Abstract
Background: Patients (pts) with relapsed/refractory AML have dismal outcomes with currently available chemotherapy regimens. Preclinical and clinical evidence suggests that the use of hypomethylating agents such as decitabine prior to standard chemotherapy may be synergistic (Qin et al. Clin Can Res 2007; Scandura et al. Blood 2011; Clozel et al. Cancer Discov 2013).
Methods: Pts aged ≥18 to 65 years with relapsed or refractory AML (up to salvage 2) were eligible. Prior therapy with hypomethylating agents was allowed. The chemotherapy regimen was as follows:
| . | Dose . | Days . |
|---|---|---|
| INDUCTION | ||
| Decitabine | 20 mg/m2 IV | Days 1-5 |
| Clofarabine | 15 mg/m2 IV | Days 6-9 (Dose level 1) Days 6-10 (Dose level 2) |
| Idarubicin | 10 mg/m2 IV | Days 6-8 |
| Cytarabine | 1 gm/m2 IV | Days 6-10 |
| CONSOLIDATION | ||
| Decitabine | 20 mg/m2 IV | Days 1-5 |
| Clofarabine | 15 mg/m2 IV | Days 6-8 |
| Idarubicin | 8 mg/m2 IV | Days 6-7 |
| Cytarabine | 1 gm/m2 IV | Days 6-8 |
| . | Dose . | Days . |
|---|---|---|
| INDUCTION | ||
| Decitabine | 20 mg/m2 IV | Days 1-5 |
| Clofarabine | 15 mg/m2 IV | Days 6-9 (Dose level 1) Days 6-10 (Dose level 2) |
| Idarubicin | 10 mg/m2 IV | Days 6-8 |
| Cytarabine | 1 gm/m2 IV | Days 6-10 |
| CONSOLIDATION | ||
| Decitabine | 20 mg/m2 IV | Days 1-5 |
| Clofarabine | 15 mg/m2 IV | Days 6-8 |
| Idarubicin | 8 mg/m2 IV | Days 6-7 |
| Cytarabine | 1 gm/m2 IV | Days 6-8 |
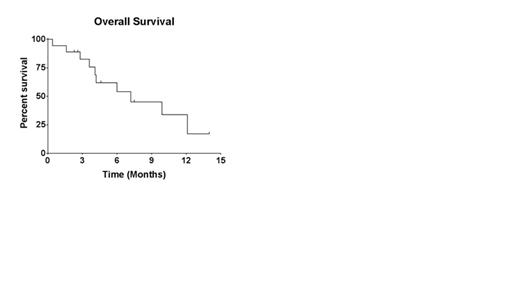
Results: Eighteenpts (female, n=4) have been treated so far. Median age was 43 years (range 20-61). Majority of the pts (10/18, 56%) were in salvage 2. Four had a prior allogeneic stem cell transplant (SCT). Cytogenetics were diploid (n=4), complex (n=11), 11q abnormality (n=2), trisomy 8 (n=1). Five pts had a TP53 mutation and 3 pts had an NRAS mutation. Median number of cycles administered was 1 (range 1-2). Grade 3-4 non-hematological toxicity included grade 3 mucositis (DLT) in 2 pts during the induction cycle. Due to mucositis and prolonged cytopenias (prolonged cytopenias didn’t meet the DLT definition), dose level 2 was not pursued, and all patients were treated at dose level 1. Grade 3 transaminitis was seen in 4 pts. Fifteen pts had one or more infections. Six (33%) pts achieved CR, of whom 5 were able to proceed to SCT (one pt with CR opted not to pursue SCT). Median time to CR was 35 days. An additional 3 pts had a >50% decrease in bone marrow blast count (marrow response) and all three proceeded with SCT. All 4 patients with diploid cytogenetics achieved CR. Only 1/5 (20%) patients with mutated TP53 achieved a CR. Two patients have relapsed (one 3 months after SCT and the other after consolidation cycle 1). Six patients are alive (4 after stem cell transplant, 2 are receiving further salvage regimens after DAC-CIA failure). Median survival of the entire group is 7.2 months (see figure 1).
Conclusions: The sequential treatment of decitabine followed by chemotherapy is safe and effective with a CR rate of 33% and with 8/18 patients able to proceed to an allogeneic SCT. The phase II part of the study is enrolling patients at this time.
Off Label Use: Decitabine and clofarabine are not approved for AML..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal