Abstract
Background: Intravenous immunoglobulin (IVIG) has been shown to reduce neutrophil adhesion to post capillary venular endothelium, reduce adherent neutrophil interactions with circulating red blood cells (RBCs), increase microcirculatory blood flow, and increase survival in murine models of sickle cell acute pain crisis in a dose dependent manner. We report results of a phase I, double blind, placebo controlled, randomized, dose escalation study of Gamunex brand of IVIG administered to pediatric and adult sickle cell disease (SCD) patients during acute pain events.
Methods: Patients received a single, one-time dose of IVIG (Gamunex, Talecris Biotherapeutics/ Grifols) or equivalent volume normal saline placebo in addition to standard care. A dose-escalation strategy was used, with 4 patients (3 treatment, 1 control) randomized at each dose level of 100 mg/kg, 200 mg/kg, 400 mg/kg, 600mg/kg and 800 mg/kg. Primary endpoints were drug safety and time to end of vaso-occlusive crisis (VOC). The activation status of neutrophils was examined before and 24 hours after infusion.
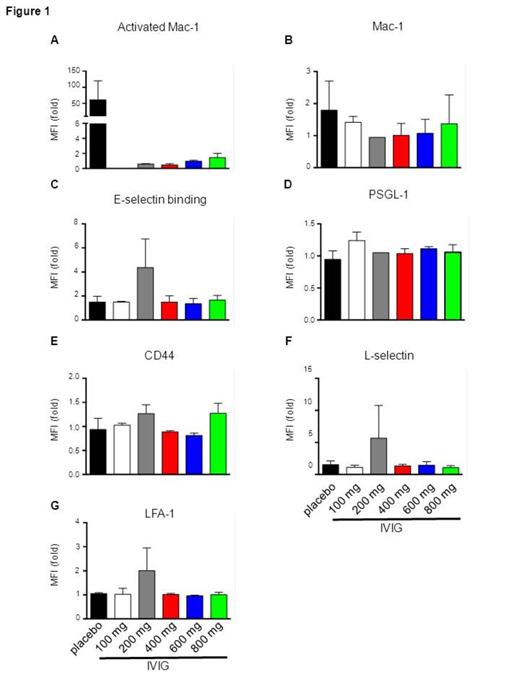
Results: There were no Grade 4-5 adverse events and no Grade 3 adverse events likely to be IVIG-related (Table 1). As expected in a Phase I study, numbers of patients were insufficient to assess clinical efficacy (Table 2). There were smaller increases in LDH and CRP in the IVIG group, suggesting a potential salutary effect on hemolysis and inflammation. Although significance was not reached, IVIG treatment was associated with down-regulated activated Mac-1 (Figure 1A) that was greater than down-regulation of the total amount of Mac-1 (Figure 1B), consistent with murine data on IVIG’s effect on RBC-neutrophil interactions mediated by Mac-1. E-selectin binding (Figure 1C), which mediates neutrophil recruitment/rolling, was not consistently decreased in the IVIG-treated group. These results are consistent with our previous observations in the murine model, where no down regulation of E-selectin binding, PSGL-1, or LFA-1 was observed after IVIG administration despite its effect on neutrophil adhesion and RBC interactions. IVIG inhibits Mac-1 activation through an independent antagonistic pathway involving Fc gammaRIII-mediated recruitment of Src-homology 2-containing tyrosine phosphatase-1.
Conclusions: Our studies demonstrate that IVIG is well tolerated in SCD patients during acute VOC and provide proof of concept that the mechanism of action of IVIG in human sickle cell pain crisis indeed proceeds via the down-regulation of activated Mac-1 as observed in the murine model. We propose to conduct a phase II study to further evaluate these findings.
Safety Outcomes
| . | IVIG (n=15) . | Control (n=5) . | p-value . |
|---|---|---|---|
| Pre-specified serious adverse events | |||
| Acute chest syndrome (%) | 7 | 0 | 1.0 |
| Thrombosis (%) | 7 | 0 | 1.0 |
| Other pre-specified adverse events | |||
| Pain crisis re-admission (%) | 40 | 20 | 0.63 |
| Red cell transfusion (%) | 13 | 40 | 0.25 |
| 24 h serum creatinine | 0.5 (0.2-0.6) | 0.6 (0.4-0.8) | 0.28 |
| Possibly IVIG-related Grade 1-2 adverse events | |||
| Fever (%) | 20 | 40 | 0.56 |
| Hypertension (%) | 20 | 20 | 1.00 |
| Headache (%) | 27 | 0 | 0.53 |
| . | IVIG (n=15) . | Control (n=5) . | p-value . |
|---|---|---|---|
| Pre-specified serious adverse events | |||
| Acute chest syndrome (%) | 7 | 0 | 1.0 |
| Thrombosis (%) | 7 | 0 | 1.0 |
| Other pre-specified adverse events | |||
| Pain crisis re-admission (%) | 40 | 20 | 0.63 |
| Red cell transfusion (%) | 13 | 40 | 0.25 |
| 24 h serum creatinine | 0.5 (0.2-0.6) | 0.6 (0.4-0.8) | 0.28 |
| Possibly IVIG-related Grade 1-2 adverse events | |||
| Fever (%) | 20 | 40 | 0.56 |
| Hypertension (%) | 20 | 20 | 1.00 |
| Headache (%) | 27 | 0 | 0.53 |
Efficacy Outcomes
| . | IVIG (n=15) . | Control (n=5) . | p-value . |
|---|---|---|---|
| Clinical endpoint outcomes | |||
| Time to crisis resolution* (h) | 54 (1,215) | 63 (31,164) | 0.61 |
| Cumulative opioid use* (ME/kg) | 2.2 (0,8.8) | 1.6 (1.1,15.6) | 1.0 |
| Time to hospital discharge (days) | 4.4 (1,10) | 4.8 (3,5) | 0.67 |
| Clinical laboratory indicators | |||
| % Δ in hemoglobin (Hb)# | -17 (-33,0) | -17 (-25, -3) | 0.76 |
| % Δ in lactate dehydrogenase (LDH)# | 4 (-48, 85) | 14 (-11, 679) | 0.46 |
| % Δ in C-reactive protein (CRP)# | 38 (-16, 868) | 192 (1.5, 1427) | 0.43 |
| % Δ in white blood cell count (WBC) | -15 (-48,13) | -22 (-41, 37) | 0.96 |
| % Δ in absolute neutrophil count (ANC) | -18 (-65, 45) | -16 (-51,49) | 0.81 |
| . | IVIG (n=15) . | Control (n=5) . | p-value . |
|---|---|---|---|
| Clinical endpoint outcomes | |||
| Time to crisis resolution* (h) | 54 (1,215) | 63 (31,164) | 0.61 |
| Cumulative opioid use* (ME/kg) | 2.2 (0,8.8) | 1.6 (1.1,15.6) | 1.0 |
| Time to hospital discharge (days) | 4.4 (1,10) | 4.8 (3,5) | 0.67 |
| Clinical laboratory indicators | |||
| % Δ in hemoglobin (Hb)# | -17 (-33,0) | -17 (-25, -3) | 0.76 |
| % Δ in lactate dehydrogenase (LDH)# | 4 (-48, 85) | 14 (-11, 679) | 0.46 |
| % Δ in C-reactive protein (CRP)# | 38 (-16, 868) | 192 (1.5, 1427) | 0.43 |
| % Δ in white blood cell count (WBC) | -15 (-48,13) | -22 (-41, 37) | 0.96 |
| % Δ in absolute neutrophil count (ANC) | -18 (-65, 45) | -16 (-51,49) | 0.81 |
Flow-cytometric analysis of surface expression of (A) activated Mac-1; (B) Mac-1; (C) E-selectin binding; (D) PSGL-1; (E) CD44; (F) L-selectin; (G) LFA-1 on CD16b-positive leukocytes. MFI, mean fluorescence intensity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal