Key Points
Maurer's clefts are P falciparum–derived membranous structures within the host erythrocyte that are essential for parasite survival.
PfPTP1 functions in a large complex of proteins and is required for linking of Maurer's clefts to the host actin cytoskeleton.
Abstract
Following invasion of human red blood cells (RBCs) by the malaria parasite, Plasmodium falciparum, a remarkable process of remodeling occurs in the host cell mediated by trafficking of several hundred effector proteins to the RBC compartment. The exported virulence protein, P falciparum erythrocyte membrane protein 1 (PfEMP1), is responsible for cytoadherence of infected cells to host endothelial receptors. Maurer clefts are organelles essential for protein trafficking, sorting, and assembly of protein complexes. Here we demonstrate that disruption of PfEMP1 trafficking protein 1 (PfPTP1) function leads to severe alterations in the architecture of Maurer's clefts. Furthermore, 2 major surface antigen families, PfEMP1 and STEVOR, are no longer displayed on the host cell surface leading to ablation of cytoadherence to host receptors. PfPTP1 functions in a large complex of proteins and is required for linking of Maurer's clefts to the host actin cytoskeleton.
Introduction
Plasmodium falciparum causes the most severe form of malaria in humans. Circulating red blood cells (RBCs) are invaded by P falciparum merozoites, which develop into schizonts generating more merozoites that in turn are released to invade new erythrocytes. Profound structural and morphological changes occur in parasite-infected erythrocytes that alter their physical properties and impair circulation in vivo.1,2 Parasitized RBCs become rigid and adhere to a variety of cell types. This can lead to perturbation or obstruction of blood flow in the microcirculation of organs and is related to parasite-induced alterations of both their biomechanical and adhesive properties, central to survival and pathogenicity of P falciparum.3
Cytoadherence is conferred by the P falciparum erythrocyte membrane protein 1 (PfEMP1) that binds to various ligands displayed on the microvasculature of organs (reviewed in Arnot and Jensen4 ). To reach the erythrocyte surface, PfEMP1 traverses the endoplasmic reticulum (ER) of the parasite and is transported across the parasite plasma membrane and parasitophorous vacuolar membrane (PVM), which surrounds the parasite. The further route of PfEMP1 is largely unknown; it is either trafficked through the erythrocyte cytosol via a vesicular pathway or in a complex where it transiently associates with parasite-induced membranous compartments, named Maurer's clefts (MC).5 The protein is subsequently translocated onto RBC membranes and anchored in protrusions of the membrane (knobs).6 Knobs are points of elevation and concentration for PfEMP1 anchoring to host cytoskeleton facilitating binding to receptors on host cells.
Central to survival of P falciparum is extensive remodeling of the RBC, a host cell lacking an established protein trafficking network. To provide protein trafficking routes in the host cell the parasite exports many proteins. These cross the parasite plasma membrane and the PVM to reach their subcellular destination in the RBC. Most exported proteins contain a signal sequence for entry into the ER7-9 and a Protein Export ELement/Vacuolar Transport Signal (PEXEL/VTS) motif,10,11 which confers trafficking to the RBC.12,13 These proteins move through the parasitophorous vacuole and PVM via a translocator machine.14-16 A number of exported proteins do not contain a PEXEL/VTS motif and are thus called PEXEL-negative exported proteins (PNEPs); their transport pathways are unknown.5,17
Various proteins play a role in trafficking PfEMP1 to the P falciparum–infected RBC membrane and its assembly into knob structures (reviewed in Maier et al18 ). In the absence of PNEPs, skeleton-binding protein 1 (SBP1),19,20 MC-associated histidine-rich protein 1 (MAHRP1),21 or ring-exported protein 1 (REX1),22 PfEMP1 cannot be displayed on the RBC membrane. Additionally, a large-scale gene-knockout screen in P falciparum identified other proteins required for trafficking and function of PfEMP1.23 MC are parasite-derived structures that appear in the RBC cytosol in the early ring stage of the parasite blood stage life cycle.5 It has been suggested that PfEMP1 trafficking from the MC to the RBC membrane occurs through vesicular transport \along actin filaments.24-26 There are a number of different vesicles described in P falciparum including electron dense vesicles (EDVs), J-dots, and vesicle-like structures (VLSs), which could play a role in PfEMP1 trafficking.27-31
We have characterized a novel PEXEL-containing protein that we have named P falciparum PfEMP1 trafficking protein.
Materials and methods
See supplemental Material (available on the Blood Web site) for full methods.
Parasites and cell culture of P falciparum
Parasites used in this study were P falciparum strain CS2 and CS2/PTP1–hemagglutinin (HA) (HA/streptavidin-tagged; supplemental Figure 1C), CS2/PTP1–green fluorescent protein (GFP) and CS2ΔPTP1 transfectants.23
Fluorescence microscopy
Thin blood smears of parasitized cells were fixed in acetone/methanol (90/10). Cells were labeled with antibodies: α-PTP1 (1:500), α-SBP1 (1:500), α-REX1 (1:2000),32 α-GFP (1:500), α-HA (1:100), α-MAHRP1 (1:100),33 α-Pf332 (1:500),34 α-STEVOR (1:500),35 α-Rif29 (1:200),36 α-Hsp70-x (1:200),37 α-PTP2 (1:250).38 Cells were viewed with a Zeiss Plan-Apochromat 100×/1.4 numeric aperture oil-immersion lens on a Zeiss Axioskop 2 microscope equipped with a PCO SensiCam (12-bit) camera.
Subcellular fractionation analysis, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and western blots
Immunoblots were probed with either mouse α-HA antibodies (Roche; 1:1000) or rabbit α-PfHSP70 antibodies (1:1000). Solubility assays were performed as described6,39,40 and probed with mouse α-HA antibodies (Roche; 1:1000), stripped and reprobed with control antibody SBP1 (rabbit; 1:500). The trypsin cleavage assays were performed as described.41
Blue native and 2-dimensional-PAGE analysis and EM and tomography
The blue native–polyacrylamide gel electrophoresis (PAGE) was performed as described.42
For cryoelectron tomography, cells were plunge-frozen and tilt series were recorded in an FEI Polara electron microscope (EMBL, Heidelberg), with an acceleration voltage of 300 kV. The tomograms were calculated by back-projection of 50 to 60 images aligned by fiducial markers using the electron microscopy (EM) image processing package and the TOM package on MATLAB. The volumes were denoised with nonlinear anisotropic diffusion methods, and the surface rendering was done using the AMIRA volume processing software (Amira TGS Europe SA).
For the in vitro actin polymerization assay, the PfEMP1 trafficking protein 1 (PfPTP1) peptide (JPT) was resuspended in 5mM Tris-HCl; pH 8.0, 0.2mM CaCl2. Actin polymerization assays were conducted as described in the Actin Polymerization Biochem kit (Cytoskeleton Inc.).
Results
PfPTP1 is exported and associates with membranous vesicles
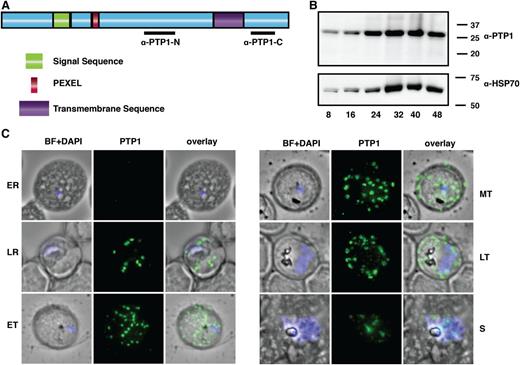
PfPTP1 is required for trafficking and display of PfEMP1 on the surface of P falciparum–infected RBCs but its function is unknown.23 PfPTP1 is a protein of 290 amino acids with a signal sequence, PEXEL,10,11 and a putative transmembrane domain consistent with export to parasite-infected RBCs (Figure 1A). To address PfPTP1 function, we showed it was expressed across the asexual cycle and located in punctate structures, surrounding the intracellular parasite, that bud from the PVM (Figure 1B-C). During development, these structures increase in size contacting the RBC membrane in the mature parasite (Figure 1C). In rupturing schizonts, PfPTP1-associated organelles appear as remnants between merozoites (Figure 1C). Live imaging of GFP-tagged PfPTP1 showed large and smaller punctate organelles with the larger structures likely being MC8 (supplemental Figure 1B; supplemental Movies 1-2). This suggests PfPTP1 is associated with MC in the P falciparum–infected RBCs.
Domain structure and localization of PfPTP1 in P falciparum–infected RBCs. (A) Schematic of the PfPTP1 protein with its signal sequence in green, its PEXEL in red and the putative transmembrane domain in purple. Regions against which antibodies were raised are indicated with a black line. (B) PfPTP1 expression during the intracellular cycle (8-48 hours) in infected RBCs. (C) Localization of PfPTP1 during the intracellular cycle in immunofluorescence assay. The fixed cells were incubated with α-PTP1 antibody. Parasite nucleus was stained with DAPI. BF, bright field; ER, early ring; ET, early trophozoite; LR, late ring; LT, late trophozoite; MT, mid trophozoite, S, rupturing schizont.
Domain structure and localization of PfPTP1 in P falciparum–infected RBCs. (A) Schematic of the PfPTP1 protein with its signal sequence in green, its PEXEL in red and the putative transmembrane domain in purple. Regions against which antibodies were raised are indicated with a black line. (B) PfPTP1 expression during the intracellular cycle (8-48 hours) in infected RBCs. (C) Localization of PfPTP1 during the intracellular cycle in immunofluorescence assay. The fixed cells were incubated with α-PTP1 antibody. Parasite nucleus was stained with DAPI. BF, bright field; ER, early ring; ET, early trophozoite; LR, late ring; LT, late trophozoite; MT, mid trophozoite, S, rupturing schizont.
PfPTP1 is an integral MC membrane protein
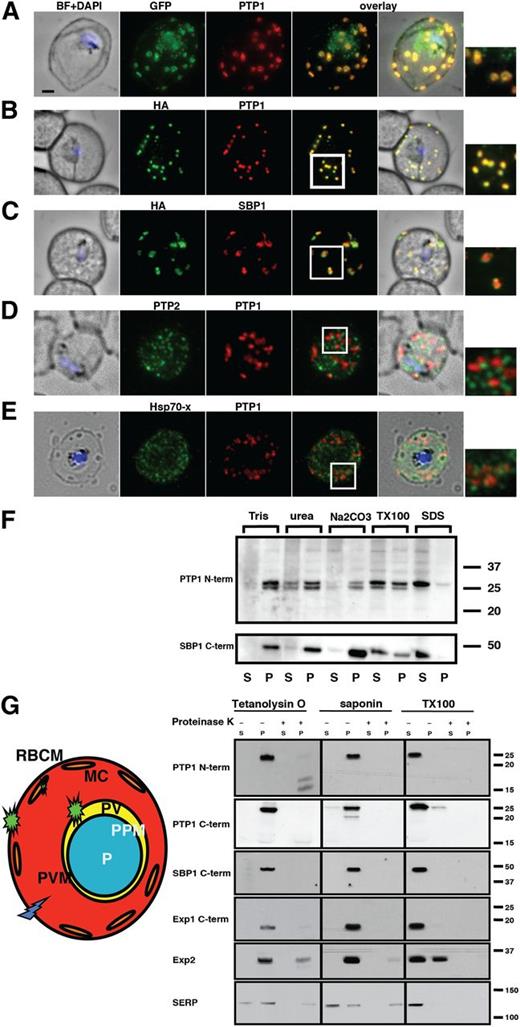
To confirm PfPTP1 localization to MC, P falciparum lines expressing PfPTP1-GFP or PfPTP1-HA (supplemental Figure 1C) were used in immunofluorescence experiments. Antibodies against PfPTP1 recognized the same structures as α-GFP and α-HA antibodies reflecting the localization of the endogenous protein (Figure 2A-B). Other antibodies raised against the C terminus of PfPTP1 showed an identical localization.
PfPTP1 is localized on Maurer's clefts in Pfalciparum–infected RBCs. (A) Immunofluorescence assay on CS2/PfPTP1-GFP cell line: the GFP signal (green) colocalizes with that of the endogenous protein (PfPTP1; red); inset: enlargement of colocalization pattern. (B) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA signal (green) colocalizes with the α-PfPTP1 signal (red); inset: enlargement of colocalization pattern. (C) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA-signal (green) colocalizes partially with the MC resident protein SBP1 (red); inset: enlargement of colocalization pattern. (D) Immunofluorescence assay on CS2 parental cell line: the PfPTP2-signal (green) does not overlap with the PfPTP1-signal (red); inset: enlargement of area where MC (PfPTP1) and electron dense vesicles (PfPTP2) are in close proximity. (E) Immunofluorescence assay on CS2 parental cell line: the PfHsp70-x-signal (green) colocalizes partially with the PfPTP1-signal (red); inset: enlargement of partial colocalization on J-dots (PfHsp70-x). Scale bar: 1μm; same size of ROI in each image. (F) Western blot of solubility study on CS2/PfPTP1-HA cell line: top panel: parasite-infected RBC samples were treated with different detergents and supernatants (S) and pellets (P) detected on a western blot with α-HA antibodies; bottom panel; blot from top panel was stripped and probed with an antibody against the MC resident transmembrane protein SBP1. The 15 kDa is non-specific since it is found in the other blots treated with α-PfPTP1 antibodies. (G) Schematic and western blot of protease protection assay on CS2/PfPTP1-HA-infected RBCs; parasite-infected RBC samples were lysed and either treated with Tetanolysin O, saponin or Triton X-100 (1%). Each of the samples was then treated with either PBS or Proteinase K. The western blot was then either probed with an antibody recognizing the N-terminal (top panel) or C-terminal part (bottom panel) of PfPTP1. SBP 1 is a MC marker with its C-terminus exposed to the RBC cytosol, EXP 1 and EXP 2 are integral membrane proteins of the PVM and SERP is a soluble marker of the PV.
PfPTP1 is localized on Maurer's clefts in Pfalciparum–infected RBCs. (A) Immunofluorescence assay on CS2/PfPTP1-GFP cell line: the GFP signal (green) colocalizes with that of the endogenous protein (PfPTP1; red); inset: enlargement of colocalization pattern. (B) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA signal (green) colocalizes with the α-PfPTP1 signal (red); inset: enlargement of colocalization pattern. (C) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA-signal (green) colocalizes partially with the MC resident protein SBP1 (red); inset: enlargement of colocalization pattern. (D) Immunofluorescence assay on CS2 parental cell line: the PfPTP2-signal (green) does not overlap with the PfPTP1-signal (red); inset: enlargement of area where MC (PfPTP1) and electron dense vesicles (PfPTP2) are in close proximity. (E) Immunofluorescence assay on CS2 parental cell line: the PfHsp70-x-signal (green) colocalizes partially with the PfPTP1-signal (red); inset: enlargement of partial colocalization on J-dots (PfHsp70-x). Scale bar: 1μm; same size of ROI in each image. (F) Western blot of solubility study on CS2/PfPTP1-HA cell line: top panel: parasite-infected RBC samples were treated with different detergents and supernatants (S) and pellets (P) detected on a western blot with α-HA antibodies; bottom panel; blot from top panel was stripped and probed with an antibody against the MC resident transmembrane protein SBP1. The 15 kDa is non-specific since it is found in the other blots treated with α-PfPTP1 antibodies. (G) Schematic and western blot of protease protection assay on CS2/PfPTP1-HA-infected RBCs; parasite-infected RBC samples were lysed and either treated with Tetanolysin O, saponin or Triton X-100 (1%). Each of the samples was then treated with either PBS or Proteinase K. The western blot was then either probed with an antibody recognizing the N-terminal (top panel) or C-terminal part (bottom panel) of PfPTP1. SBP 1 is a MC marker with its C-terminus exposed to the RBC cytosol, EXP 1 and EXP 2 are integral membrane proteins of the PVM and SERP is a soluble marker of the PV.
PfPTP1 localizes to the same structures as MC protein SBP1; however, there was not complete overlap suggesting differences within these organelles (Figure 2C). MC are composed of subcompartments and resident proteins can display different labeling patterns.29,43 Similar results were obtained for MAHRP1, a second MC protein (supplemental Figure 1A). To determine whether PfPTP1 was localized to transport vesicles within the RBC cytoplasm, we performed colocalization studies with PfPTP2, a marker for EDVs38 and PfHsp70-x, which is associated with J-dots.37 PfPTP1 did not overlap with PfPTP2 indicating it was not localized to EDVs (Figure 2D). In some instances, EDVs were closely associated with PfPTP1-labeled MC confirming a connection between these structures in transport processes. PfHsp70-x colocalized with PfPTP1 in some areas (Figure 2E). The small mobile vesicles found in PfPTP1-GFP time-lapse studies (supplemental Movies 1-2) combined with overlap of PfHsp70-x signal suggests localization of some PfPTP1 to J-dots as well as MC.
To determine whether the transmembrane region of PfPTP1 was embedded in membranes of MC, its solubility characteristics were determined. PfPTP1 was partially extractable with urea and Triton X-100 but insoluble in sodium carbonate (Figure 2F).39 PfPTP1 showed similar properties to SBP1, a known MC transmembrane protein40 (Figure 2F), suggesting PfPTP1 is a transmembrane protein embedded in MC membranes.
To determine orientation of PfPTP1 in MC, P falciparum–infected RBCs were treated with detergents and proteases (Figure 2G). Tetanolysin O forms pores in RBC membranes and exposes proteins present in infected host cells (Figure 2G).44 Saponin exposes proteins of the RBC cytoplasm, the PVM and MC (Figure 2G). Triton X-100 solubilizes all membranes and releases proteins from each compartment.40,45 Triton X-100 treatment solubilized PfPTP1 consistent with results from solubility assays (Figure 2F) and upon proteinase K treatment it was degraded and therefore accessible. In saponin-treated samples, most PfPTP1 was in the pellet but degraded by proteinase K as the MC lumen becomes accessible by this treatment. Tetanolysin O treatment renders the infected RBC cytoplasm accessible and PfPTP1 was present in the pellet. After proteinase K treatment, PfPTP1 was not detectable with αPTP1-C but a 17-kDa band was observed with α-PTP1-N antibodies. Control antibodies for MC, PVM, and PV gave the expected results (Figure 2G). Taken together, these data show the C-terminal region of PfPTP1 is exposed to the RBC cytosol whereas the N terminus faces the MC lumen.
PfPTP1 function is required for trafficking of PfEMP1 and STEVOR to MC
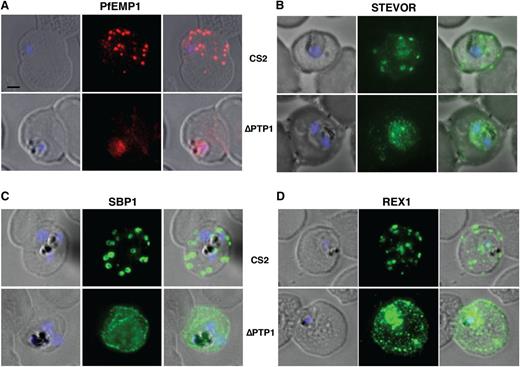
To determine whether PfPTP1 was required for subcellular localization of proteins trafficking to MC, we analyzed their subcellular localization in CS2∆PTP1.23 PfEMP1 accumulates in MC in trophozoites (Figure 3A) and then moves to the surface of parasite-infected RBCs.46 However, in CS2∆PTP1, PfEMP1 was stalled and accumulates in the parasite (Figure 3A). STEVOR, an exported protein family that traffics through MC,35 has been detected on the parasite-infected RBC surface.47 Similar to the trafficking defect observed for PfEMP1, absence of PfPTP1 function leads to accumulation of STEVOR at the PV (Figure 3B). These results show PfPTP1 is required for trafficking of PfEMP1 and STEVOR across the PVM to MC and the parasite-infected RBC surface consistent with an overlapping pathway.
Immunofluorescence assays. (A-D) Immunofluorescence assay on CS2 (top panels) vs CS2ΔPTP1 parasites (bottom panels). Immunofluorescence assay probed with α-PfEMP1 antibody (A), α-STEVOR antibody (B), α-SBP 1 antibody (C), and α-REX 1 antibody (D). Scale bar: 1 μm; same size of ROI in each image.
Immunofluorescence assays. (A-D) Immunofluorescence assay on CS2 (top panels) vs CS2ΔPTP1 parasites (bottom panels). Immunofluorescence assay probed with α-PfEMP1 antibody (A), α-STEVOR antibody (B), α-SBP 1 antibody (C), and α-REX 1 antibody (D). Scale bar: 1 μm; same size of ROI in each image.
In contrast, the MC resident protein SBP1 (Figure 3C), REX1 (Figure 3D), MAHRP 1, and Pf332, which are PNEP exported proteins (supplemental Figure 1D),48 are exported into the RBC cytoplasm but their localization was different to CS2. These proteins localize to small puncta within the RBC cytoplasm of CS2∆PTP1-infected cells instead of MC in the parental line. A similar result was observed for PEXEL-containing A-Rifin (supplemental Figure 1D), which is also an MC protein36,49 ; however, a high proportion of this protein was trapped within the parasite. The aberrant localization of MC proteins in CS2∆PfPTP1-infected RBCs shows the structure of these organelles was disturbed. We could not generate a CS2∆PfPTP1 parasite line in which the pfptp1 gene has been complemented despite several attempts. Therefore, we generated a second independent CS2∆PfPTP1 (CS2∆PfPTP1.2) parasite line (supplemental Figure 2A) and confirmed the phenotype (supplemental Figure 2B-C).23 This shows PfPTP1 function is required for export of some proteins and causes a significant disturbance of the localization of other exported proteins.
PfPTP1 function is required for MC architecture
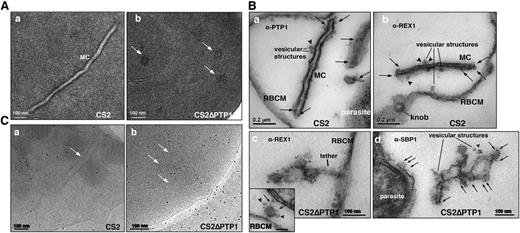
To understand the defect in MC architecture caused by loss of PfPTP1 function, the ultrastructure of CS2∆PTP1-infected RBCs was analyzed using transmission electron microscopy. In CS2, typical single lamellae for MC were observed with an electron dense coat and translucent lumen (Figure 4Aa). In contrast, CS2ΔPTP1 displayed electron dense areas randomly distributed through the RBC cytoplasm (Figure 4Ab). To identify these structures, we used equinatoxin II to selectively form pores in the RBC membrane, which left other membranes intact so hemoglobin, which obscures features in the cytoplasm, was removed.50 Antibodies to PfPTP1 and REX1 were used to identify MC in CS2. The α-PfPTP1 antibodies line the MC lamella similar to REX1 localization (Figure 4B). Vesicular structures were observed that appeared to bud off MC and these had a dense lumen and less distinct membranes. These may be vesicles involved in trafficking between PVM and MC and/or MC to the RBC membrane (ie, J-dots). These structures were decorated with PfPTP1 and REX1 antibodies (Figure 4Ba-b) consistent with PTP1-GFP fluorescence on smaller punctate structures in CS2/PTP1-GFP parasites (supplemental Movies 1-2). In CS2ΔPTP1, the architecture of MC was disrupted showing large aggregated globular structures (Figure 4Bc-d). SBP1 and REX1 were located on these structures reflecting the results obtained by immunofluorescence assay (IFA) (Figure 3). These structures contained “budding vesicular structures” that were labeled with both REX1 and SBP1 antibodies (Figure 4Bd). Therefore, loss of PfPTP1 function results in disruption of MC architecture.
Comparison of transmission electron microscopic studies on CS2 vs CS2ΔPTP1 cell line. (A) Conventional chemical fixation: (a) CS2 parental line. MC (MC) display electron dense membranes and an electron lucent lumen; (b) CS2ΔPTP1 cell line shows globular structures and no long lamellar MC structures (arrows). (B) Equinatoxin II-treated cells with pre-embedding labeling: (a) α-PfPTP1 antibodies or (b) α-REX1 antibodies label the slender MC membranes (arrows) and vesicles (arrowheads) in the CS2 parasite line; (c) α-REX1 or (d) α-SBP1 antibodies label globular structures (arrows) and vesicular structures (arrowheads) in the CS2ΔPTP1 cell line. RBCM, red blood cell membrane. (C) The frozen hydrated samples show MC (arrow) in which the lumen looks similar to the RBC cytoplasm as described earlier.61 The CS2ΔPTP1 cell line shows aggregated globular structures (arrows).
Comparison of transmission electron microscopic studies on CS2 vs CS2ΔPTP1 cell line. (A) Conventional chemical fixation: (a) CS2 parental line. MC (MC) display electron dense membranes and an electron lucent lumen; (b) CS2ΔPTP1 cell line shows globular structures and no long lamellar MC structures (arrows). (B) Equinatoxin II-treated cells with pre-embedding labeling: (a) α-PfPTP1 antibodies or (b) α-REX1 antibodies label the slender MC membranes (arrows) and vesicles (arrowheads) in the CS2 parasite line; (c) α-REX1 or (d) α-SBP1 antibodies label globular structures (arrows) and vesicular structures (arrowheads) in the CS2ΔPTP1 cell line. RBCM, red blood cell membrane. (C) The frozen hydrated samples show MC (arrow) in which the lumen looks similar to the RBC cytoplasm as described earlier.61 The CS2ΔPTP1 cell line shows aggregated globular structures (arrows).
To understand the globular structures of CS2∆PTP1 in 3-D, the parasite lines were analyzed by transmission electron tomography. Analysis of tilt series of tomograms confirmed the globular structures were in close proximity but not connected (data not shown). To exclude artifacts introduced by chemical fixation and dehydration, the cells were studied after vitrification via cryoelectron microscopy preserving the structure in a state close to live.51,52 Single lamellae of MC were observed in CS2 (Figure 4Ca) whereas globular structures were observed for CS2ΔPTP1 (Figure 4Cb). Tilt series were taken for CS2 and CS2ΔPTP1 and representative tomograms and models are shown (supplemental Figure 3A-B). Supplemental Figure 3 shows a single MC lamella vs the globular structures in CS2ΔPTP1. In the tomogram of CS2, a tethering structure was observed extending from MC toward the RBC membrane, similar to that described previously (supplemental Figure 3A, small arrowheads).31,41,53,54 Also, large globular structures with the same density were observed (supplemental Figure 3A, large arrowheads). We hypothesize these structures are transport vesicles from MC similar to the previously described EDVs.29,43
The state of actin filaments was compared between CS2ΔPTP1 and CS2-infected RBCs by iterative denoising of the tomograms. In CS2, MC linked to the RBC via actin filaments were observed consistent with a previous study.51 Foci of actin attachment were more prominent around knob structures (red; Figure 5A). In contrast, CS2ΔPTP1-infected RBCs displayed short actin filaments that did not link to the globular remnants of MC, the RBC membrane, knobs, or the cytoskeleton of the host membrane (Figure 5B). This suggested that PfPTP1 function is associated with organization of the host actin cytoskeleton.
Cryoelectron tomography depicting actin network. (A) CS2 parental line: Section through cryoelectron tomogram showing a part of an RBC infected with P falciparum (left) in a trophozoite stage, and corresponding surface rendered view (right). The insets in the middle show views extracted at different Z-planes and orientations of the tomogram, and are indicated by boxes and marked accordingly (1-4). Actin filaments are indicated with arrows. Scale bar: 100 nm. (B) CS2ΔPTP1 cell line: Section through cryo-electron tomogram showing a part of an RBC infected with P falciparum CS2ΔPTP1 (left) in a trophozoite stage, and corresponding surface rendered view (right). The inserts in the middle show views extracted at different Z-planes and orientations of the tomogram, and are indicated by boxes and marked accordingly (1-5). Actin filaments are indicated with arrows. Color code in both figures: RBC membrane (dark blue); knobs (red); vesicles (cyan); MC (cyan); filaments (yellow); indicated by arrows. *Edge of a hole in EM-grid carbon support. Scale bar: 100 nm. (C) Transmission electron micrograph image of negatively stained human actin filaments in the absence (top image) or presence (bottom image) of PTP1 C-terminal peptide. Scale bar represents 100 nm. (D) A significant difference of F-actin length polymerized in vitro is observed in the absence (green line) or presence of scrambled PfPTP1 peptide (red) vs presence of the peptide PfPTP1 (100 μM) (blue line).
Cryoelectron tomography depicting actin network. (A) CS2 parental line: Section through cryoelectron tomogram showing a part of an RBC infected with P falciparum (left) in a trophozoite stage, and corresponding surface rendered view (right). The insets in the middle show views extracted at different Z-planes and orientations of the tomogram, and are indicated by boxes and marked accordingly (1-4). Actin filaments are indicated with arrows. Scale bar: 100 nm. (B) CS2ΔPTP1 cell line: Section through cryo-electron tomogram showing a part of an RBC infected with P falciparum CS2ΔPTP1 (left) in a trophozoite stage, and corresponding surface rendered view (right). The inserts in the middle show views extracted at different Z-planes and orientations of the tomogram, and are indicated by boxes and marked accordingly (1-5). Actin filaments are indicated with arrows. Color code in both figures: RBC membrane (dark blue); knobs (red); vesicles (cyan); MC (cyan); filaments (yellow); indicated by arrows. *Edge of a hole in EM-grid carbon support. Scale bar: 100 nm. (C) Transmission electron micrograph image of negatively stained human actin filaments in the absence (top image) or presence (bottom image) of PTP1 C-terminal peptide. Scale bar represents 100 nm. (D) A significant difference of F-actin length polymerized in vitro is observed in the absence (green line) or presence of scrambled PfPTP1 peptide (red) vs presence of the peptide PfPTP1 (100 μM) (blue line).
To determine whether PfPTP1 interacts with host cell actin, we used in vitro actin polymerization assays. This showed no difference in polymerization kinetics if actin was incubated with a peptide from the C terminus of PfPTP1 (domain in the RBC cytoplasm) or a scrambled peptide (Figure 5D). However, a larger number of longer actin filaments were observed in the presence of the PfPTP1 peptide (Figure 5C, supplemental Figure 4). This reflects the cryo-EM data (Figure 5A-B) and suggests PfPTP1 plays a role in organization of host actin filaments, which is required for architecture of MC.
PfPTP1 interacts with PfEMP1 and MC resident proteins
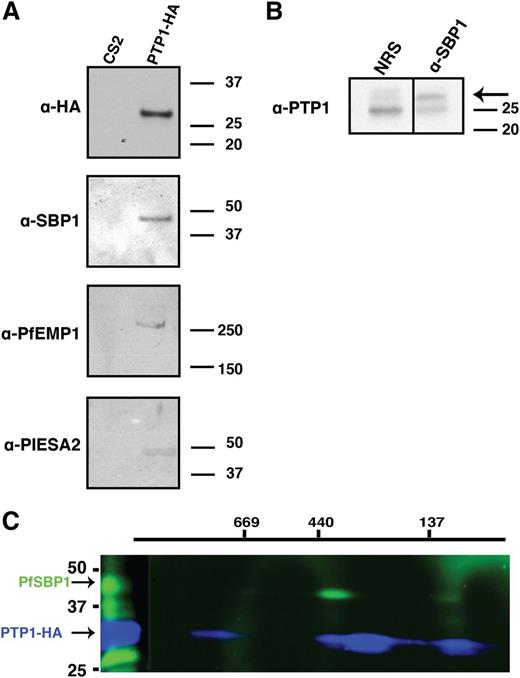
For PfPTP1 to perform its function in MC, it likely interacts with other proteins; to identify potential interacting partners, immunoprecipitation experiments were performed. CS2/PTP1-HA– and CS2-infected RBCs were solubilized and PfPTP1-HA immunoprecipitated with α-HA-antibody beads (supplemental Figure 5A-C). Specific protein bands immunoprecipitated from CS2∆PTP1-HA parasite line and corresponding regions from the CS2 lane were excised from the SDS-PAGE gel and subjected to mass spectrometry (supplemental Figure 5B; supplemental Table 1). Supplemental Table 2 shows specific proteins with a PEXEL motif or known exported proteins identified by mass spectrometry with >2 peptides and/or >5% coverage. Interestingly, exported proteins SBP1, PFE0060w (PIESP2), PFA0670c (hyp8), PF13_0076, and PFE1600w (PHISTb) were immunoprecipitated (supplemental Table 2). Fifteen peptides corresponded to VAR2CSA, the PfEMP1 variant expressed in CS2. This suggests PfPTP1 may interact directly with PfEMP1, or in a large complex that includes these proteins, to play a role in displaying this virulence protein on the surface of P falciparum–infected RBCs.
To confirm the proteins binding to PfPTP1 immunoprecipitations were performed and probed with α-SBP1, α-PfEMP1, and α-PIESA2 antibodies. This suggested binding for these proteins (Figure 6A), whereas, in comparison, other MC proteins such as MAHRP1 and REX1 showed no detectable PfPTP1 binding (data not shown). In reciprocal experiments endogenous untagged PfPTP1 was immunoprecipitated from CS2-infected RBCs with α-SBP1 antibodies confirming this interaction (Figure 6B). However, we could not detect bands in the reciprocal experiments with α-PfEMP1 (against the acidic terminal segment [ATS] region of the protein) and α-PIESA2. This might be due to masking of the PfPTP1-interacting region of the 2 proteins by the antibodies under the experimental conditions.
Interaction partners of PfPTP1. (A) Affinity purification of interacting partners on CS2/PTP1-HA cell line with α-HA coupled beads. Eluted fractions were subjected to western blot analysis and blot was probed with α-HA antibody (top panel); α-SBP1 antibody (second panel); α-PfEMP1 antibody (third panel) and α-PIESA2 (bottom panel). (B) Reciprocal immunoprecipitation of interacting partners of PfPTP1 in CS2 parental line with α-SBP1 antibody or rabbit normal serum (NRS; negative control). The western blot was probed with α-PfPTP1 antibodies. The arrow points to the specific band for PfPTP1. The 25 kDa band represents the light chain of the SBP1 antibody. (C) Blue native PAGE on CS2/PTP1-HA cell line. The first dimension was run under native conditions, the strip then excised and run in the second dimension under denaturing conditions to resolve potential complexes. The blot was first probed with α-SBP1 antibody and HRP-conjugated secondary antibody, then stripped and probed with α-HA antibody and specific complexes detected via electrochemiluminescent reagents and x-ray film.
Interaction partners of PfPTP1. (A) Affinity purification of interacting partners on CS2/PTP1-HA cell line with α-HA coupled beads. Eluted fractions were subjected to western blot analysis and blot was probed with α-HA antibody (top panel); α-SBP1 antibody (second panel); α-PfEMP1 antibody (third panel) and α-PIESA2 (bottom panel). (B) Reciprocal immunoprecipitation of interacting partners of PfPTP1 in CS2 parental line with α-SBP1 antibody or rabbit normal serum (NRS; negative control). The western blot was probed with α-PfPTP1 antibodies. The arrow points to the specific band for PfPTP1. The 25 kDa band represents the light chain of the SBP1 antibody. (C) Blue native PAGE on CS2/PTP1-HA cell line. The first dimension was run under native conditions, the strip then excised and run in the second dimension under denaturing conditions to resolve potential complexes. The blot was first probed with α-SBP1 antibody and HRP-conjugated secondary antibody, then stripped and probed with α-HA antibody and specific complexes detected via electrochemiluminescent reagents and x-ray film.
To determine the PfPTP1 complex size, 2-dimensional blue native gel analysis was used to separate proteins under native conditions in the first and under reducing conditions in the second dimension.55 A complex of ∼420 kDa was detected containing PfPTP1 (Figure 6C blue band) and SBP1 (Figure 6C green band). Furthermore, a smaller complex of ∼130 kDa was also detected that included PfPTP1 and SBP1. This indicated PfPTP1 functions as a high-molecular-weight complex with SBP1 that likely includes additional P falciparum proteins.
Discussion
MC are essential trafficking nodes for proteins conferring cytoadherence of parasite-infected RBCs to microvasculature in the host.8,27,31 In particular, they are important for assembly of PfEMP1 into a cytoadherence complex for insertion into P falciparum–infected RBC membranes.6,8,27,31 A number of proteins resident in MCs have been identified required for PfEMP1 trafficking but their functions are unknown.20,21,23 In this study, we show PfPTP1 is required for trafficking of PfEMP1 to the P falciparum–infected RBC surface and MC architecture. PfPTP1 functions in a large complex that includes at least SBP1 and is the first protein shown to have a role in linking MC to the host actin cytoskeleton.
PfPTP1 is exported and inserted into MC membranes with the N terminus located within the lumen and the short cytoplasmic tail protruding into the cytosol of P falciparum–infected RBCs adopting the same orientation as other MC-associated proteins, MAHRP1 and SBP1.27,40,43 These PNEPs are required for trafficking of PfEMP1 to the surface of the infected RBCs; however, their role is not known.19,21,23 PfPTP1 is required for this same process; however, it is a PEXEL-containing protein. In the absence of PfPTP1 function, PfEMP1 remained associated with the parasite indicating its function is required for transfer of PfEMP1 across the PVM to MC. Although PfPTP1 showed colocalization with SBP1 and MAHRP1 on MC, there were areas of nonoverlap consistent with their structural and functional compartmentalization.56 PfPTP1 was observed on small vesicles in close contact with MC lamellae. These structures were evident in absence of PfPTP1 and therefore its function is not required for their formation. They may be related vesicular structures called J-dots observed in the cytosol of parasite-infected RBCs37 and the partial colocalization of PfPTP1 with PfHsp70-x supports this notion. J-dots may be chaperone complex transport vehicles and their membrane characteristics are different from MC.57 It was suggested J-dots could be protein-cholesterol aggregates or vesicular structures of an unusual lipid composition.30 This may explain absence of a visible membrane in our EM studies around vesicular structures we observed. PfPTP1 and SBP1 occur on these structures and it has been shown that PfEMP1 is found on structures with very similar characteristics.23,26
Models have been suggested for trafficking PfEMP1 to MC and its final destination on the P falciparum–infected RBC surface including: (1) loading of PfEMP1 onto nascent MC as they bud from the PVM, (2) transport in vesicles to MC,24 (3) transport as soluble complexes that insert into fully formed MC.10,29 Recent studies show MC bud from the PVM and establish in the RBC cytoplasm after invasion before expression of proteins normally resident in or traveling through these organelles, including PfEMP1.5,31 Therefore, MC-associated proteins translated later have to make their way across the PVM and RBC cytosol to reach these organelles, arguing against the first model.
Here, we demonstrate that in the absence of PfPTP1 function, PfEMP1 is stalled before the PVM. The presence of PfPTP1 as part of a large complex associated with SBP1 is consistent with them functioning together in trafficking of PfEMP1 to MC. Complex formation explains why lack of SBP1 function also leads to loss of PfEMP1 on the RBC surface.19,20 The presence of mobile vesicular structures containing PfPTP1-GFP in the RBC cytosol supports a hybrid model of the latter 2 listed above where PfEMP1 traffics to MC in a complex associated with vesicular structures like J-dots.30 We hypothesize the complex forms upon translocation of the involved molecules across the PVM or even within certain compartments of the PV and provide a shuttling modality for PfEMP1 to reach MC. A recent report showed PfEMP1 accumulates in membranous structures in the PV in ring stages before translocation across the PVM.31 These regions may contain docking signals for proteins to travel as complexes even if they have different expression profiles. The overlapping localization of SBP1 and PfPTP1 on MC may be docking points for the complex onto these organelles. The additional presence of SBP1 and PfPTP1 in separate compartments on MC points toward further functions for these proteins.
The MC resident proteins studied (SBP1, MAHRP1, Pf332, A-Rifin, and REX1) are trafficked to the remnant MC structures in the absence of PfPTP1 function. This suggests PfPTP1 is not required for trafficking of these proteins. Interestingly, STEVOR cannot be trafficked to MC in CS2∆PTP1-infected RBCs similar to PfEMP1. STEVORs are PEXEL-containing proteins, which are processed by plasmepsin V, whereas PfEMP1 is a PNEP and not cleaved by this enzyme.58 In contrast to MC proteins, which are trafficked across the PVM in absence of PfPTP1, the final destination for STEVORs and PfEMP1 is the RBC surface. Therefore, although these proteins might be processed differently in their early trafficking pathway they share trafficking modalities once reaching the PVM. We could not identify STEVOR as one of the interacting partners of PfPTP1 so its exact transport across the RBC remains unknown.
A recent study involving cryo-EM analysis showed the parasite mines the human actin filament network to establish an efficient transport system within the cytosol of its host.51 It was demonstrated that in RBCs from patients with hemoglobinopathies the actin network was rudimentary, and MC, which in normal RBCs were hinged on the actin network, are no longer stretched lamellae but large globular structures. It was concluded that MC are linked to the RBCs via actin filaments from the trophozoite stage onwards and transport vesicles are shuffled along these actin filaments to deliver cargo to RBC membranes. We have demonstrated in absence of PfPTP1 function, MC are reduced to globular structures and the actin network was not intact with fewer long filaments. Our interpretation is that the peptide does not change the total amount of actin filaments, but influences actin filament length or organization, possibly due to actin filament stabilization of the PfPTP1 peptide. An in vitro actin polymerization assay confirmed that although the presence of the C terminus of PfPTP1 does not change the total amount of rabbit actin filaments, actin forms longer filaments than in the presence of PTP1-scrambled/no peptide. Interestingly, we readily observed vesicular structures in close proximity to remnant MC in CS2ΔPTP1-infected RBCs; however, if these structures were budding off the MC they would not be able to effectively transport any proteins to the RBC membrane because the extended actin filaments spanning the distance between MC and the RBC membrane were aberrant. Therefore, our data suggest that in addition to transport of PfEMP1 to MC, PfPTP1 functions in physically linking MC to the host actin skeleton and that in its absence these organelles lose their normal architecture. Previous data has suggested that SBP1 binds actin and the direct link for interaction of the detected higher order complex may be required for F-actin nucleation at MC.59
This is the first report where both tethers and actin filaments have been found in the same EM preparation (cryo-EM). Both structures play a role in docking MC to the RBC membrane.31,41,53,60 However, only the actin network seems to be disturbed in the absence of PfPTP1 whereas the tethers are still present. Therefore, the initiation of the docking process takes place but it may not fulfill trafficking requirements for proteins destined for the RBC surface like STEVORs and PfEMP1.
In summary, we have shown that PfPTP1 plays a critical role in MC architecture and trafficking of PfEMP1 and STEVOR. PfPTP1 forms a complex with SBP1 (and possibly PfEMP1) and these interact with other exported proteins to form a large complex involved in trafficking to and/or from the MC membrane. Furthermore, the actin network linking MC and transport vesicles for transport of proteins to the RBC membrane was disrupted in the absence of PfPTP1. Therefore, PfPTP1 is a key protein required for the export of the cytoadherence complex of P falciparum–infected RBCs.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Red Cross Blood Service (Melbourne, Australia) for supply of blood. The authors also thank Simon Crawford for technical advice and Matthew Dixon, Peter Beck, Peter Preiser, Michaela Petter, Klaus Lingelbach, Catherine Braun-Breton, and Michael Ryan for antibodies. EMBL Heidelberg (Germany) is acknowledged for access to the FEI Polara microscope.
This work was supported by the National Health and Medical Research Council (NHMRC; grant numbers: 1011453 and 637406), Victorian State Government Operational Infrastructure Support, and Australian Government NHMRC IRIISS. M.C. was supported by a Bill and Melinda Gates grant (Grand Challenges Explorations: OPP1069409); L.L. by a postdoctoral fellowship from the University of Heidelberg Cluster of Excellence CellNetworks; F.F. by the Chica and Heinz Schaller Foundation; and S.K. by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft.
A.G.M. is an Australian Research Fellow supported by the Australian Research Council and A.F.C. is a Howard Hughes International Scholar.
Authorship
Contribution: M.R. performed parasite culturing, transfection, light and electron microscopy experiments, orientation, solubility, and pulldown studies; M.C. and L.L. performed cryoelectron tomography and in vitro actin assays and analyzed the data; A.M. performed blue native gel experiments; S.K. performed orientation assays; C.P.S. performed actin in vitro assays; J.T. generated the independent knock-out cell line; E.H. performed electron tomography; M.O. and C.L. performed transfections; M.L. and F.F. analyzed and interpreted in vitro actin assays; M.R., A.F.C., and A.G.M. designed and interpreted experiments; M.R. and A.F.C. wrote the manuscript; and all other authors contributed to the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan F. Cowman, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3052, Australia; e-mail: cowman@wehi.edu.au; and Melanie Rug, Centre for Advanced Microscopy, The Australian National University, Canberra 0200, Australia; e-mail: melanie.rug@anu.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal