Abstract
Introduction: FMS- like tyrosine kinase gene 3 (FLT3) internal tandem duplications (ITD) are associated with poor outcome in AML. They are less frequent in MDS, and their prognostic impact is not clear (Daver et al. Am J Hematolo. 2013; 88 (1): 56-9). Although RAS (NRAS and KRAS) mutations occur at higher frequency, their presence is not associated with outcome in MDS. Several studies have reported the dynamic acquisition and prognostic value of FLT3-ITD and RAS mutations (FLT3-ITDm and RASm) in patients with MDS (Takahashi et al. Leukemia. 2013; 27 (10): 2081-3). We further studied the acquisition FLT3-ITDm and/or RASm at the time of transformation to AML in a larger group of patients and assessed its impact on survival.
Method: We retrospectively analyzed 289 MDS patients who had wild type (wt) FLT3- ITD and RAS at diagnosis and were referred to our institution between 2002 and 2013. Among them, 102 (35%) patients had repeat mutation testing at the time of transformation to AML. We evaluated these patients for the frequency of FLT3-ITDm and/or RASm acquisition at transformation and its clinical implications.
Result: Twenty nine (28%) out of 102 patients acquired FLT3- ITDm or RASm at the time of transformation to AML (23 [79%] RASm and 6 [21%] FLT3- ITDm), but none of the patients acquired both mutations.
The median age for our patients was 65 years (range, 40-83). Three (11%), 13 (46%), 11 (39%), and 2 (7%) patients had IPSS low, int-1, int-2, and high-risk disease, respectively. Eleven (38%) patients had complex cytogenetics (CG), including 8 (28%) diploid and 4 (14%) -7/7q- , and 5 (17%) patients had other CG abnormalities. Eight (28%) patients had therapy-related MDS.
Fifteen (52%) patients received hypomethylating agents (HMA) alone as initial therapy for MDS, with 3 (10%) patients receiving lenalidomide, 3 (10%) HMA and investigational agents, and 3 (10%) pts received growth factors. Five (17%) patients did not receive any therapy for MDS. Four (14%) patients had stem cell transplant (SCT) at MDS diagnosis. The median number of therapies patients received for MDS was 1(range, 0-3), and 6 (21%) patients received more than 2 therapies for MDS.
At the time of transformation to AML in patients who acquired FLT3-ITDm or RASm, 8 (28%) patients had CG, including 7 (24%) diploid, 3 (10%) -7/7q- , and 4 (14%) trisomy 8, and 6 (21%) patients had other CG abnormalities. Seven (24%) patients received cytarabine + nucleoside analogues (clofarabine/cladarabine/fludarabine) as induction therapy for AML, 7 (24%) idarubicin + cytarabine (IA), 6 (21%), HMA with or without investigational agents, 5 (17%) investigational agents alone, and 4 (14%) patients received cytarabine or the IA regimen with or without investigational agents as induction therapy for AML. The median number of therapies patients received for AML was 1 (range, 1-5). Six (21%) patients had stem cell transplant (SCT) during the course of their AML therapy.
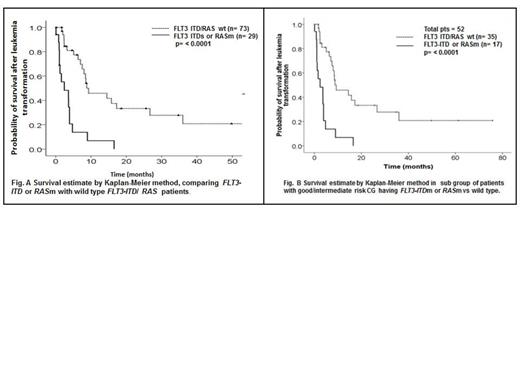
The median survival after leukemia transformation in patients with FLT3-ITDm and/or RASm was 2.43 months compared to 7.5 months in FLT3-ITD/RAS wt patients (p= <0.0001) (Fig. 1A). The median overall survival (OS) was 24 months, with no significant difference between groups (25.6 months for FLT3-ITDm and/or RASm patients and 23.7 months for FLT3-ITD/RAS wt; p= 0.93). Patients > 60 years old had survival time of 4.7 months after AML transformation compared to 7.3 months in patients < 60 years old (p= 0.26). Patients with poor-risk CG at AML transformation (according to IPSS) had a median survival of 4.6 months compared to 7.5 months for patients with good/intermediate risk CG (p= 0.023).
On multivariable analysis, FLT3-ITDm/RASm and poor-risk CG retained independent prognostic significance (hazard ratio [HR] 3.11, p= <0.0001, and HR 1.81, p= 0.014, respectively). In subset analysis of 52 (51%) patients with good/intermediate risk CG, 17 (33%) patients acquired mutations (12 [71%] RASm and 5 [29%] FLT3-ITDm). The median survival in FLT3-ITDm/RASm patients was 2.4 months compared to 9.3 months in wt patients (p= ≤ 0.0001) (Fig.1B).
Conclusion: The temporal acquisition of FLT3-ITD or RAS mutations at transformation to AML is associated with poorer outcome. This occurs in close to a third of patients at the time of transformation and may provide opportunities for targeted therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal