Key Points
The HCT-CI stratifies patients into 3 groups for risks of grades 3 to 4 GVHD regardless of conditioning intensity, donor, or graft types.
Comorbidity burden and development of grades 2 to 4 acute GVHD have cumulative effects on mortality rates.
Abstract
Whether the hematopoietic cell transplantation comorbidity index (HCT-CI) can provide prognostic information about development of acute graft-versus-host disease (GVHD) and subsequent mortality is unknown. Five institutions contributed information on 2985 patients given human leukocyte antigen-matched grafts to address this question. Proportional hazards models were used to estimate the hazards of acute GVHD and post-GVHD mortality after adjustment for known risk variables. Higher HCT-CI scores predicted increased risk of grades 3 to 4 acute GVHD (P < .0001 and c-statistic of 0.64), and tests of interaction suggested that this association was consistent among different conditioning intensities, donor types, and stem cell sources. Probabilities of grades 3 to 4 GVHD were 13%, 18%, and 24% for HCT-CI risk groups of 0, 1 to 4, and ≥5. The HCT-CI was statistically significantly associated with mortality rates following diagnosis of grade 2 (hazard ratio [HR] = 1.24; P < .0001) or grades 3 to 4 acute GVHD (HR = 1.19; P < .0001). Patients with HCT-CI scores of ≥3 who developed grades 3 to 4 acute GVHD had a 2.63-fold higher risk of mortality than those with scores of 0 to 2 and did not develop acute GVHD. Thus, pretransplant comorbidities are associated with the development and severity of acute GVHD and with post-GVHD mortality. The HCT-CI could be useful in designing trials for GVHD prevention and could inform expectations for GVHD treatment trials.
Introduction
Acute graft-versus-host disease (GVHD) develops in the majority of recipients of allogeneic hematopoietic cell transplantation (HCT) and can lead to significant posttransplant morbidity and mortality. Recipient-donor human leukocyte antigen (HLA) mismatch, grafts from unrelated donors, donor alloimmunization, and sex mismatch and donor parity have often been associated with increased risks for acute GVHD.1-6
High-intensity myeloablative conditioning regimens confer relatively high risk for acute GVHD due to the resultant substantial tissue damage that may initiate a “cytokine storm.”7,8 The cytokine storm is thought to be involved in the initial phase of acute GVHD development where antigen-presenting cells (APCs) are activated.9 Although intriguing, this hypothesis alone fails to explain the still relatively high incidence of acute GVHD (up to 60% for grades 2-4)10 following reduced-intensity or nonmyeloablative conditioning regimens, which by definition are associated with less global tissue damage. Overall, lower intensity regimens tend to be offered to a population that is typically older and with a significant burden of comorbidities; hence, other mechanisms might be responsible for initiation of T-cell responses.
The effect of patient age on acute GVHD has been an area of controversy. Whereas some studies showed an adverse impact of aging on development of acute GVHD,4,11 others did not.12 Moreover, there is no clear explanation for an impact of age on acute GVHD. Age-related thymic atrophy and defective negative selection of newly generated donor T cells by the thymus are thought to play a role in developing chronic but not acute GVHD, because the latter is triggered mostly by the thymic-independent peripheral expansion of mature donor T cells.13,14 On the other hand, aging is known to be associated with increasing comorbidity burden.15,16 The role of impaired health status or pretransplant organ damage on development of acute GVHD has not been examined.
Organ dysfunctions (comorbidities) affect the outcomes of cancer treatment by initiating or aggravating treatment-related morbidities.17,18 The HCT-comorbidity index (HCT-CI) was developed as a measure of pretransplant organ dysfunction that was adapted specifically for transplant recipients. The HCT-CI has been validated in large prospective patient cohorts.19,20 Further, comorbidity scoring has been standardized to ensure excellent reproducibility across investigators and institutions.21 We and others have shown the HCT-CI to be strongly associated with nonrelapse mortality after HCT.22-27 A better understanding of associations between pretransplant comorbidity burden and specific posttransplant complications could pave the way for future trials aiming to improve outcomes of patients with clinically significant comorbidities before HCT. Here, we studied the associations between pretransplant comorbidities and development of acute GVHD and subsequent mortality.
Patients and methods
Patients
This is a multi-institutional retrospective study that was approved by the internal review boards of the Fred Hutchinson Cancer Research Center, City of Hope, Oregon Health and Science University, University of Utah, and Colorado Blood Cancer Institute. Informed consent was obtained from all patients at the time of transplantation in accordance with the Declaration of Helsinki.
Data were collected from consecutive patients with the following criteria: 1) diagnoses of hematological malignant or nonmalignant diseases, 2) allogeneic HCT between 1 January 2000 and 31 December 2006, 3) inclusion of all types of conditioning regimens, 4) grafts from HLA-matched related or unrelated donors, and 5) marrow or granulocyte-colony stimulating factor-mobilized peripheral blood mononuclear cells. No upper limit was stated for the number of patient charts to be reviewed, and data were collected from 3335 patients. Of those, 350 (10%) were excluded due to lack of information on any or all of the HCT-CI components. Therefore, a final sample of 2985 patients contributed to the analyses.
Patients and donors were matched for HLA-A, -B, and -C antigens by either intermediate resolution DNA typing (to a level at least as sensitive as serology) or high-resolution techniques. HLA matching for -DRB1 and -DQB1 was at the allele level.28 Infection prophylaxis and treatment were done according to each institution’s standard practice guidelines.
Data collection and assessment of pretransplant comorbidities
Data on recipient age, Karnofsky performance status (KPS) scores, diagnoses and disease status, donor type, stem cell source, and recipient and donor cytomegalovirus (CMV) serology and outcome data were retrieved from the computer databases of each of the 5 institutions. The number of prior regimens and comorbidities were assessed by comprehensive review of medical records and laboratory values. Evaluation of comorbidities and assignment of scores were done using consistent definitions for coding the 17 components of the HCT-CI.3,4 Acute GVHD was graded according to the previously described criteria.29
Definitions
Post-acute GVHD mortality was defined as any death after the date of diagnosis (onset) of acute GVHD. Low disease risk included acute leukemia in first complete remission, chronic myeloid leukemia in first chronic phase, chronic lymphocytic leukemia or lymphoma in complete remission, and myelodysplasia-refractory anemia or refractory anemia with ringed sideroblasts. All remaining diagnoses were considered high risk. Nonmalignant diseases were considered as a separate category for disease risk. Conditioning regimens were classified into high dose, reduced intensity, or nonmyeloablative intensity based on the previously published criteria.30
Statistical methods
Cumulative incidence estimates were used to summarize the probabilities of grade 2 or grades 3 to 4 acute GVHD as stratified by the HCT-CI scores. For purposes of estimating the probability of GVHD, deaths without GVHD are treated as competing risks. Kaplan-Meier estimates were used to estimate the probability of survival in landmark analyses dating from the diagnosis of acute GVHD.
Proportional hazards regression models were used to assess the associations between HCT-CI scores and development of acute GVHD and subsequent mortality. The models were adjusted for patient-related risk factors: age, KPS score, CMV serology results, and donor/recipient gender combinations; disease-related risk factors: diagnosis category, disease risk, and number of prior regimens; and transplant-related risk factors: donor type, stem-cell source, degree of conditioning intensity, inclusion of anti-thymocyte globulin in conditioning, and GVHD prophylaxis regimen. In addition, all models were adjusted for center effect.
All P values from regression models were derived from the Wald test and are 2-sided.
We developed a GVHD-specific comorbidity index by assigning integer weights for associations between individual comorbidities and onset of acute GVHD that were derived from Cox proportional hazards modeling with grades 3 to 4 as the outcome. Hazard ratios (HRs) for each individual comorbidity were calculated after controlling for the presence of all previously mentioned covariates and all other pretransplant comorbidities. The adjusted HRs were converted to integer weights per the following equation: comorbidities with adjusted HR of 0.8 to 1.2 were dropped from consideration, comorbidities with an adjusted HR of 1.3 to 2.0 were assigned a weight of 1, comorbidities with an adjusted HR of 2.1 and greater were assigned a weight of 2, comorbidities with an adjusted HR of 0.5 to 0.8 were assigned a score of −1, and comorbidities with HR of <0.5 a score of −2. These scores were then summated to generate a GVHD-specific CI (GVHD-CI) total score.
In order to assess the capacity of both indices (HCT-CI and GVHD-CI) to discriminate outcomes, we computed concordance probability estimates using the c-statistic.31 Unlike confidence intervals and P values from regression models, c-statistic estimates have the advantage of not being influenced by sample size or scale marker. The c-statistic was computed to discriminate risks for development of grades 3 to 4 acute GVHD as a time-to-event outcome over the first year following HCT. Bootstrapping validation, which has been demonstrated to be a more efficient method for model validation than cross validation or splitting data into 2 groups,32-34 was used to derive a bias-corrected c-statistic estimate for the GVHD-CI. The c-statistic was estimated for each of 200 bootstrap samples of size n = 2985, where each sample was generated randomly with replacement from the original data set. A GVHD-CI for each sample was created and the resulting c-statistic estimated; the same weighting scheme that produced this GVHD-CI was applied to the original group of patients, and a GVHD-CI along with the c-statistic estimated from this original population. The difference between these 2 c-statistics was then calculated, and these differences were averaged over the 200 bootstrap samples to arrive at an estimate of the bias in the c-statistic that results from calculating the c-statistic on the same data that was used to create the GVHD-CI. Our intent was to favor the new GVHD-CI if the bias-corrected c-statistic estimate associated with GVHD-CI was higher than the c-statistic estimate for HCT-CI.
We investigated whether the association of the HCT-CI with overall mortality is dependent on the development of acute GVHD by modeling acute GVHD as a time-dependent covariate and assessing the interaction of this variable with HCT-CI scores.
Results
Patient characteristics
Patient-, disease-, and transplant-related characteristics of 2985 patients with available HCT-CI scores are described in Table 1. Median age was 45 (range 0.1-74.5) years. HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 were assigned to 32%, 32%, 26%, and 10% of patients, respectively. Conditioning regimens were high dose (62%), reduced intensity (15%), or nonmyeloablative (23%). Compared with patients treated with high-dose regimens, patients treated with reduced-intensity and nonmyeloablative conditioning regimens more frequently had HCT-CI scores of ≥3 (50% and 45% vs 30%), were ≥60 years of age (22% and 31% vs 3%), had KPS scores of ≤80% (29% and 36% vs 23%), received ≥4 preceding regimens (26% and 35% vs 18%), received peripheral blood mononuclear cells (85% and 94% vs 71%), and were at high risk for relapse (66% and 69% vs 56%). The 3 groups were comparable for the frequency of receiving unrelated grafts, and having positive CMV serology status. Cyclosporine (CSP) and mycophenolate mofetil combination was the most frequent GVHD prophylaxis regimen for recipients of reduced-intensity (28%) and nonmyeloablative (79%) regimens, whereas CSP/methotrexate combination was used more frequently for high-dose recipients (52%). Myeloid malignancies were the most frequent diagnoses among recipients of reduced-intensity (61%) and high-dose (67%) regimens, whereas lymphoid malignancies were the most frequent diagnoses among those of nonmyeloablative regimens (51%).
Patient, transplant, and disease characteristics
| Characteristics . | All patients (n = 2985) . | Conditioning regimens as classified per intensity . | ||
|---|---|---|---|---|
| High dose (N = 1844) . | Reduced intensity (N = 460) . | Nonmyeloablative (N = 681) . | ||
| n (%) . | n (%) . | |||
| HCT-CI scores | ||||
| 0 | 953 (32) | 694 (38) | 102 (22) | 157 (23) |
| 1 | 437 (15) | 300 (16) | 57 (12) | 80 (12) |
| 2 | 493 (17) | 289 (16) | 72 (16) | 132 (19) |
| 3 | 531 (18) | 290 (16) | 104 (23) | 137 (20) |
| 4 | 255 (9) | 131 (7) | 53 (12) | 71 (10) |
| ≥5 | 316 (10) | 140 (7) | 72 (15) | 104 (15) |
| Age, years | ||||
| 0-19 | 410 (14) | 333 (18) | 35 (8) | 42 (6) |
| 20-39 | 760 (25) | 597 (32) | 85 (18) | 78 (11) |
| 40-49 | 684 (23) | 467 (25) | 96 (21) | 121 (18) |
| 50-59 | 765 (26) | 390 (21) | 143 (31) | 232 (34) |
| ≥60 | 366 (12) | 57 (3) | 101 (22) | 208 (31) |
| KPS | ||||
| 100% | 1104 (37) | 776 (42) | 143 (31) | 185 (27) |
| 90-95% | 1090 (37) | 655 (35) | 183 (40) | 252 (37) |
| 80-85% | 543 (18) | 310 (17) | 89 (19) | 154 (23) |
| ≤75% | 248 (8) | 103 (6) | 45 (10) | 90 (13) |
| Prior regimens | ||||
| 0 | 470 (16) | 288 (16) | 100 (22) | 82 (12) |
| 1 | 510 (17) | 380 (21) | 60 (13) | 70 (10) |
| 2 | 735 (25) | 480 (26) | 99 (22) | 156 (23) |
| 3 | 587 (20) | 368 (20) | 84 (18) | 135 (20) |
| ≥4 | 683 (23) | 328 (18) | 117 (26) | 238 (35) |
| Patient CMV serology-status* | ||||
| Positive | 1835 (61) | 1140 (62) | 286 (62) | 409 (60) |
| Negative | 1122 (38) | 690 (37) | 169 (37) | 263 (39) |
| Unknown | 28 (1) | 14 (1) | 5 (1) | 9 (1) |
| Stem cell source | ||||
| Marrow | 641 (21) | 527 (29) | 70 (15) | 44 (6) |
| PBMC | 2344 (79) | 1371 (71) | 390 (85) | 637 (94) |
| Donor | ||||
| Related | 1648 (55) | 1041 (56) | 238 (52) | 369 (54) |
| Unrelated | 1337 (45) | 803 (44) | 222 (48) | 312 (46) |
| GVHD prophylaxis regimen† | ||||
| CSP/MMF | 760 (25) | 95 (5) | 129 (28) | 536 (79) |
| CSP/MTX | 1066 (36) | 954 (52) | 89 (19) | 23 (3) |
| Tacrolimus-based | 480 (16) | 340 (18) | 68 (15) | 72 (11) |
| Triple drugs | 342 (11) | 280 (15) | 52 (11) | 10 (1) |
| Sirolimus-based | 147 (5) | 76 (4) | 70 (15) | 1 (<1) |
| Others | 38 (1) | 32 (2) | 5 (1) | 1 (<1) |
| Unknown | 152 (5) | 67 (4) | 47 (10) | 38 (6) |
| Diagnoses‡ | ||||
| Myeloid | 1802 (60) | 1241 (67) | 279 (61) | 282 (41) |
| Lymphoid | 1053 (35) | 566 (31) | 137 (30) | 348 (51) |
| Nonmalignant diseases | 130 (5) | 37 (2) | 42 (9) | 51 (8) |
| Disease risk for relapse§ | ||||
| High | 1797 (60) | 1025 (56) | 302 (66) | 470 (69) |
| Low | 1128 (38) | 789 (43) | 150 (33) | 189 (28) |
| Nonmalignant diseases | 60 (2) | 30 (2) | 8 (2) | 22 (3) |
| Characteristics . | All patients (n = 2985) . | Conditioning regimens as classified per intensity . | ||
|---|---|---|---|---|
| High dose (N = 1844) . | Reduced intensity (N = 460) . | Nonmyeloablative (N = 681) . | ||
| n (%) . | n (%) . | |||
| HCT-CI scores | ||||
| 0 | 953 (32) | 694 (38) | 102 (22) | 157 (23) |
| 1 | 437 (15) | 300 (16) | 57 (12) | 80 (12) |
| 2 | 493 (17) | 289 (16) | 72 (16) | 132 (19) |
| 3 | 531 (18) | 290 (16) | 104 (23) | 137 (20) |
| 4 | 255 (9) | 131 (7) | 53 (12) | 71 (10) |
| ≥5 | 316 (10) | 140 (7) | 72 (15) | 104 (15) |
| Age, years | ||||
| 0-19 | 410 (14) | 333 (18) | 35 (8) | 42 (6) |
| 20-39 | 760 (25) | 597 (32) | 85 (18) | 78 (11) |
| 40-49 | 684 (23) | 467 (25) | 96 (21) | 121 (18) |
| 50-59 | 765 (26) | 390 (21) | 143 (31) | 232 (34) |
| ≥60 | 366 (12) | 57 (3) | 101 (22) | 208 (31) |
| KPS | ||||
| 100% | 1104 (37) | 776 (42) | 143 (31) | 185 (27) |
| 90-95% | 1090 (37) | 655 (35) | 183 (40) | 252 (37) |
| 80-85% | 543 (18) | 310 (17) | 89 (19) | 154 (23) |
| ≤75% | 248 (8) | 103 (6) | 45 (10) | 90 (13) |
| Prior regimens | ||||
| 0 | 470 (16) | 288 (16) | 100 (22) | 82 (12) |
| 1 | 510 (17) | 380 (21) | 60 (13) | 70 (10) |
| 2 | 735 (25) | 480 (26) | 99 (22) | 156 (23) |
| 3 | 587 (20) | 368 (20) | 84 (18) | 135 (20) |
| ≥4 | 683 (23) | 328 (18) | 117 (26) | 238 (35) |
| Patient CMV serology-status* | ||||
| Positive | 1835 (61) | 1140 (62) | 286 (62) | 409 (60) |
| Negative | 1122 (38) | 690 (37) | 169 (37) | 263 (39) |
| Unknown | 28 (1) | 14 (1) | 5 (1) | 9 (1) |
| Stem cell source | ||||
| Marrow | 641 (21) | 527 (29) | 70 (15) | 44 (6) |
| PBMC | 2344 (79) | 1371 (71) | 390 (85) | 637 (94) |
| Donor | ||||
| Related | 1648 (55) | 1041 (56) | 238 (52) | 369 (54) |
| Unrelated | 1337 (45) | 803 (44) | 222 (48) | 312 (46) |
| GVHD prophylaxis regimen† | ||||
| CSP/MMF | 760 (25) | 95 (5) | 129 (28) | 536 (79) |
| CSP/MTX | 1066 (36) | 954 (52) | 89 (19) | 23 (3) |
| Tacrolimus-based | 480 (16) | 340 (18) | 68 (15) | 72 (11) |
| Triple drugs | 342 (11) | 280 (15) | 52 (11) | 10 (1) |
| Sirolimus-based | 147 (5) | 76 (4) | 70 (15) | 1 (<1) |
| Others | 38 (1) | 32 (2) | 5 (1) | 1 (<1) |
| Unknown | 152 (5) | 67 (4) | 47 (10) | 38 (6) |
| Diagnoses‡ | ||||
| Myeloid | 1802 (60) | 1241 (67) | 279 (61) | 282 (41) |
| Lymphoid | 1053 (35) | 566 (31) | 137 (30) | 348 (51) |
| Nonmalignant diseases | 130 (5) | 37 (2) | 42 (9) | 51 (8) |
| Disease risk for relapse§ | ||||
| High | 1797 (60) | 1025 (56) | 302 (66) | 470 (69) |
| Low | 1128 (38) | 789 (43) | 150 (33) | 189 (28) |
| Nonmalignant diseases | 60 (2) | 30 (2) | 8 (2) | 22 (3) |
MMF, mycophenolatemofetil; MTX, methotrexate. *CMV serology status was not known for 34 patients (1.1%).
GVHD prophylaxis regimen was not known for 156 (5%) of the total patient population.
Myeloid malignancies included acute myeloid leukemia, biphenotypic leukemia, chronic myeloid leukemia, and myelodysplastic syndromes; lymphoid/plasma cell malignancies included acute lymphocytic leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma, and plasma cell leukemia; and nonmalignant disease included aplastic anemia, sickle cell anemia, autoimmune disease, and other hematological nonmalignant diseases.
Low disease risk for relapse included acute leukemia in first complete remission; chronic myeloid leukemia in first chronic phase; myelodysplastic syndromes with refractory anemia or refractory anemia with ringed sideroblasts; chronic lymphocytic leukemia, lymphoma, or multiple myeloma in complete remission; and nonmalignant diseases. High disease risk for relapse included all other disease status.
There were no differences in the distribution of HCT-CI risk groups per GVHD prophylaxis regimens. Among recipients of CSP-based regimens, 32% had a score of 0, 33% scores of 1 to 2, 25% scores of 3 to 4, and 9% scores of ≥5. Among recipients of tacrolimus-based regimens, 30% had a score of 0, 33% scores of 1 to 2, 24% scores of 3 to 4, and 13% scores of ≥5. Among recipients of sirolimus-based regimens, 31% had a score of 0, 26% scores of 1 to 2, 31% scores of 3 to 4, and 12% scores of ≥5.
Associations between the HCT-CI scores and incidence and risk of acute GVHD
Increasing HCT-CI scores, when modeled as a continuous linear variable in the regression model, were statistically significantly associated with an increased risk for grades 2 to 4 acute GVHD (HR = 1.03 per unit of the HCT-CI score, CI 95%: 1.00-1.06, P = .04). The association between increasing HCT-CI scores and risks for grades 3 to 4 acute GVHD was of a higher HR (HR = 1.12 per unit of the HCT-CI score, CI 95%: 1.07-1.18) and a higher statistical significance (P < .0001). Therefore, we have focused subsequent analyses on prediction of grades 3 to 4 acute GVHD.
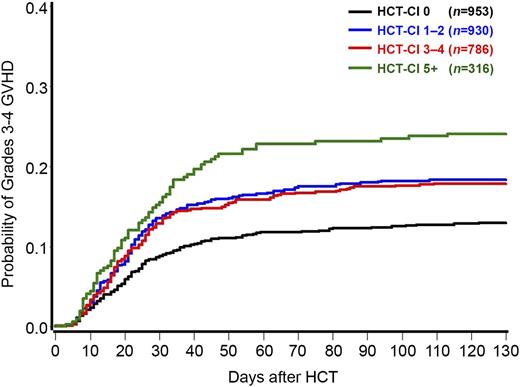
Relaxing the above assumption of a linear association between HCT-CI and grades 3 to 4 acute GVHD, HCT-CI was modeled as a categorical variable. Compared with the group of patients with a score of 0, groups with scores of 1 to 2, 3 to 4, and ≥5 had HRs of 1.47 (CI 95%: 1.16-1.86, P = .002), 1.47 (CI 95%: 1.15-1.90, P = .003), and 2.10 (CI 95%: 1.55-2.83, P < .0001), respectively, for development of grades 3 to 4 acute GVHD. The corresponding rates of grades 3 to 4 acute GVHD at 120 days were 13%, 18%, 18%, and 24%, respectively (Figure 1). Therefore, and for prognostication purposes, the HCT-CI scores could be collapsed into 3 risk groups for prediction of grades 3 to 4 acute GVHD (0 vs 1-4 vs ≥5).
Probabilities of grades 3 to 4 acute GVHD as stratified by the HCT-CI scores among 2985 recipients of allogeneic HCT. Patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 had 13%, 18%, 18%, and 24% probabilities, respectively, of grades 3 to 4 acute GVHD at 120 days after HCT.
Probabilities of grades 3 to 4 acute GVHD as stratified by the HCT-CI scores among 2985 recipients of allogeneic HCT. Patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 had 13%, 18%, 18%, and 24% probabilities, respectively, of grades 3 to 4 acute GVHD at 120 days after HCT.
Because GVHD was modeled as a time-to-event end point, the fact that the risk of GVHD was lower for the group with HCT-CI of 0 implies that the onset was, on average, later for this group. One way to demonstrate this is to pick a common percentile of GVHD occurrence, for example 50%, and compare the median time to GVHD. However, given the overall low frequency of grades 3 to 4 acute GVHD, the median time of GVHD onset (ie, the time at which the estimate of GVHD reaches 50%) was not reached for any HCT-CI group. If we instead look at the 10th percentile of time to GVHD (the time at which the estimate of GVHD reaches 10%), this time was 37 days for the group with an HCT-CI score of 0, 23 days for the group with HCT-CI scores of 1 to 4, and 18 days for the group with HCT-CI scores of ≥5.
Next, we evaluated possible interaction between age and HCT-CI scores in prediction of grades 3 to 4 acute GVHD. Higher comorbidity scores were associated with, on average, increasing age (median ages for patients with scores of 0, 1-2, 3-4, and ≥5 were 39.1, 45.6, 47.6, and 53.1 years, respectively). However, age, modeled as a continuous variable, in the regression model was not statistically significantly associated with increased risks for grades 3 to 4 acute GVHD (HR = 1.004, CI 95%: 0.998-1.010, P = .225). Tests of interactions showed no suggestion that the impact of HCT-CI on the risk of GVHD varied in different age groups (P value for interaction between age and HCT-CI with each modeled as continuous variables, P = .99; P value for interaction between age (continuous variable) and HCT-CI of 1-4 vs HCT-CI of 0, P = .62; and P value for interaction between age (continuous variable) and HCT-CI of ≥5 vs HCT-CI of 0, P = .81).
Subpopulation analyses of the performance of the HCT-CI in risk stratification of grades 3 to 4 acute GVHD
There was no suggestion that the association between HCT-CI scores and the risks of grades 3 to 4 acute GVHD differed across the 3 levels of regimen intensity (interaction P = .42 for comparison of high-dose vs nonmyeloablative regimen intensity and P = .73 for comparison of high-dose vs reduced-intensity regimens).
In particular, the HCT-CI, when modeled as a continuous linear variable, was associated with higher risks for grades 3 to 4 acute GVHD consistently among recipients of high-dose (HR = 1.11), reduced-intensity (HR = 1.16), and nonmyeloablative regimens (HR = 1.15), respectively. The HCT-CI scores stratified the observed probabilities of grades 3 to 4 acute GVHD among each of the 3 groups of regimen intensity (Table 2).
Probabilities of grades 3 to 4 acute GVHD as stratified by the HCT-CI scores and conditioning intensity
| HCT-CI group . | High dose . | Reduce-intensity conditioning . | Nonmyeloablative . |
|---|---|---|---|
| 0 | 15% | 8% | 9% |
| 1-2 | 20% | 15% | 18% |
| 3-4 | 18% | 15% | 22% |
| 5+ | 26% | 21% | 24% |
| HCT-CI group . | High dose . | Reduce-intensity conditioning . | Nonmyeloablative . |
|---|---|---|---|
| 0 | 15% | 8% | 9% |
| 1-2 | 20% | 15% | 18% |
| 3-4 | 18% | 15% | 22% |
| 5+ | 26% | 21% | 24% |
Similar analyses revealed that the associations between HCT-CI scores and risks of grades 3 to 4 acute GVHD did not differ by donor type (related vs unrelated donor grafts, interaction P value = .78) or by stem cell source (marrow vs granulocyte-colony stimulating factor-mobilized peripheral blood mononuclear cells, interaction P value = .83).
Association of individual comorbidities with development of grades 3 to 4 acute GVHD
Because the HCT-CI was developed based on its association with nonrelapse mortality, not GVHD, we investigated whether all or some of the 17 comorbidities within the HCT-CI were associated with grades 3 to 4 acute GVHD.
In a new Cox regression risk model for time-to-event grades 3 to 4 acute GVHD, 9 comorbidities had HRs between 1.289 and 1.637, and a single additional comorbidity had HR of 0.301 for prediction of grades 3 to 4 acute GVHD (Table 3). Based on the described equation under “Patients and methods,” each of the 10 comorbidities was assigned a weighted score to formulate a more concise comorbidity index that is particularly specific for prediction of grades 3 to 4 acute GVHD (GVHD-CI).
Prevalence and adjusted HRs for the associations between each of the 17 comorbidities included in the HCT-CI and risks of grades 3 to 4 acute GVHD among 2985 recipients of allogeneic HCT from 5 institutions
| Comorbidity category . | Prevalence, % . | HR* . |
|---|---|---|
| Peptic ulcer | 1.1 | 0.301 |
| Obesity | 8.2 | 0.974 |
| Diabetes | 4.5 | 1.028 |
| Heart valve disease | 1.5 | 1.047 |
| Prior malignancy | 5.9 | 1.081 |
| Renal | 0.7 | 1.089 |
| Psychiatric disorders | 11.4 | 1.181 |
| Cardiac | 5.4 | 1.186 |
| Cerebro-vascular disease | 1.4 | 1.289 |
| Severe pulmonary | 14.1 | 1.289 |
| Hepatic, mild | 15.5 | 1.313 |
| Moderate pulmonary | 26.7 | 1.352 |
| Arrhythmia | 3.0 | 1.440 |
| Moderate-severe hepatic | 4.4 | 1.463 |
| Rheumatologic | 3.0 | 1.488 |
| Inflammatory bowel disease | 1.0 | 1.606 |
| Infection | 5.6 | 1.637 |
| Comorbidity category . | Prevalence, % . | HR* . |
|---|---|---|
| Peptic ulcer | 1.1 | 0.301 |
| Obesity | 8.2 | 0.974 |
| Diabetes | 4.5 | 1.028 |
| Heart valve disease | 1.5 | 1.047 |
| Prior malignancy | 5.9 | 1.081 |
| Renal | 0.7 | 1.089 |
| Psychiatric disorders | 11.4 | 1.181 |
| Cardiac | 5.4 | 1.186 |
| Cerebro-vascular disease | 1.4 | 1.289 |
| Severe pulmonary | 14.1 | 1.289 |
| Hepatic, mild | 15.5 | 1.313 |
| Moderate pulmonary | 26.7 | 1.352 |
| Arrhythmia | 3.0 | 1.440 |
| Moderate-severe hepatic | 4.4 | 1.463 |
| Rheumatologic | 3.0 | 1.488 |
| Inflammatory bowel disease | 1.0 | 1.606 |
| Infection | 5.6 | 1.637 |
HRs were calculated for the association between each individual comorbidity and grades 3 to 4 acute GVHD, controlling for the presence of all coexisting comorbidities as well as for age, KPS score, and CMV serology results, diagnosis category, disease risk, and number of prior regimens, donor type, stem cell source, degree of conditioning intensity, inclusion of anti-thymocyte globulin in conditioning, and GVHD prophylaxis regimen.
Not surprisingly, GVHD-CI, when modeled as a continuous variable, had an adjusted HR of 1.41 per unit of the score (CI 95%: 1.28-1.55, P < .0001) for association with grades 3 to 4 acute GVHD.
We then compared the performance of the HCT-CI vs the GVHD-CI by estimating the c-statistic associated with HCT-CI and the bias-corrected c-statistic for GVHD-CI as described under “Patients and methods.” The 2 indices had similar predictive power for grades 3 to 4 acute GVHD, with c-statistic estimates of 0.64 for each.
The HCT-CI and mortality following acute GVHD
Increasing HCT-CI scores, when modeled as a continuous linear variable in the regression model, were statistically significantly associated with an increased risk of mortality following grade 2 (HR = 1.24 per unit of the score, CI 95%: 1.18-.29, P < .0001) as well as following grades 3 to 4 acute GVHD (HR = 1.19 per unit of the score, CI 95%: 1.13-1.25, P < .0001).
In a new regression model using the HCT-CI as a categorical variable, patients with scores of 1 to 2, 3 to 4, and ≥5 had 1.67-, 2.52-, and 3.37-fold higher risks for mortality following diagnosis of grade 2 acute GVHD compared with patients with a score of 0. Similarly, compared with patients with score 0, those with scores of 1 to 2, 3 to 4, and ≥5 had 1.59-, 2.35-, and 2.77-fold higher risks for mortality following diagnosis of grades 3 to 4 acute GVHD (Table 4).
Adjusted HRs for associations between the HCT-CI scores and risks of mortality following grades 2 and 3 to 4 acute GVHD among 2985 recipients of allogeneic HCT from 5 institutions
| HCT-CI scores . | Risk of mortality following grade 2 acute GVHD . | Risk of mortality following grades 3 to 4 acute GVHD . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| 0 | 1 | 1 | ||
| 1-2 | 1.67 (1.32-2.11) | <.0001 | 1.59 (1.15-2.20) | .006 |
| 3-4 | 2.52 (1.99-3.20) | <.0001 | 2.35 (1.68-3.27) | <.0001 |
| 5+ | 3.37 (2.48 −4.56) | <.0001 | 2.77 (1.90-4.05) | <.0001 |
| HCT-CI scores . | Risk of mortality following grade 2 acute GVHD . | Risk of mortality following grades 3 to 4 acute GVHD . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| 0 | 1 | 1 | ||
| 1-2 | 1.67 (1.32-2.11) | <.0001 | 1.59 (1.15-2.20) | .006 |
| 3-4 | 2.52 (1.99-3.20) | <.0001 | 2.35 (1.68-3.27) | <.0001 |
| 5+ | 3.37 (2.48 −4.56) | <.0001 | 2.77 (1.90-4.05) | <.0001 |
The models were adjusted for age, KPS, CMV serology results, donor/recipient gender combinations, diagnosis category, disease risk, number of prior regimens, donor type, stem-cell source, degree of conditioning intensity, inclusion of anti-thymocyte globulin in conditioning, and GVHD prophylaxis regimen.
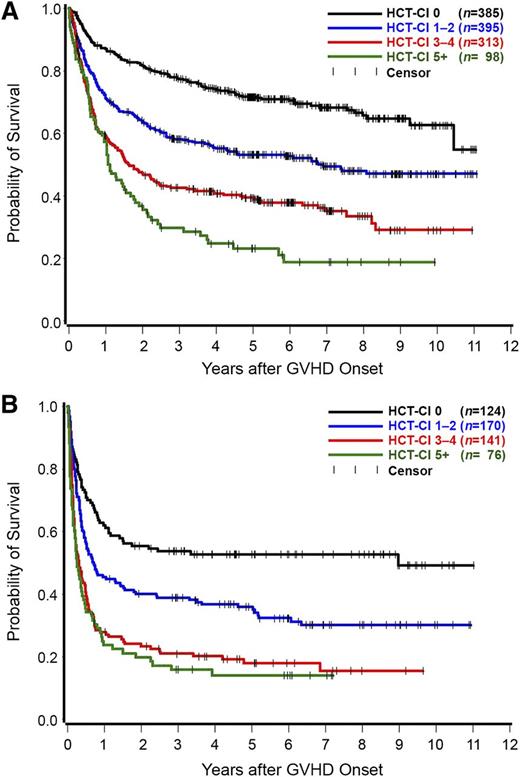
Overall, 1191 patients developed grade 2 and 511 patients grades 3 to 4 acute GVHD. In a landmark analysis dating from the onset of acute GVHD, patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 had 3-year rates of survival of 78%, 58%, 42%, and 30%, respectively, following diagnosis of grade 2 acute GVHD (Figure 2A). The figures for survival rates following diagnosis of grades 3 to 4 acute GVHD were 54%, 39%, 21%, and 16%, respectively (Figure 2B).
Kaplan Meier estimates of overall survival. A landmark analysis dating from the onset of grade 2 (number of patients = 1191) (A) or grades 3 to 4 (number of patients = 511) (B) acute GVHD as stratified by the HCT-CI scores. Patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 had 3-year rates of survival of 78%, 58%, 42%, and 30%, respectively, following diagnosis of grade 2 acute GVHD. The figures for survival rates following diagnosis of grades 3 to 4 acute GVHD were 54%, 39%, 21%, and 16%, respectively.
Kaplan Meier estimates of overall survival. A landmark analysis dating from the onset of grade 2 (number of patients = 1191) (A) or grades 3 to 4 (number of patients = 511) (B) acute GVHD as stratified by the HCT-CI scores. Patients with HCT-CI scores of 0, 1 to 2, 3 to 4, and ≥5 had 3-year rates of survival of 78%, 58%, 42%, and 30%, respectively, following diagnosis of grade 2 acute GVHD. The figures for survival rates following diagnosis of grades 3 to 4 acute GVHD were 54%, 39%, 21%, and 16%, respectively.
The cumulative effects of comorbidity burden and diagnosis of acute GVHD on mortality
We performed 3 different analyses to test whether the comorbidity burden and diagnosis of grades 2 to 4 acute GVHD have an additive effect on subsequent mortality. First, both the diagnosis of grades 2 to 4 acute GVHD (HR = 2.16, 95% CI: 1.93-2.42, P < .0001) and increasing HCT-CI scores (P < .0001; HR = 1.47 per unit of the score) were statistically significantly associated with mortality when both were included in a regression model.
Secondly, a formal interaction test between HCT-CI scores, as a continuous variable, and grades of acute GVHD for overall morality yielded a P value of .74 for grades 2 vs 0 to 1 and a P value of .33 for grades 3 to 4 vs 0 to 1.
Finally, we dichotomized HCT-CI into 2 groups (scores 0-2 vs ≥3) and acute GVHD into 2 groups (grades 0-1 vs 2-4), where acute GVHD was treated as a time-dependent covariate, so that the composition of these 2 groups changed as the GVHD status of a particular patient changed. This analysis yielded 4 risk groups for stratification of mortality risks: group I (low HCT-CI and no acute GVHD) had an HR of 1; group 2 (low HCT-CI scores and grades 2-4 acute GVHD) had a HR of 1.32 (95% CI: 1.13-1.54, P = .0005); group 3 (high HCT-CI scores and no acute GVHD) had an HR of 2.37 (95% CI: 2.02-2.77, P < .0001); and group 4 (high HCT-CI scores and grades 2-4 acute GVHD) had an HR of 2.63 (95% CI: 2.25-3.08, P < .0001) for overall mortality.
Discussion
The overarching goal of this study was to investigate the associations between an established transplant-specific comorbidity index (the HCT-CI) and a common posttransplant complication such as acute GVHD. We made 3 key findings. The HCT-CI was informative for the risks of development of grades 2 to 4 and, more strongly, of grades 3 to 4 acute GVHD. Previous studies identified different profiles of risk factors11 and cytokine gene expressions35 for grades 3 to 4 vs grade 2 acute GVHD, suggesting the possibility of distinct pathogenic pathways. In the current study, HCT-CI scores stratified patients into low (score 0), intermediate (scores 1-4), and high (scores ≥5) risk groups, with the latter 2 possessing 1.47- and 2-fold, respectively, higher risks for grades 3 to 4 acute GVHD compared with the lower risk group. The second finding was related to post-GVHD mortality. In general, mortality rates are higher following grades 3 to 4 acute GVHD than those following grade 2, but the HCT-CI scores stratified survival rates well following diagnosis of both grade 2 and grades 3 to 4 acute GVHD. Third, the prognostic impact of comorbidities on mortality was additive to that of grades 2 to 4 acute GVHD. Thus, our results suggest the HCT-CI can enhance the ability of clinicians and investigators to accurately predict the risks of acute GVHD and subsequent mortality, so that we can factor this information into decision making.
Assessment of the association between pretransplant organ function and risks of acute GVHD has been very limited in the literature. In a single report, elevated alanine aminotransferase was found to independently predict grades 2 to 4 acute GVHD, and moderate-severe liver cirrhosis or fibrosis predicted grades 3 to 4.36 Pretransplant infections were thought to promote initiation of acute GVHD through activation of APCs.9,37 KPS of <90% was found to be associated with higher risks of grades 2 to 4 and 3 to 4 acute GVHD.11 Here, we showed that an aggregate comorbidity index stratifies the probabilities of severe acute GVHD with a range of 13% to 24% among patients with HCT-CI scores of 0 and ≥5, respectively. Increasing age was not predictive of acute GVHD in this analysis once comorbidities were taken into account. The earlier observations of an impact of aging on acute GVHD4,11,38 might in fact have been reflective of the impact of increased number and/or severity of comorbidities with aging.
Patients with different comorbidity profiles were equally distributed among the various GVHD prophylaxis regimens, and these regimens were included in the multivariate models, suggesting an independent impact of comorbidities on acute GVHD. Nevertheless, current results emphasize the need for prospective studies assessing tolerance of patients with different comorbidity scores to GVHD prophylaxis and treatment agents and how this tolerance contributes to the fate of acute GVHD.
Our study included a relatively large sample of patients from 5 institutions to enhance generalizability of our conclusions. Despite the retrospective nature of the study, we had a relatively low rate of missing data (10%), suggesting that most comorbidity information is well documented in the medical record. Inclusion of the HCT-CI in future clinical trials focusing on prophylaxis or treatment of acute GVHD would allow prospective evaluation of the current results. The recognition of the HCT-CI as one of the predictors for acute GVHD and subsequent mortality is new to the field. However, the additional burden would be minimal, because HCT-CI scores are currently calculated regularly prior to HCT, making them readily available for clinicians evaluating patients for risks of GVHD or subsequent mortality. Of note, the HCT-CI was shown to retain an equal predictive strength for severe GVHD compared with a modified GVHD-specific index. Moreover, the collection of comorbidity data per the HCT-CI has been standardized and facilitated in a validated Web-based application (www.hctci.org).21 These characteristics will encourage routine use of the HCT-CI during management of acute GVHD.
Understanding the previous limitations, our results could lead to a number of significant clinical applications. The HCT-CI could be routinely incorporated into randomized trials comparing regimens for prophylaxis or treatment of acute GVHD39-41 to ensure balanced distribution of patients with comorbidity burden among comparison groups. This would improve our abilities to identify regimens that are successful in preventing or treating GVHD but also reasonably tolerated by vulnerable patients. Second, the HCT-CI could standardize our statistical methods for detection and validation of candidate genetic or biomarker associations with acute GVHD by accounting for the confounding effect of comorbidities across studies.42,43 For clinicians and patients, the HCT-CI could be an important asset for counseling patients about acute GVHD and its outcomes. For example, among nonmyeloablative patients, rates of severe acute GVHD ranged from 8% in patients with low comorbidity scores to 24% in patients with high scores (Table 2). Among all patients, 3-year rates of survival ranged between 78% and 30%, respectively, following grade 2 and between 54% and 16%, respectively, following grades 3 to 4 acute GVHD. Finally, improvement in the treatment of acute GVHD has been limited by a lack of prognostic models.44 The HCT-CI could be used, with other risk factors,45 to accurately distinguish patients with higher risks for GVHD or subsequent mortality who might benefit from intensified treatments from those with standard risks who might experience more harm than benefit from such therapies.
Biologically, there could be 2 plausible hypotheses for the associations between comorbidities and the development of severe acute GVHD. Tissue injury, mainly caused by high-dose regimens, has been implicated in the release of certain cytokines leading to activation of APCs and contributing to the development and/or severity of acute GVHD.9,43,46 The most commonly reported group of these cytokines47-50 has also been implicated in the pathogenesis of the set of comorbidities identified in our analysis to predict severe acute GVHD.51-61 In particular, higher pretransplant levels of soluble IL-2 receptor α chain and interleukin-18 were correlated with severity of acute GVHD.47,62 Therefore, it could be hypothesized that allogeneic HCT for patients with significant comorbidities, usually using reduced-intensity or nonmyeloablative regimens, might result in entry of donor cells in an already established cytokine-rich environment. This might also explain the different kinetics of acute GVHD between recipients of high-dose vs reduced-intensity conditioning regimens. Whereas the former group of patients tends to develop early-onset acute GVHD due to the acute tissue injury with abrupt release of cytokines, the latter patients, usually with heavy comorbidity burden, tend to have chronic tissue injury and ongoing cytokine elevations resulting in delayed-onset acute GVHD.63-65 Along those lines, the stratification of probabilities of severe acute GVHD improved as the conditioning intensity lessened (Table 2). Target tissue-specific injury before HCT might also have been responsible for the severity of post-HCT GVHD. For example, the regenerating islet-derived 3-α correlates with disease severity in both inflammatory bowel disease66 before HCT and colonic GVHD67,68 after HCT.
Pretransplant endothelial vulnerability, as detected by high angipoietin-2, was shown to increase the chances of developing steroid refractory acute GVHD.69 In that study, all patients with steroid refractory disease had grades 3 to 4 acute GVHD.69 Investigators suggested that although immunosuppressive salvage therapy can efficiently eradicate T cells, endothelial mechanisms perpetuate damage of target organs specifically among patients who develop severe acute GVHD.69 Interestingly, angipoietin-2, by sensitizing endothelial cells to proinflammatory cytokines, correlates with severity of a number of organ dysfunction syndromes that are characterized by systemic inflammation.70-75 Both the results by Luft et al and our observations in the current study suggest that endothelial vulnerability could constitute a link between pretransplant organ dysfunction and the severity of acute GVHD.
Comorbidities, as quantified in the HCT-CI, are key factors in determining risks of severe acute GVHD and the prognosis of patients diagnosed with acute GVHD. In addition to the prognostic and clinical benefits, future research on the biological association between pretransplant organ dysfunctions and acute GVHD could identify new avenues for intervention to reduce morbidity and mortality following allogeneic transplants in patients with significant comorbidity burden.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Gary Schoch, Cara Hanby, Kayo Togawa, Nan Subbiah, Rachel Frires, Juli Murphy, Araki Kristen, and Jennifer Sheldrake for their help in obtaining internal review board approvals and acquisition of data from computerized databases at the different institutions. We thank Bonnie Larson and Helen Crawford for their assistance with manuscript preparation. We are grateful to the many physicians, nurses, research nurses, physician assistants, nurse practitioners, pharmacists, data coordinators, and support staff who cared for our patients and to the patients who allowed us to care for them and who participated in our ongoing clinical research.
This study was supported by grants HL088021, CA018029, HL036444, CA078902, and CA015704 from the National Institutes of Health, National Heart Lung Blood Institute. M.L.S. is also supported by a Research Scholar Grant from the American Cancer Society as well as a Patient-Centered Outcome Research Institute contract.
Authorship
Contribution: M.L.S., P.J.M., and T.A.G. contributed to study design; M.L.S. collected data and obtained funding for the study; S.B., R.T.M., M.A.P., and M.B.M. coordinated the study at respective centers; T.A.G. performed statistical analysis; M.L.S., P.J.M., R.F.S., R.T.M., M.A.P., M.B.M., H.J.D., S.J.L., F.R.A., and B.M.S. contributed to interpretation of results; M.L.S. wrote the manuscript; and P.J.M., R.F.S., R.T.M., M.A.P., M.B.M., D.J.M., H.J.D., S.J.L., F.R.A., B.M.S., and T.A.G. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamed L. Sorror, Clinical Research Division (D5-280), Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, Seattle, WA 98109-1024; e-mail: msorror@fhcrc.org.