Abstract
Introduction: Sickle Cell Disease (SCD) is an inherited blood disorder, afflicting 1 in 400 African Americans, clinically characterized by hemolytic anemia, painful vaso-occlusive crises and endothelial dysfunction resulting in chronic organ damage.1 Hydroxyurea (HU) is a myelosuppressive agent that has been shown to reduce the number of vaso-occlusive crises, acute chest syndromes, blood transfusions and hospital admissions and is currently being investigated as an alternative to transfusion therapy for primary stroke prevention.2,3 HU's protective properties are primarily thought to be a result of its ability to increase HbF levels, which reduces sickle hemoglobin polymerization.4 However, HU requires a minimum of 3-6 months for sufficient HbF induction and previous studies have shown improved clinical performance well before a detectable rise in HbF.3,4 The HbF-independent clinical improvement is hypothesized to be a result of HU acting as a nitric oxide (NO) donor, a potent vasodilator, whose bioavailability is reduced in SCD.5,6 Reduced NO has a direct effect on the dilation of cerebral vessels, which warrants investigation into the impact of HU treatment length on cerebral hemodynamics. To assess cerebral hemodynamics we obtained dynamic MR measurements of cerebrovascular reactivity (CVR), which reflect the capacity of vascular endothelia to dilate in the presence of a vasoactive stimulus. In addition, we also obtained CBF data using arterial-spin labelling (ASL) to assess its relationship to CVR. We hypothesized that CVR will be higher in long-term HU-treated patients compared to short-term HU-treated patients. Furthermore, we hypothesized that CBF will be inversely correlated with CVR and will be lower in patients on long-term HU compared to short-term HU-treated patients.
Methods: 21 Patients (11M/10F; avg age 14±2.45) with no history of stroke, were imaged on a 3T MRI system. 15 patients were on HU for more than 1 year (long-term) and 6 were on HU less than 5 months (short-term). The hematocrit of both groups was similar. CVR data of the entire brain was obtained using a standard blood-oxygen-level-dependent (BOLD) MRI sequence in combination with a CO2 breathing challenge. CO2 was delivered via a re-breathing mask in 4 alternating cycles of 40 mmHg PETCO2 for 60 seconds and 45mmHg PETCO2 for 45 seconds. Correlations of voxel-based BOLD changes to end-tidal CO2 waveforms were analyzed using FSL v4.1 and yielded CVR maps that were co-registered to anatomical images. CBF was obtained with a standard pulsed ASL protocol and was quantified from the mean signal difference between ASL tag and control images using a kinetic model. The Student's t-test was used to assess whether the mean global CVR and CBF were significantly different (p<0.05) between patients on short-term and long-term HU treatment.
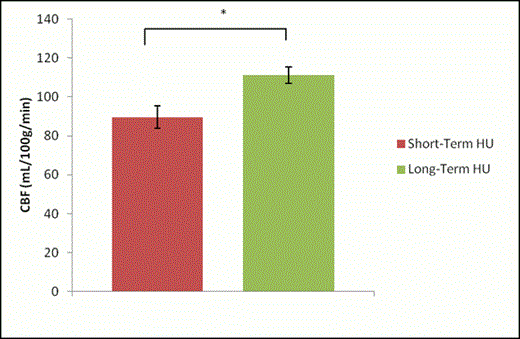
Results: Global CVR was significantly higher in long-term HU-treated patients compared to short-term HU-treated patients (p=0.034) [Figure 1]. However, global CBF was significantly higher in long-term HU-treated patients compared to short-term HU-treated patients (p=0.032) [Figure 2].
Conclusions: As expected, long-term HU-treated patients had higher CVR compared to the short-term HU-treated patients. This could be due to the rise in HbF which reduces sickling, thereby buffering the impact of hemolysis-associated endothelial dysfunction thereby increasing NO more compared to the short-term group. However, CBF in long-term HU-treated patients was significantly higher compared to the short-term treated patients. This was unexpected and not in line with our previous studies, which have shown that CVR is inversely correlated with CBF, both in children with SCD as well as healthy controls, which is characteristic of functioning endothelia. The increase in both CVR and CBF in long-term HU-treated patients may indicate an uncoupling of CVR and CBF, which could be exacerbated with continued treatment and should be monitored. However, further studies are needed to verify this.
Effect of HU treatment on CVR
Effect of HU treatment on CBF
References
1. Switzer J, et al. Lancet Neurol. 2006;5:501-5012.
2. Rodgers GP, et al. N Engl J Med. 1990; 322:1037–1045.
3. Charache S, et al. N Engl J Med. 1995;332:1317– 1322.
4. Halsey C, et al. Br J Haematol. 2003;120: 177-186.
5. Nahavandi M, et al. Hematology. 2000;5:235–239.
6. Morris C, et al. J Pediat Hematol Onc. 2003;28:629-634.
Off Label Use: Hydroxyurea is FDA approved for adults with HbSS. However, it is prescribed off-label at the Hospital for Sick Children for children with HbSS. .
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal