Abstract
Background: The clinical utility of mutation profiling in hematologic malignancies requires robust detection of all classes of genomic alterations in a single analysis across different tumor types. We developed a novel, hybrid capture-based NGS assay (FoundationOne® Heme) designed to comprehensively assess the genomic landscape of hematologic malignancies from archived FFPE, blood and bone marrow aspirate samples, sequencing both DNA and RNA to improve sensitivity for driver fusion events. We have analyzed the genomic alterations including substitutions, indels, copy number alterations and genomic rearrangements of 795 samples with various hematolymphoid malignancies including lymphoma, leukemia and multiple myeloma to demonstrate the diagnostic, therapeutic and prognostic utility of this test in routine clinical practice.
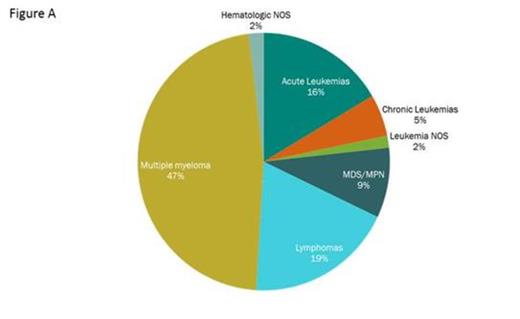
Method: Genomic profiles of 746 out of 795 (94%) consecutive samples received in a CLIA-certified laboratory (Foundation Medicine) were successfully characterized by the FoundationOne® Heme test including 527 from bone marrow aspirates, 176 FFPE samples and 92 samples derived from blood. The clinical cohort is composed of 375 multiple myeloma cases, 185 leukemia cases, 150 lymphoma cases, 75 MDS/MPN cases, and 15 unknown hematologic disorder cases (Figure A). Adaptor-ligated sequencing libraries were captured by solution hybridization using a custom bait-set targeting 405 blood cancer-related genes and 31 frequently rearranged genes by DNA-seq, and 265 frequently-rearranged genes by RNA-seq. All captured libraries were sequenced to high depth (Illumina HiSeq), averaging 498x for DNA and ~7M on-target unique pairs for RNA, to enable the sensitive and specific detection of substitutions, indels, copy number alteration and gene rearrangements.
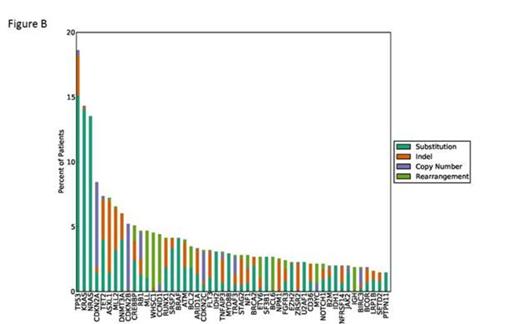
Results: 705/746 (95%) patients had at least one alteration reported as a somatic driver mutation, 68% of cases harbored at least one alteration linked to a targeted therapy or would inform enrollment in a clinical trial consistent with therapeutic relevance. These potentially actionable alterations included mutations in KRAS (14% of cases), NRAS (13%), CDKN2A (8%), DNMT3A (6%), IDH1/2 (5%), BRAF (4%) and FLT3 (3%) (Figure B). In addition, 64% of cases harbored at least one alteration that have been shown to predict outcome and have prognostic relevance, including TP53 (14% of cases), TET2 (7%), ASXL1 (7%), CDKN2B (5%), CREBBP (5%), MLL (5%) and NPM1 (3%)(Figure B). We also detected 344 genomic rearrangements in 280/746 (38%) patients. Of the 344 reported rearrangements, 166 were known fusions in various disease types and 178 were novel rearrangements involving known oncogenes and tumor suppressor genes, including a novel RCSD1-ABL2 fusion in a patient with B-cell ALL who will derive benefit from kinase inhibitor therapy as part of their anti-leukemic regimen. Genomic rearrangements (detected in 38% of cases) can lead to various functional consequences: 260 (76%) rearrangements detected in this cohort are predicted to create activating fusions; 19 (6%) rearrangements are predicted to be activating intragenic rearrangements in MLL, FLT3 and CARD11; and 60 (17%) rearrangements are likely truncating events in tumor suppressors.
Conclusions: We demonstrated that hybrid capture-based targeted DNA and RNA sequencing can be used to identify all classes of genomic alterations in genes known to be therapeutic targets or prognostic predictors in a broad spectrum of hematologic malignancies. Moreover, our data suggests that the FoundationOne® Heme comprehensive genomic profiling test can alter therapeutic strategy in routine clinical practice.
He:Foundation Medicine: Employment, Equity Ownership. Nahas:Foundation Medicine, Inc: Employment. Wang:Foundation Medicine, Inc.: Employment, Equity Ownership. Young:Foundation Medicine: Employment, Equity Ownership. Brennan:Foundation Medicine, Inc: Employment. Donahue:Foundation Medicine: Employment. Young:Foundation Medicine, Inc: Employment. Ross:Foundation Medicine, Inc.: Employment, Equity Ownership. Morosini:Foundation Medicine, Inc. : Employment, Equity Ownership. van den Brink:Foundation Medicine, Inc: Consultancy. Intlekofer:Foundation Medicine, Inc: Consultancy. Dogan:Foundation Medicine, Inc: Consultancy. Armstrong:Epizyme, Inc: Consultancy. Yelensky:Foundation Medicine, Inc.: Employment, Equity Ownership. Otto:Foundation Medicine, Inc.: Employment. Lipson:Foundation Medicine, Inc.: Employment, Equity Ownership. Stephens:Foundation Medicine, Inc.: Employment, Equity Ownership. Miller:Foundation Medicine, Inc: Employment. Levine:Foundation Medicine, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal