 3).
The mAbs inhibitory potential was assessed in a Bethesda-like assay, where purified mAbs were preincubated with a normal human plasma pool at a final mAb concentration of 10 or 100 nM. At 100 nM final concentration, 27/29 (93%) of anti-ADAMTS13 mAbs inhibited plasma ADAMTS13 activity by 91% to 100% (pat A) and 60% to 100% (pat B; Tables 2 and 3). Two mAbs were noninhibitory at this concentration and both originated from patient A (Fab clone 1g and whole IgG1 A-6). At a 10 nM final concentration, 11/29 mAbs still exhibited an inhibitory potential of >1 Bethesda units/ml (residual ADAMTS13 activity in the mixture <50%). Nine of these 11 mAbs were whole IgGs derived from EBV-transformed switched B cells.
Epitope mapping
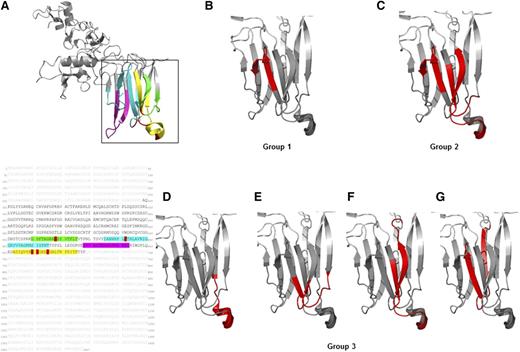
Epitope mapping using overlapping peptides of the ADAMTS13 spacer domain (residues 556-685; Figure 4A) revealed that 23/29 mAbs recognized conformational epitopes within this domain (Figure 4B-G). Binding pattern of the mAbs allowed classification into 3 groups (Tables 2 and 3). Group 1 (Figure 4B; 12 mAbs) targeted residues 595AVGRIGGRYVV605 (within the blue loop in Figure 4A), whereas group 2 (Figure 4C; 7 mAbs) targeted in addition residues 564AGRAREYVTFL574 (within the green loop in Figure 4A) and 655IQVRRYGEEY665 (within the yellow loop in Figure 4A). Unique binding patterns were observed for 4/23 mAbs (group 3), with mAb B-2 binding to an exposed part of the yellow loop 661YGEEYGNLTRP671 (Figure 4D). The discontinuous epitope of Fab 1h (Figure 4E) was restricted to residues 562FTAGRAREY570 (green loop) and 587NHRPLFTHL595 (blue loop). The discontinuous epitope of Fab 2a (Figure 4F) encompassed residues 568REVTFLTVTP578 (green loop) and 655IQVRRYGEEY665 (yellow loop), whereas the epitope of Fab 2h (Figure 4G) was composed of residues 580LTSVYIANHRPLFTHLAVRIG600 (blue loop).
Mapping of the binding sites of the human anti-ADAMTS13 mAbs to the spacer domain using conformational CLIPS peptide technology. (A) Crystal structure and amino acid spacer domain of ADAMTS13 with residues 560 to 575 (green loop), residues 585 to 615 (blue loop), residues 653 to 675 (yellow loop), and residues 628 to 643 (magenta loop) highlighted. Red amino acid residues are part of the key epitope recognized by autoantibodies published previously by others. (B-G) Mapped binding sites of the anti-ADAMTS13 mAbs are highlighted in red. The binding patterns were categorized into 3 groups, with group 1 and 2 consisting of several mAbs recognizing the same conformational epitope. (B) Group 1 (12/23 mAbs: Fabs 1a, 1d, 1e, 2c, 3h and whole IgG A-1, A-2, A-3, A-5, B-1, B-4, B-8) binding to residues 595 to 605 (within the blue loop, A). (C) Group 2 (7/23 mAbs: Fab 2i, 2j, 3b, 3j, 4a and whole IgG B-3, B-7) binding to residues 595 to 605 (within the blue loop), residues 564 to 574 (within the green loop), and residues 655 to 665 (within the yellow loop). (D-G) Group 3 (4/23 mAbs): each with an unique epitope pattern, for whole (D) IgG B-2 residues 661 to 671 (within the yellow loop); (E) Fab 1h residues 562 to 570 (within the green loop) and 587 to 595 (within blue loop); (F) Fab 2a residues 655 to 665 (within the yellow loop) and residues 568 to 578 (within the green loop); and (G) Fab 2h residues 580 to 600 (blue loop only). Six of the 29 anti-ADAMTS13 mAbs could not be mapped: Fabs 1f, 1g, and 3c and whole IgGs A-6, B-5, and B-6.
Mapping of the binding sites of the human anti-ADAMTS13 mAbs to the spacer domain using conformational CLIPS peptide technology. (A) Crystal structure and amino acid spacer domain of ADAMTS13 with residues 560 to 575 (green loop), residues 585 to 615 (blue loop), residues 653 to 675 (yellow loop), and residues 628 to 643 (magenta loop) highlighted. Red amino acid residues are part of the key epitope recognized by autoantibodies published previously by others. (B-G) Mapped binding sites of the anti-ADAMTS13 mAbs are highlighted in red. The binding patterns were categorized into 3 groups, with group 1 and 2 consisting of several mAbs recognizing the same conformational epitope. (B) Group 1 (12/23 mAbs: Fabs 1a, 1d, 1e, 2c, 3h and whole IgG A-1, A-2, A-3, A-5, B-1, B-4, B-8) binding to residues 595 to 605 (within the blue loop, A). (C) Group 2 (7/23 mAbs: Fab 2i, 2j, 3b, 3j, 4a and whole IgG B-3, B-7) binding to residues 595 to 605 (within the blue loop), residues 564 to 574 (within the green loop), and residues 655 to 665 (within the yellow loop). (D-G) Group 3 (4/23 mAbs): each with an unique epitope pattern, for whole (D) IgG B-2 residues 661 to 671 (within the yellow loop); (E) Fab 1h residues 562 to 570 (within the green loop) and 587 to 595 (within blue loop); (F) Fab 2a residues 655 to 665 (within the yellow loop) and residues 568 to 578 (within the green loop); and (G) Fab 2h residues 580 to 600 (blue loop only). Six of the 29 anti-ADAMTS13 mAbs could not be mapped: Fabs 1f, 1g, and 3c and whole IgGs A-6, B-5, and B-6.
With the exception of Fab 2h and mAb B-2, the discontinuous epitope of 21/23 mAbs encompassed residues 655 to 665 (yellow loop, Figure 4A) and 568 to 574 (green loop, Figure 4A), with some differences in the individual mAb binding strength. Although linear epitopes were recognized by 20/29 anti-ADAMTS13 mAbs targeting residues located in the metalloprotease, thrombospondin type 1 repeat 1, and thrombospondin type 1 repeat 6 domains, this binding was very weak. The remaining 6/29 anti-ADAMTS13 mAbs could not be mapped by CLIPS technology (Figure 4), despite their high affinity toward full-length ADAMTS13 observed by ELISA (Figure 3), Biacore, and dot blot analysis (with the exception of the A-6 by dot-blot; Tables 2 and 3).
Discussion
Splenectomy has been a therapeutic option for patients with refractory or relapsing acquired TTP for >3 decades.17,18,45-47 Given the importance of inhibitory anti-ADAMTS13 Abs in the pathophysiology of acquired TTP, these authors hypothesized that the beneficial effect of splenectomy might be ascribed to the removal of a large reservoir of B cells producing pathogenic autoantibodies.18,47 The clinical course of frequently relapsing TTP in our 2 patients changed following splenectomy, after which both have remained in remission to this day, for 11 and 8 years, respectively. In our study, using mononuclear cells of the 2 patients’ spleens, the phage display technique, and EBV transformation of switched memory B cells, we found noticeable numbers of pathogenic B cells (eg, 5% of switched memory B cells in patient A and 18% in patient B) capable of producing anti-ADAMTS13 autoantibodies with the same inhibitory capacity as present in plasma of patients with an acute TTP episode.
The isolated anti-ADAMTS13 mAbs of our 2 patients demonstrated an IgG variable H-chain use limited to 4 VH germ-line families: VH1-3 in 55%, VH4-28 in 21%, VH3-30 in 7%, and VH1-69 in 17% of mAbs (Table 1; Figure 2B). Use of VH1-69, previously reported to be present in plasma of a considerable number of acquired TTP patients,32,34 was seen only in patient B, who had been repeatedly treated with rituximab during acute TTP episodes or prophylactically when ADAMTS13 activity dropped to <10% of the normal. The IgG subclasses of the whole anti-ADAMTS13 mAbs were IgG1 (pat A, 40%; pat B, 75%) and IgG4 (pat A, 60%; pat B, 25%) and matches in part the IgG subclass distribution observed in plasma of TTP patients, where IgG4 is the most prevalent, followed by IgG1, IgG2, and IgG3.32 The anti-ADAMTS13 mAbs showed somatic hypermutation typical for an antigen-driven, affinity matured immune response, which is a process dependent on CD4+ T-cell help.40-42,48 The average nucleic acid mutation rate was 12% for the VH and the VL chain regions, with the highest mutation rate in mAbs using the VH1-3 germ-line gene (average, 15%; range, 13.5-18.4%) and the lowest in VH1-69 mAbs (average, 4.8%; range, 2.9-5.7%).
The most important finding is the fact that the anti-ADAMTS13 mAbs were characterized by a limited number of VH CDR3 signatures, labeled CDR3 motifs 1-8, of which 4 (CDR3 motifs 1-4) were shared by the 2 patients. The 2 patients were neither related nor from the same geographical region but shared 1 HLA DRB1*allele: HLA DRB1*1501. The other HLA DRB1* alleles were HLA DRB1*0301 (pat A) and HLA DRB1*1101 (pat B). The probability of 2 B cells arranging their IgG germ-line segments very similarly is very low (8.5 × 10−8) and that of almost identical CDR3 regions in addition is even lower (<10−9). Our findings thus point at the discovery of pathologically relevant CDR3 motifs in inhibitory anti-ADAMTS13 mAbs and are in line with observations in other (autoimmune) diseases, where it was shown that shared CDR3 signatures are critical for the pathogenicity of autoantibodies.49-51
Despite the high similarity of the 2 noninhibitory anti-ADAMTS13 mAbs (clones 1g and A-6) belonging to the same VH1-3-CDR3 motif 1 as the inhibitory mAbs, they differed in their L-chain use and their binding pattern toward rADAMTS13 (Tables 2 and 3), with no epitope recognized in the spacer domain of ADAMTS13 by CLIPS technology. The binding pattern of the inhibitory mAbs, although in close proximity and slightly overlapping with the previously published epitopes in the ADAMTS13 spacer domain,52-57 were composed of 6 thus far not described distinct conformational discontinuous epitopes (Figure 4B-G), possibly contributing to their greater inhibitory capacity than reported by others.32-34
The splenic anti-ADAMTS13 B-cell response was genetically distinct from the naïve immune response found in the IgM cord blood library, suggesting that the ADAMTS13 autoimmune response is likely acquired and does not evolve from a low-affinity naïve repertoire. Recent findings of linear epitopes shared by noninhibitory antibodies from healthy individuals and inhibitory antibodies from acquired TTP patients hint at the noninhibitory anti-ADAMTS13 antibodies as serving as a template for pathogenic antibodies, but the trigger to set off the maturation process still needs to be elucidated.58
For the first time, we show that an antibody repertoire selected by 2 techniques, phage display and EBV transformation of memory B cells, contains very similar antibodies regarding the VH gene use, VH CDR3 regions, functionality (ADAMTS13 inhibitory potential), and epitope recognition, indicating that both techniques reflect the in vivo situation. Pairing of H- and L-chain in whole IgG derived from EBV-transformed switched memory B cells was, however, more uniform and restricted than in the phage display Fabs. As mAbs generated by both techniques showed similar functional properties, this may suggest a limited contribution of the L-chain to the pathogenicity of the anti-ADAMTS13 autoantibodies.
The anti-ADAMTS13 repertoire of patient B, clinically noticeable for a disease onset at a young age and repeated rituximab treatment, was characterized by more diverse VH gene use (including IgVH1-69) and 4 additional CDR3 motifs (motifs 5-8), a high percentage of anti-ADAMTS13–specific switched memory B cells in the spleen (18%), and of anti-ADAMTS13 IgG1 B cells, the IgG subclass possibly associated with an adverse outcome in acquired TTP.59 Whether these remarkable observations are the consequence of rituximab treatment remains to be elucidated but are in line with a recent study showing that anti-CD20 therapy promoted differentiation and selection of pathogenic, long-lived plasma cells in the spleen in immune thrombocytopenia.60
Our discovery that the spleen harbors a limited autoimmune repertoire against ADAMTS13, marked by 4 antigen-binding signatures (CDR3 motifs 1-4) shared by 2 unrelated patients with relapsing acquired TTP, if confirmed in other acquired TTP patients, is a step forward toward the development of diagnostic assays and/or therapeutic approaches based on binding and neutralizing the pathogenically relevant autoantibodies in acquired TTP. Further studies are needed to elucidate the role of T cells in shaping the anti-ADAMTS13 B-cell repertoire.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the 2 TTP patients for enabling this project by donating their spleens for research; the surgeons performing the splenectomy; and Dr Elisabetta Traggiai (Novartis) for her advice with setting up the EBV transformation of the IgG-switched memory B cells. The expert technical assistance of Irmela Sulzer and our diploma students, Karin Sprecher and Gianna Tanno, is gratefully acknowledged.
This research was supported by Swiss National Science Foundation grant 32003B_124892 (to J.A.K.H. and B.L.) and Marie Heim-Vögtlin fellowship PMPDP3_139794/1 (to M.S.), as well as the ISTH 2007 Presidential Fund (to M.S. and J.A.K.H.) and the Josephine Clark Fund of the University of Bern (to M.S.).
Authorship
Contribution: M.S. and J.A.K.H. were responsible for the study concept and design; J.A.K.H. prepared the splenocytes; M.S. performed the experiments to generate the human mAbs (phage display and EBV transformation) and functional characterization of the mAbs with the help of Irmela Sulzer, Karin Sprecher, and Gianna Tanno; M.V. generated the IgM-naïve phage library; M.S., J.A.K.H., and M.V. analyzed the data; J.A.K.H., K.K., and B.L. were responsible for patient care; M.S. and J.A.K.H. wrote the manuscript; and all authors edited the final version of the manuscript.
Conflict-of-interest disclosure: J.A.K.H. and B.L. received research support from Baxter Vienna for the investigator-initiated setup of the hereditary TTP registry (www.ttpregistry.net). The remaining authors declare no competing financial interests.
The current affiliation for B.L. is Center for Thrombosis and Hemostasis, University Medical Center, Mainz, Germany.
Correspondence: Monica Schaller, University Hospital Bern, Inselspital, Department of Hematology, Hemostasis Research Laboratory, Freiburgstrasse, CH-3010 Bern, Switzerland; e-mail: monica.schaller@insel.ch.


![Figure 3. Specificity of all 29 anti-ADAMTS13 mAbs toward recombinant ADAMTS13 and control antigens at equimolar concentrations assessed by ELISA. Black bars represent absorbance at 450 nm as an average of the signal obtained for each mAb separately within the corresponding CDR3 motifs M-1 to M-8. Antigens (human rADAMTS13, human factors VII [hVII], VIII [hVIII], and IX [hIX]) and tetanus toxoid (TT) were coated at equimolar concentration on an ELISA plate. Anti-ADAMTS13 mAb I-9 (gift of Jan Voorberg, Amsterdam, The Netherlands) served as a positive control and the secondary antibody (II-Ab) as a negative control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/23/10.1182_blood-2014-04-561142/4/m_3469f3.jpeg?Expires=1769126366&Signature=sMDF3XSAPv9~q1ExTGDiACsvIOenKqCMiw9N~--pDpjs5NSu8W9nIFTECnE6cWMxCWnLzvqn~sexroCpsIKZ-2h9TMZo7aI-zIbOLgfXFaWlYSB~6K0HQBqp6qO2rxFv4em2UpGvRu4nwdAUb-u1hVhBGxJjhNHvfqLWWgok2nE5sxe6Yhw9bDxo1S7KqtwWVvJxryxttkr6Esvoj7YeT0TtibnvQBwCcl1Y0xX-sy2n96M4xGpET8cN78zJso61rOmpnW-RTOU~cOzlYBMt7RAQwMNQ8GhSPNdRsTcEn90aV0-f0TWhPt0JygVodZNoZr3k32f1YCcrc41aae8WsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)