Abstract
Introduction:
Peripheral T-cell lymphomas (PTCL) are heterogeneous diseases, lightly sensitive to chemotherapy, with an adverse outcome and a 5-year survival rate of about 30%. Allogeneic hematopoietic stem cell transplant (allo-SCT) can lead to durable remission even for patients (pts) who show poor response to chemotherapy. Several observations argue for a Graft Versus Lymphoma (GVL) effect in PTCL. However, allo-SCT is not consensually recommended, at least in first line therapy. The aim of the current study is to highlight GVL effect in PTCL. To do so, we evaluated the benefits of immune modulation on disease control (donor lymphocyte infusions (DLI) and immunosuppressive therapy tapering) with analysis of outcome in pts who relapsed after allo-SCT.
Patient selection and methods:
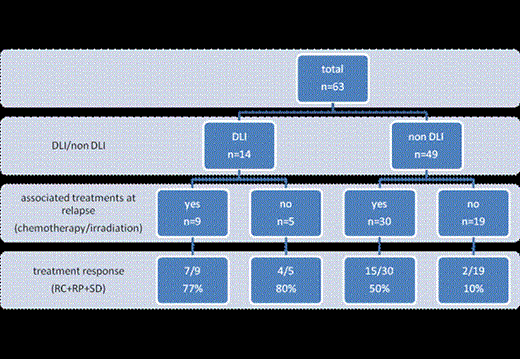
Between 1988 and 2012, 373 pts received an allo-SCT for treatment of PTCL. 80 pts in relapse after allo-SCT were identified in 27 SFGM-TC(French Society of Bone Marrow Transplantation and Cell Therapies) transplant centers. Exhaustive data were collected for 63 pts. Median age at transplant was 45 years; 67% were male. The main histopathologic subtypes were T-nos (20, 32%), primary cutaneous TCL (13, 21%) and anaplastic large TCL (11, 17%). Others were angioimmunoblastic TCL (8), T/NK lymphomas (5), HTLV1 lymphomas (5) and EIATL (1). At transplant, 38% were in complete remission (CR), 43% in partial remission (PR) and 19% had progressive disease. Median number of previous lines of treatment was 2 and 25% of the pts had previously received an autologous SCT. Conditioning regimen was myeloablative for 23 pts and non-myeloablative for 40. At relapse, pts were treated with either non-immunologic-based strategy (chemotherapy, radiotherapy) or immune modulation (DLI and/or discontinuation of immunosuppressive therapy), or both (Figure 1). We analyzed their outcomes according to the treatment they received at relapse.
Results:
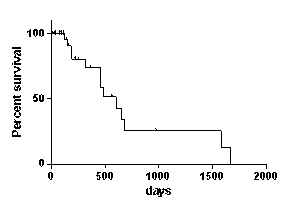
Relapse occurred at a median time of 204 days after transplant (interquartile range: 49-190 days); seventeen pts had a localized cutaneous relapse (7 Mycosis fungoides, 2 angioimmunoblastic TCL, 3 T-nos, 3 anaplastic TCL and 2 NK/TCL). Among the 14 pts (22%) who received DLI (DLI alone: 5, DLI + radiation and/or chemotherapy: 9), 9 obtained a response (7CR, 2 PR) and 2 had a stable disease (SD) status (overall response rate: 78%). Six of these 14 pts remained alive at last follow up. Median overall survival (OS) of these 14 pts was 23.6 months. Among the 49 pts who did not receive DLI, immunosuppressive therapy was tapered or stopped for 23. Sustained CR after tapering immunosuppressive drugs was observed in2 pts along with extensive chronic graft versus host disease (cGVHD). In the non-DLI group, 30 out of 49 pts received a radio/chemotherapy treatment with a response rate of 50% and a 1-year survival rate of 25% after relapse. Among the 13/49 pts who experienced a response in this group, six developed a cGVHD after relapse. Median OS of these 49 pts was 3.6 months. Median follow up was 40 months for the whole population.
Conclusion:
DLI alone or in association with other therapies, when possible, seems to be an efficient salvage option. This study suggests the benefit of DLI in cases of post transplant relapse for PTCL and supports the existence of a GVL effect in these diseases. These findings could potentially lead to the set up of a proactive immune modulation strategy after transplant for pts with high-risk disease. However, the short time frame from transplant to relapse currently observed, might limit the feasibility of this therapeutic option.
Combination treatments at relapse and response:
Overall survival in non- DLI group (n=49)
Overall survival in DLI group (n=14):
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal