Abstract
Introduction: Despite 40 years of intense clinical research, there remain no new approved treatments or standard of care for patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). New safe and effective salvage treatments are urgently needed. Vosaroxin is a first-in-class anticancer quinolone derivative that is active in AML. Vosaroxin is minimally metabolized, evades P glycoprotein receptormediated efflux and has activity independent of p53 status. VALOR is a rigorously designed and conducted phase 3, adaptive design, randomized, double-blind, placebo-controlled trial evaluating vosaroxin plus cytarabine (vos/cyt) vs placebo plus cytarabine (pla/cyt) in patients with R/R AML (NCT01191801).
Methods: Patients were randomized 1:1 to receive cytarabine (1 g/m2 IV over 2 hr, d 1-5) plus either vosaroxin (90 mg/m2 IV over 10 min d 1 and 4; 70 mg/m2 in subsequent cycles) or placebo. Up to 2 induction and 2 consolidation cycles were administered. Eligible patients had refractory disease (persistent disease after induction, or first complete remission [CR1] < 90 d) or were in first relapse (early relapse: CR1 of 90 d to 12 mo; late relapse: CR1 of 12 mo to 24 mo). Patients had received 1-2 cycles of prior induction chemotherapy including at least 1 cycle of anthracycline (or anthracenedione) and cytarabine. Randomization was stratified by disease status (refractory, early relapse, late relapse), age (< 60, ≥ 60 years), and geographic location (US, non-US). Primary efficacy and safety endpoints were overall survival (OS) and 30- and 60-day mortality; secondary endpoints were complete remission (CR) rate and incidence of adverse events (AEs).
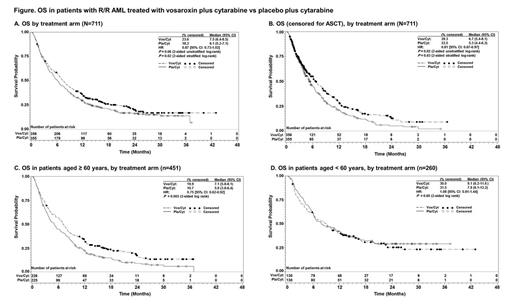
Results: Between Dec 2010 and Sept 2013, 711 patients were randomized to receive vos/cyt (n = 356) or pla/cyt (n = 355) at 124 sites; per the adaptive design, a prespecified 1-time sample size increase of 225 patients was implemented after the interim analysis. At the final analysis, median OS was 7.5 mo (95% CI: 6.4-8.5) with vos/cyt vs 6.1 mo (95% CI: 5.2-7.1) with pla/cyt (HR = 0.866 [95% CI: 0.73-1.02]; 2-sided unstratified log-rank P = 0.06) (Figure). The OS difference was statistically significant in a preplanned analysis accounting for the stratification factors at randomization (2-sided stratified log-rank P = 0.02).
Overall, 29.5% of patients underwent allogeneic stem cell transplant (ASCT), including 45.8% of patients < 60 years and 20.2% of patients ≥ 60 years. Transplant rates were comparable between the 2 treatment arms (30.1% with vos/cyt and 29.0% with pla/cyt). In a predefined analysis censoring for subsequent ASCT, median OS was improved with vos/cyt (6.7 mo vs 5.3 mo with pla/cyt; HR = 0.81 [95% CI: 0.67-0.97]; P = 0.02; stratified P = 0.03) (Figure). In predefined subgroup analyses, OS benefit was greatest in patients aged ≥ 60 years (7.1 mo with vos/cyt vs 5.0 mo with pla/cyt; HR = 0.75; P = 0.003) (Figure) and those with early relapse (6.7 mo vs 5.2 mo; HR = 0.77; P = 0.04). OS with vos/cyt vs pla/cyt was 9.1 mo vs 7.9 mo in patients < 60 years (HR = 1.08; P = 0.60); 6.7 mo vs 5.0 mo in patients with refractory disease (HR = 0.87; P = 0.23); and 14.1 mo vs 12.3 mo in patients with late relapse (HR = 0.98; P = 0.96), respectively. A CR was achieved in 30.1% of patients treated with vos/cyt vs 16.3% treated with pla/cyt (P = 0.00001).
Thirty-day and 60-day all-cause mortality was similar in the 2 arms (30-day: 7.9% vs 6.6%; 60-day: 19.7% vs 19.4% with vos/cyt vs pla/cyt, respectively). Most common serious AEs were febrile neutropenia (11.3% with vos/cyt vs 7.4% with pla/cyt), sepsis (8.7% vs 4.3%), pneumonia (7.6% vs 4.9%), bacteremia (8.5% vs 2.9%), and stomatitis (3.4% vs 1.4%). Serious and non-serious cardiac, renal, neurologic, and hepatic AEs were comparable between treatment groups.
Conclusion: Vos/cyt demonstrated improved OS and higher CR rates in patients with R/R AML without increased early mortality. In the primary OS analysis, the overall clinical benefit associated with vosaroxin may be underestimated, particularly in younger patients, due to the confounding effect of high transplant rates, a methodological limitation of AML trials. Vosaroxin-containing therapy had acceptable tolerability. VALOR results represent one of the largest datasets available in this setting, and the OS benefit was confirmed by a robust sensitivity analysis. These data support the use of this combination as a new option for salvage therapy in patients with R/R AML.
Ravandi:Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sayar:Sunesis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Strickland:Sunesis: Membership on an entity's Board of Directors or advisory committees. Schiller:Sunesis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Erba:Sunesis: Consultancy; Seattle Genetics: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Celgene: Consultancy; Amgen: Consultancy. Pigneux:Sunesis: Consultancy. Horst:Sunesis: Research Funding. Recher:Sunesis: Consultancy; Celgene: Consultancy, Research Funding; Chugai: Research Funding. Klimek:Sunesis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Craig:Sunesis: Equity Ownership. Fox:Sunesis: Consultancy, Equity Ownership. Ward:Sunesis: Employment, Equity Ownership. Smith:Sunesis: Employment, Equity Ownership. Acton:Sunesis: Consultancy. Mehta:Sunesis: Consultancy. Stuart:Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal