Abstract
BACKGROUND: The prognosis for patients with hematologic malignancies (HM) who relapse after allo-HCT is dismal, and more effective treatments are urgently needed. Immune checkpoint modulation was previously explored in a pilot study of a single low dose of the anti-CTLA-4 monoclonal antibody ipilimumab in patients relapsed after allo-HCT, and durable anti-tumor activity was seen in lymphoid malignancies without inducing clinical GVHD (Bashey et al., Blood, 2009). Here, we report for the first time on the results of a phase I study of ipilimumab given for multiple doses over an extended time course for patients with relapsed HM after allo-HCT.
METHODS: The primary objectives were to determine the MTD and evaluate safety. Secondary objectives included preliminary evaluation of efficacy and changes in immune cell phenotype. Patients were required to be off all immune suppression for > 4 weeks prior to study entry, with no history of grades 2-4 acute or extensive chronic GVHD. Ipilimumab was given at 3 mg/kg or 10 mg/kg IV every 3 weeks for 4 cycles of induction, followed by maintenance dosing every 12 weeks up to 1 year. The DLT observation period was the 12 week induction period. Standard response criteria for each disease were assessed at the mid-point (7 weeks), end of induction (13 weeks), and throughout maintenance. Immunophenotyping was performed by 8-color flow cytometry and analyzed by FACSDiva. This study is sponsored by CTEP (protocol 9204) and run through the Blood Cancer Research Partnership (BCRP) of the Leukemia & Lymphoma Society.
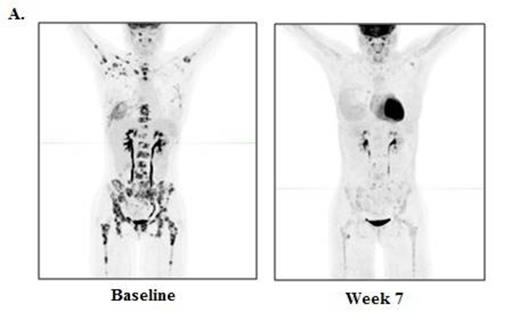
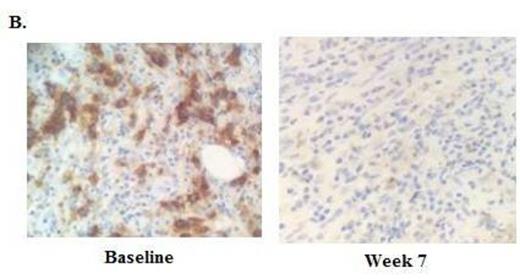
RESULTS: Thirteen patients were enrolled, with 6 patients at dose level 1 (3 mg/kg) and 7 patients in dose level 2 (10 mg/kg). The median age at time of transplant was 52 (range 20-70), and the median time from allo-HCT to study enrollment was 19.3 months (range 6.2 - 49.3). The median number of prior therapies was 7 (range 2-11), and 9 patients had received prior therapy for their post transplant relapse, including 4 patients with prior DLI. Seven patients had related donors and 6 patients had unrelated donors. Histologies included cHL (n=4), NHL (n=3), AML (n=2), and 1 patient each had MM, MDS, MPN, and ALL. In the safety analysis, there was no acute GVHD and one case of chronic GVHD (liver, mild) at 3 mg/kg, which was the only DLT. Immune-related adverse events (irAEs) were observed in 2 patients, and included grade 3 thrombocytopenia due to ITP (n=1), grade 2 pneumonitis (n=2), and grade 2 diarrhea (n=1), all of which were rapidly reversible with steroids and did not preclude further ipilimumab dosing. Eight patients discontinued due to progressive disease, 1 patient due to cGVHD, and 4 patients remain on treatment. The MTD was not reached. In the preliminary efficacy analysis, 4/11 (36.4%) patients evaluable for response had clinical benefit, including 1 cHL patient with a formal response, and 3 patients with stable disease with evidence of anti-tumor activity. The cHL patient achieving PR had a dramatic reduction in nodal and extranodal disease (Figure 1A), with a complete marrow response at 7 weeks (baseline 90% involement) (Figure 1B). A second cHL patient had a substantial reduction in disease burden and remains on study > 7 months, after progressing through 11 prior lines of therapy. A patient with CTCL also continues on study with stable disease > 6 months. A patient with AML/myeloid sarcoma had a tumor flare at 7 weeks followed by a reduction in tumor size at 13 weeks. The median follow-up time among survivors is 6.2 months, and 6 month overall survival is currently 65%. Immunophenotyping studies revealed that the ratio of regulatory T cells to conventional T cells decreased between 24% and 41% after ipilimumab treatment.
CONCLUSIONS: Multiple doses of ipilimumab given to patients with relapsed HM after allo-HCT were generally well-tolerated, with only 1 DLT (mild cGVHD), and organ-specific irAE's that were managable with steroids. Anti-tumor activity was observed, particularly in lymphoid malignanices. The ratio of regulatory to conventional T cells decreased with CTLA-4 blockade. The 10 mg/kg dose was chosen for a phase Ib expansion cohort to provide additional efficacy, safety, and correlative data.
Baseline and week 7 re-staging studies in a classical Hodgkin lymphoma patient after 2 doses of ipilimumab at 10 mg/kg. A. PET/CT scan and B. Bone marrow (CD30 stain)
Baseline and week 7 re-staging studies in a classical Hodgkin lymphoma patient after 2 doses of ipilimumab at 10 mg/kg. A. PET/CT scan and B. Bone marrow (CD30 stain)
Davids:Infinity Pharmaceuticals: Consultancy, Research Funding; Genentech: Consultancy. Off Label Use: ipilimumab is FDA-approved for the treatment of melanoma and is being studied here in hematologic malignancies. Armand:Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal