Key Points
SDF-1 acutely affects megakaryocyte spatial distribution in the bone marrow at steady state and in the setting of radiation injury.
SDF-1-directed localization of megakaryocytes into the vascular niche increases platelet output.
Abstract
Megakaryocyte (MK) development in the bone marrow progresses spatially from the endosteal niche, which promotes MK progenitor proliferation, to the sinusoidal vascular niche, the site of terminal maturation and thrombopoiesis. The chemokine stromal cell-derived factor-1 (SDF-1), signaling through CXCR4, is implicated in the maturational chemotaxis of MKs toward sinusoidal vessels. Here, we demonstrate that both IV administration of SDF-1 and stabilization of endogenous SDF-1 acutely increase MK-vasculature association and thrombopoiesis with no change in MK number. In the setting of radiation injury, we find dynamic fluctuations in marrow SDF-1 distribution that spatially and temporally correlate with variations in MK niche occupancy. Stabilization of altered SDF-1 gradients directly affects MK location. Importantly, these SDF-1-mediated changes have functional consequences for platelet production, as the movement of MKs away from the vasculature decreases circulating platelets, while MK association with the vasculature increases circulating platelets. Finally, we demonstrate that manipulation of SDF-1 gradients can improve radiation-induced thrombocytopenia in a manner additive with earlier TPO treatment. Taken together, our data support the concept that SDF-1 regulates the spatial distribution of MKs in the marrow and consequently circulating platelet numbers. This knowledge of the microenvironmental regulation of the MK lineage could lead to improved therapeutic strategies for thrombocytopenia.
Introduction
Platelet-producing megakaryocytes (MKs) are derived from megakaryocyte progenitors (MKPs), which are defined functionally by their capacity to form colonies in vitro.1,2 MKPs are thought to reside near the bone surface in an “endosteal niche,” where environmental cues encourage expansion, but suppress terminal maturation.3-6 Polyploid MKs mature cytoplasmically, extrude proplatelets in association with sinusoidal vasculature, and shed platelets into the peripheral blood.7-9 This process results in past-maturity “exhausted” MKs comprised of a nucleus with a thin layer of cytoplasm surrounded by a cell membrane.10,11 Megakaryopoiesis is primarily regulated by the cytokine thrombopoietin (TPO), which signals through its receptor Mpl to promote MKP proliferation and MK maturation.12-14
Although the physical association of MKs with sinusoidal vasculature was first appreciated several decades ago,15-17 the functional significance of the “vascular niche” for MK maturation and thrombopoiesis has only more recently begun to be elucidated.4,18-21 Several studies have implicated the chemokine stromal cell-derived factor-1 ([SDF-1] or CXCL12) signaling through receptor CXCR4 in the maturational localization of MKs to the vascular niche. CXCR4 is expressed throughout the MK lineage, and in vitro SDF-1 stimulation results in intracellular calcium mobilization, matrix metalloproteinase 9 expression, surface CXCR4 polarization, and ultimately migration along an SDF-1 gradient.22-27 Several cell types within the bone marrow produce SDF-1, including osteoblasts, endothelial cells, and perivascular mesenchymal stromal cell populations.28-32 Additionally, in vivo studies demonstrate that sustained elevation of SDF-1 in the blood increases thrombopoiesis, with images indicating enhanced MK association with vasculature.20,33 Recently, VEGF-A treatment was shown to increase MK-vasculature interactions in vivo through upregulation of CXCR4 on MKs.34 Despite this growing body of evidence indicating a role for SDF-1/CXCR4 in megakaryopoiesis, the acute and endogenous effects of SDF-1 on MK localization and platelet production remain unknown.
Radiation causes significant damage to the hematopoietic system, and thrombocytopenia can be a life-threatening consequence of radiation exposure.35 Seminal studies in rodents identified an initial persistence of polyploid MKs after both sublethal and lethal doses of total body irradiation (TBI).36-39 Accordingly, radioresistant MKs persist within an injured marrow microenvironment established by differential radiation damage to surrounding cell populations.40-43 The sinusoidal vasculature, which supports platelet production, can dilate as surrounding cells succumb to damage and become leaky at higher levels of injury.44,45 Intriguingly, SDF-1 levels increase in the marrow after TBI and radioresistant MKs relocate to the endosteal niche.39,46,47 It is not known whether the spatial changes of MKs in the setting of TBI are regulated by microenvironmental changes in SDF-1 or have functional consequences for platelet production.
Here, we investigate the ability of SDF-1 to acutely control MK location in the bone marrow both at steady state and after radiation injury. Importantly, we demonstrate that endogenous SDF-1 regulates the association of MKs with vasculature, which contributes to platelet production in uninjured bone marrow. After sublethal TBI, fluctuations in marrow SDF-1 dynamically affect the distribution of MKs. Our data give insight into the spatial regulation of megakaryopoiesis and demonstrate that changes in MK location have functional consequences for thrombopoiesis.
Materials and methods
Mice and irradiation
Seven- to 9-week-old female C57BL/6J mice (Jackson Laboratory) were used for all experiments. Unanesthetized mice, confined in a Plexiglass restraint, were exposed to 4 Gy TBI at a dose rate of 1.6 Gy/min using a Shephard Irradiator with 6000 Ci 137Cs source and collimating equipment. All animal experiments were approved by the University of Rochester Committee on Animal Resources.
In vivo treatments
Recombinant murine SDF-1a (400 ng; Peprotech) in 100 μL phosphate-buffered saline (PBS) was injected IV into unirradiated mice, or mice at day 2 or day 4 post-TBI. Diprotin A ([ILE-PRO-ILE]; 1.7mg; Enzo) in 250 μL PBS was injected intraperitoneally into unirradiated mice, or mice at day 2 or day 3 post-TBI. Recombinant murine TPO (0.3μg; Peprotech) in 250 μl PBS was injected intraperitoneally 2 hours after 4 Gy TBI.
Blood and marrow isolation
Mice were killed by CO2 narcosis and peripheral blood collected from the inferior vena cava. Femoral marrow was flushed into PB248 with 25 μg/mL heparin and titurated cells counted by hemocytometer.
MK lineage analysis
Platelet counts were obtained using a HemaTrue Veterinary Hematology Analyzer (Heska). For platelet RNA analysis, EDTA-collected blood was stained in PBS with 1000 U/mL heparin, 1 μg/mL thiozole orange (Sigma-Aldrich), and 1:100 eFluor450-CD41 and phycoerythrin (PE) Cy7-Ter119 (eBioscience), fixed with 1% formaldehyde, and analyzed by ImagestreamX (Amnis).
MKPs were quantified by colony assay, as previously described.49 MK analysis was performed by imaging flow cytometry, as recently described.50 PE-CXCR4 (eBioscience) was used to determine median fluorescent intensity per unit area (MFI/A) for each individual MK with the median pixel intensity feature (IDEAS [version 4.0]; Amnis).
In vitro differentiation of MKs
Marrow cells were stained with biotinylated Kit antibody (EBioscience), incubated with IMag streptavidin magnetic particles, and magnetically separated (iMagnet; BD Biosciences). Kit+ cells were cultured at 106/mL with Iscove modified Dulbecco medium (Invitrogen), 20% BIT 9500 (Stem Cell Technologies), and 100 ng/mL TPO (Peprotech). After 4 days at 37°C/5% CO2, 300 ng/mL SDF-1 (Peprotech) or vehicle was added for 1 hour. Cells were washed in PB2, stained, and analyzed by imaging flow cytometry.50
Immunohistochemistry
Hindlimbs were fixed in 4% paraformaldehyde for 24 hours, decalcified in either 10% EDTA for 10 to 14 days or Richard Allen Decalcifying Solution (Thermo-Fisher) for 5 hours, and embedded in paraffin. Femoral sections (5 μm) underwent heat antigen retrieval (Dako), blocking with 5% goat serum (Vector Labs) and 5% bovine serum albumin (Gemini Bio-Products), and sequential antibody staining: 1:100 anti-panendothelial cell antigen (MECA32; Biolegend), 1:500 AlexaFluor647 goat anti-rat (Life Technologies), 1:300 anti-Gp1Bβ (Emfret), and 1:500 IRDye 800CW goat anti-rat (LI-COR). Images were acquired with an ORCA-R2 digital camera (Hamamatsu) with Nikon NIS-Elements software on an Eclipse 80i microscope (Nikon) with a ×20 objective. Total Gp1Bβ+ MKs and MKs physically associated with MECA32+ vessels were manually enumerated for 8 to 12 images per section (mean, 94 total MK/sample; range, 45-148). MECA32+ vessels were demarcated and vascular area/total marrow area was determined by NIS-Elements.
RNA in situ hybridization
Analysis of SDF-1 transcripts was performed on femoral sections, as previously described.51,52 Darkfield and brightfield images were acquired with an ORCA-R2 digital camera (Hamamatsu) with Nikon NIS-Elements software on an Eclipse 80i microscope (Nikon) with a ×4 objective. For each darkfield image, signal threshold was set to capture single autoradiographic grains, and the grain area determined in a 100 μm × 1250 μm region at the endosteal boundary of diaphyseal marrow, as well as an immediately adjacent region in central marrow. The endosteal:central grain area ratio was obtained for each image. The images were processed and pseudocolored using Photoshop 6.0 (Adobe).
Gene expression analysis
RNA was isolated from flushed marrow and complementary DNA prepared, as previously described.53 Quantitative polymerase chain reaction (qPCR) was performed with Taqman Gene Expression Assays (Life Technologies). CXCL12 (Mm00445553_m1) expression was normalized to 18S (Hs99999901_s1).
Results
Exogenous SDF-1 acutely increases MKs in the vascular niche and platelet output
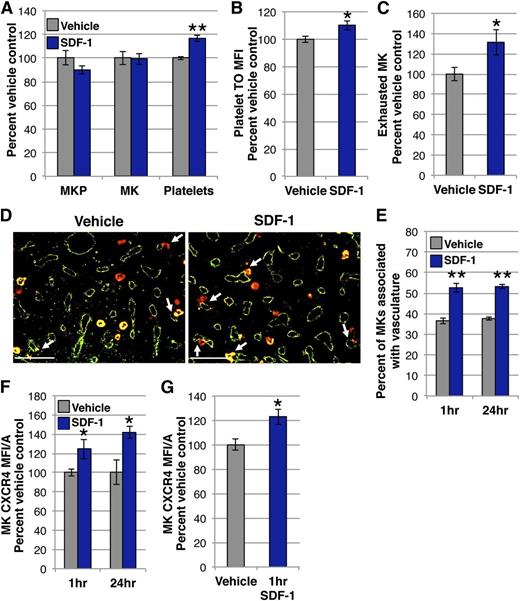
Studies utilizing a murine model of constitutive plasma elevation of SDF-1 indicate that a sustained gradient of SDF-1 toward the vasculature results in increased MKs associated with marrow vasculature.20 However, the extended timeframe of SDF-1 exposure, coupled with the capacity of the vascular niche to influence MK maturation4,18,19 makes it difficult to determine direct SDF-1 effects on MK location. Given that the chemotactic response of MKs to SDF-1 in vitro occurs rapidly,24,54 we asked if an exogenous gradient of SDF-1 toward the vasculature acutely regulates MK migration. A single 400 ng dose of SDF-1 was administered IV and the compartments of the MK lineage were analyzed 24 hours later. There was no change in the number of MKPs, defined functionally by colony assay, or MKs, determined by imaging flow cytometry, in the femurs of the mice treated with SDF-1 compared with vehicle controls (Figure 1A). Surprisingly, however, mice receiving SDF-1 had a 17% increase in platelet count (Figure 1A). This increase in circulating platelets was likely due to enhanced production, as platelets isolated from mice treated with SDF-1 had higher thiozole orange staining, which can label both RNA and dense granules, and indicates an increase in young, reticulated platelets55,56 (Figure 1B). Further supporting an acute increase in thrombopoiesis, mice receiving SDF-1 had more exhausted MKs at 24 hours by imaging flow cytometry10,11,50 (Figure 1C). Although there was no change in the total number of MKs (Figure 1A), as early as 1 hour after IV SDF-1 there was a dramatic shift in their location, with a >40% increase in the proportion of Gp1Bβ+ MKs associated with MECA32+ vasculature by immunohistochemistry (IHC) (Figure 1D-E).57
Vascular elevation of SDF-1 by IV administration acutely promotes the association of MKs with vasculature and thrombopoiesis. (A) MKP, MK, and platelet kinetics 24 hours after 400 ng IV SDF-1 (blue) or vehicle control (gray). SDF-1-treated mice have an acute increase in circulating platelets with no change in MK or MKP number in the marrow. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values. Mean absolute numbers for vehicle controls: 16 905 MKP/femur, 45 939 MK/femur, 430 × 103 platelets/μL. (B) MFI of thiozole orange (TO) in platelets identified as CD41+Ter119- by imaging flow cytometry 24 hours after IV SDF-1 or vehicle. Platelets from SDF-1–treated mice have increased TO staining. (C) The proportion of exhausted MKs was identified by imaging flow cytometry and presented as percent of vehicle control for each experiment. Mean proportion of exhausted MKs for vehicle controls: 0.14. (D) Representative images of femoral marrow immunohistochemistry (IHC) for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) 24 hours after IV treatment with vehicle (left panel) or SDF-1 (right panel). White arrows indicate examples of MKs physically associated with MECA32+ vessels. Some MKs express the pan-endothelial antigen recognized by MECA32.57 Images were processed as described in the “Immunohistochemistry” section. (E) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by manual counting of IHC 1 hour and 24 hours after IV SDF-1 (blue) or vehicle (gray). IV SDF-1 acutely increases MK association with the vasculature. (F) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared 1 hour and 24 hours after IV SDF-1 (blue) or vehicle (gray). (G) MFI/A of surface CXCR4 on in vitro–derived MKs 1 hour after SDF-1 treatment (blue) by imaging flow cytometry. SDF-1 treatment increases MK surface CXCR4 both in vivo (F) and in vitro (G). Error bars represent standard error of the mean of ≥3 independent experiments (n = 6-18 total mice per group). Statistical analyses comparing SDF-1 to vehicle controls were performed using a 2-tailed Student’s t test. Bar represents 100 μm (D). *P < .04; **P < .005.
Vascular elevation of SDF-1 by IV administration acutely promotes the association of MKs with vasculature and thrombopoiesis. (A) MKP, MK, and platelet kinetics 24 hours after 400 ng IV SDF-1 (blue) or vehicle control (gray). SDF-1-treated mice have an acute increase in circulating platelets with no change in MK or MKP number in the marrow. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values. Mean absolute numbers for vehicle controls: 16 905 MKP/femur, 45 939 MK/femur, 430 × 103 platelets/μL. (B) MFI of thiozole orange (TO) in platelets identified as CD41+Ter119- by imaging flow cytometry 24 hours after IV SDF-1 or vehicle. Platelets from SDF-1–treated mice have increased TO staining. (C) The proportion of exhausted MKs was identified by imaging flow cytometry and presented as percent of vehicle control for each experiment. Mean proportion of exhausted MKs for vehicle controls: 0.14. (D) Representative images of femoral marrow immunohistochemistry (IHC) for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) 24 hours after IV treatment with vehicle (left panel) or SDF-1 (right panel). White arrows indicate examples of MKs physically associated with MECA32+ vessels. Some MKs express the pan-endothelial antigen recognized by MECA32.57 Images were processed as described in the “Immunohistochemistry” section. (E) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by manual counting of IHC 1 hour and 24 hours after IV SDF-1 (blue) or vehicle (gray). IV SDF-1 acutely increases MK association with the vasculature. (F) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared 1 hour and 24 hours after IV SDF-1 (blue) or vehicle (gray). (G) MFI/A of surface CXCR4 on in vitro–derived MKs 1 hour after SDF-1 treatment (blue) by imaging flow cytometry. SDF-1 treatment increases MK surface CXCR4 both in vivo (F) and in vitro (G). Error bars represent standard error of the mean of ≥3 independent experiments (n = 6-18 total mice per group). Statistical analyses comparing SDF-1 to vehicle controls were performed using a 2-tailed Student’s t test. Bar represents 100 μm (D). *P < .04; **P < .005.
As changes in CXCR4 expression have been noted after SDF-1 stimulation in many cell types,58-62 we investigated CXCR4 surface expression on MKs by imaging flow cytometry. MKs isolated 1 hour and 24 hours after IV SDF-1 demonstrated increased surface expression of the CXCR4 receptor (Figure 1F). In vitro–derived primary MKs displayed a similar increase in CXCR4 expression at 1 hour, suggesting this is a direct response of MKs to SDF-1 stimulation (Figure 1G). Taken together, these results indicate that acute elevation of vascular SDF-1 with IV administration rapidly enhances the MK-vasculature association, which leads to increased platelet production.
Stabilization of endogenous SDF-1 enhances MK-vasculature interactions and increases circulating platelets
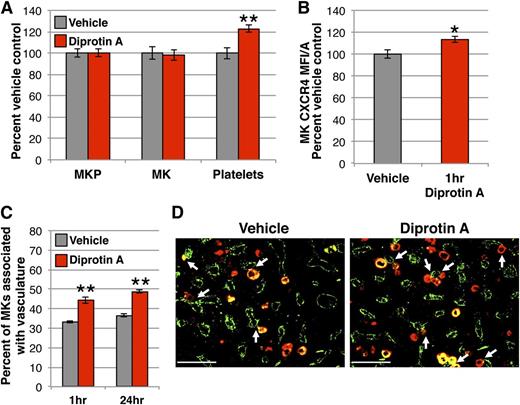
Next, we sought to determine if the migration of MKs during steady-state megakaryopoiesis is regulated by a native, endogenous SDF-1 gradient. In vivo, SDF-1 contributes to chemotactic gradients in close proximity to its synthesis, as it binds many glycosaminoglycan extracellular matrix components upon secretion and can undergo rapid proteolysis.63,64 To stabilize endogenously-produced SDF-1, we used Diprotin A, a small peptide that inhibits dipeptidyl peptidase 4 (DPP4), a soluble and cell-surface protease present in the bone marrow that truncates SDF-l and abolishes its chemotactic activity.65-68 There were no changes in the number of marrow MKPs or MKs by 24 hours after administration of Diprotin A; however, there was a 20% increase in circulating platelets (Figure 2A). Similar to the response of MKs to exogenous SDF-1 (Figure 1F), marrow MKs had increased surface CXCR4 1 hour after Diprotin A-mediated stabilization of SDF-1 (Figure 2B). Significantly, mice receiving Diprotin A also demonstrated a 30% increase in MKs associated with the vasculature by IHC (Figure 2C-D). These results mirror the MK shift toward the vascular niche and increased platelet production seen with the acute administration of IV SDF-1 (Figure 1), supporting the concept that SDF-1 regulates the maturational movement of MKs toward the vasculature during normal megakaryopoiesis.
Native SDF-1 enhances MKs in the vascular niche and increases circulating platelets. (A) MKP, MK, and platelet kinetics 24 hours after 1.7 mg (5 μmol) intraperitoneal Diprotin A (red) or vehicle control (gray). Stabilization of SDF-1 with Diprotin A acutely increases circulating platelets with no change in MK or MKP number in the marrow. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of vehicle control. Mean absolute numbers for vehicle controls: 11 593 MKP/femur, 49 856 MK/femur, 436 × 103 platelets/μL. (B) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared 1 hour after treatment with Diprotin A (red) or vehicle control (gray). (C) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by IHC 1 hour and 24 hours following treatment with Diprotin A (red) or vehicle (gray). Diprotin A-mediated stabilization of SDF-1 acutely increases MK association with the vasculature. (D) Representative images of femoral marrow IHC for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) 24 hours after administration of vehicle (left panel) or Diprotin A (right panel). White arrows indicate examples of MKs physically associated with MECA32+ vessels. Images were processed as described in the “Immunohistochemistry” section. Error bars represent standard error of the mean of ≥3 independent experiments (n = 7-8 total mice per group). Statistical analyses comparing Diprotin A to vehicle controls were performed using a 2-tailed Student’s t test. Bar represents 100 μm (D). *P < .02; **P < .003.
Native SDF-1 enhances MKs in the vascular niche and increases circulating platelets. (A) MKP, MK, and platelet kinetics 24 hours after 1.7 mg (5 μmol) intraperitoneal Diprotin A (red) or vehicle control (gray). Stabilization of SDF-1 with Diprotin A acutely increases circulating platelets with no change in MK or MKP number in the marrow. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of vehicle control. Mean absolute numbers for vehicle controls: 11 593 MKP/femur, 49 856 MK/femur, 436 × 103 platelets/μL. (B) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared 1 hour after treatment with Diprotin A (red) or vehicle control (gray). (C) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by IHC 1 hour and 24 hours following treatment with Diprotin A (red) or vehicle (gray). Diprotin A-mediated stabilization of SDF-1 acutely increases MK association with the vasculature. (D) Representative images of femoral marrow IHC for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) 24 hours after administration of vehicle (left panel) or Diprotin A (right panel). White arrows indicate examples of MKs physically associated with MECA32+ vessels. Images were processed as described in the “Immunohistochemistry” section. Error bars represent standard error of the mean of ≥3 independent experiments (n = 7-8 total mice per group). Statistical analyses comparing Diprotin A to vehicle controls were performed using a 2-tailed Student’s t test. Bar represents 100 μm (D). *P < .02; **P < .003.
Sublethal TBI induces temporal and spatial changes in marrow SDF-1
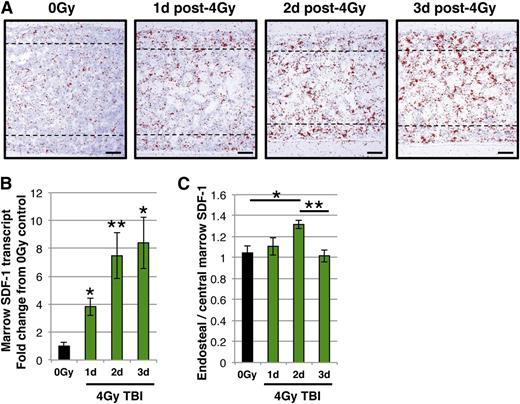
As SDF-1 contributes to MK localization to the thrombopoietic niche at steady state, we asked whether changes in marrow SDF-1 have consequences for the MK lineage. Given the importance of the location of SDF-1 production for its chemotactic function,63 and the varied injury and recovery kinetics of different marrow cell populations after sublethal TBI,40-43 we reasoned there might be changes in marrow SDF-1 gradients in this injury setting. To address this, we investigated the location of SDF-1 transcripts in the marrow of mice after sublethal 4 Gy TBI by radioactive in situ hybridization (Figure 3A). As expected, there was an overall increase in SDF-1 message in the marrow following TBI,39,46 which was confirmed by qPCR (Figure 3B). There were, however, no changes in plasma SDF-1 protein levels in the first 4 days after injury (supplemental Figure 1 available on the Blood Web site). Changes in marrow distribution were quantified by comparing SDF-1 transcript levels within the endosteal region in the diaphyseal marrow to the levels in an adjacent region within the central marrow (Figure 3C). Interestingly, we detected the development of an SDF-1 gradient toward the endosteum at day 2 that was no longer present at day 3 (Figure 3C).
Temporal and spatial changes in marrow SDF-1 following sublethal radiation. (A) Representative images of radioactive in situ hybridization with SDF-1 antisense probe (red pseudocolor indicates SDF-1 transcripts) on femoral marrow sections from (left to right panels): unirradiated (0 Gy), and days 1, 2, and 3 post-4 Gy TBI. Dotted lines delineate the endosteal region. Images were acquired and processed as described in the “RNA in situ hybridization.” (B) Expression of SDF-1 transcripts by qPCR in flushed marrow cells from unirradiated mice (black) and days 1, 2, and 3 post-4 Gy TBI (green). SDF-1 transcripts increase in the marrow following TBI. (C) Ratio of SDF-1 transcript area in the endosteal region (between 0-100 μm from the endosteal surface within the diaphysis) compared to an immediately adjacent region of the same size (between 100-200 μm from the endosteal surface) for each biologic replicate of unirradiated mice (black), and days 1, 2, and 3 post-4 Gy TBI (green). An endosteal gradient of SDF-1 transcript is apparent at day 2, but is lost at day 3. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-7 total mice per group). Statistical analyses either comparing irradiated samples to unirradiated control (B) or indicated samples (C) were performed using a 2-tailed Student’s t test. Bar represents 100 μm (A). *P < .02; **P < .007.
Temporal and spatial changes in marrow SDF-1 following sublethal radiation. (A) Representative images of radioactive in situ hybridization with SDF-1 antisense probe (red pseudocolor indicates SDF-1 transcripts) on femoral marrow sections from (left to right panels): unirradiated (0 Gy), and days 1, 2, and 3 post-4 Gy TBI. Dotted lines delineate the endosteal region. Images were acquired and processed as described in the “RNA in situ hybridization.” (B) Expression of SDF-1 transcripts by qPCR in flushed marrow cells from unirradiated mice (black) and days 1, 2, and 3 post-4 Gy TBI (green). SDF-1 transcripts increase in the marrow following TBI. (C) Ratio of SDF-1 transcript area in the endosteal region (between 0-100 μm from the endosteal surface within the diaphysis) compared to an immediately adjacent region of the same size (between 100-200 μm from the endosteal surface) for each biologic replicate of unirradiated mice (black), and days 1, 2, and 3 post-4 Gy TBI (green). An endosteal gradient of SDF-1 transcript is apparent at day 2, but is lost at day 3. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-7 total mice per group). Statistical analyses either comparing irradiated samples to unirradiated control (B) or indicated samples (C) were performed using a 2-tailed Student’s t test. Bar represents 100 μm (A). *P < .02; **P < .007.
Radioresistant MKs display altered marrow niche occupancy after sublethal TBI
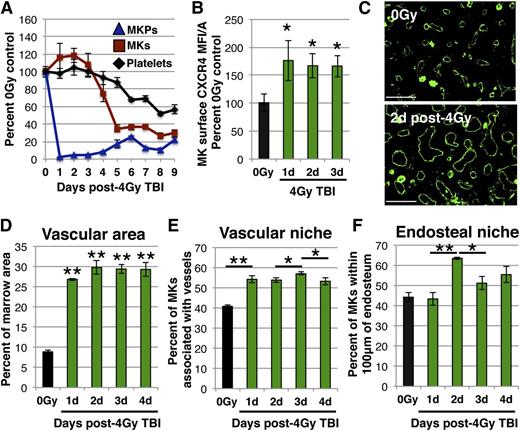
To determine whether MKs respond to the observed shifts in SDF-1 expression following 4 Gy TBI, we first characterized the injury kinetics of the MK lineage (Figure 4A). Platelets remained at steady-state levels for 4 days, followed by the development of thrombocytopenia, with a nadir in platelet count at day 8 following TBI (Figure 4A). MKs persisted during the first 3 days postinjury (Figure 4A), despite the loss of >85% of nucleated marrow cells (data not shown). Importantly, these radioresistant MKs had increased surface expression of CXCR4, indicating they retained the ability to respond to SDF-1 (Figure 4B; supplemental Figure 1). Megakaryocytopenia and thrombocytopenia eventually develop as a consequence of the rapid, near complete loss of the upstream MKP compartment (Figure 4A).
Altered niche occupancy of radioresistant MKs following sublethal radiation. (A) MK lineage injury kinetics following 4 Gy TBI. Circulating platelets (black) and marrow MKs (red) remain at steady-state levels for 3 days (one-way analysis of variance, P = .45), while upstream MKPs (blue) are rapidly lost. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 13 550 MKP/femur, 56 022 MK/femur, 484 × 103 platelets/μL. (B) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared from unirradiated controls (black) or days 1 to 3 post-4 Gy TBI (green). MKs from irradiated mice display increased surface CXCR4 expression. (C) Representative images of femoral IHC for MECA32 (vasculature, green) with hindlimbs isolated from unirradiated mice (top panel) or day 2 post-4 Gy TBI (bottom panel). The marrow vasculature dilates after TBI. (D) Quantification of vascular area within MECA32+ marrow vessels by IHC. Vascular dilation occurs by day 1 after injury and remains constant from days 1 to 4 post-4 Gy TBI. (E) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by femoral IHC prepared from unirradiated control mice (black) or days 1 to 4 post-4 Gy TBI (green). MK association with vasculature changes dynamically following radiation, increasing between days 0 and 1 and between days 2 and 3, but decreasing between days 3 and 4. (F) Quantification of Gp1Bβ+ MKs in the endosteal niche (within 100 μm of the endosteal surface within diaphysis) by IHC in unirradiated control mice (black) and days 1 to 4 post-4 Gy TBI (green). MKs increase in the endosteal niche between days 1 and 2, and decrease between days 2 and 3. Image processing and analysis is described in the “Immunohistochemistry” section. The vascular niche and endosteal niche measurements were not made in a mutually exclusive manner and there may be overlap in occupancy between these niches. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-12 total mice per group). Statistical analyses either comparing irradiated samples to unirradiated control (B and D) or indicated samples (E and F) were performed using a 2-tailed Student’s t test. Bar represents 100 μm (C). *P < .04; **P < .001.
Altered niche occupancy of radioresistant MKs following sublethal radiation. (A) MK lineage injury kinetics following 4 Gy TBI. Circulating platelets (black) and marrow MKs (red) remain at steady-state levels for 3 days (one-way analysis of variance, P = .45), while upstream MKPs (blue) are rapidly lost. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 13 550 MKP/femur, 56 022 MK/femur, 484 × 103 platelets/μL. (B) MFI/A of surface CXCR4 on primary MKs by imaging flow cytometry of flushed marrow samples prepared from unirradiated controls (black) or days 1 to 3 post-4 Gy TBI (green). MKs from irradiated mice display increased surface CXCR4 expression. (C) Representative images of femoral IHC for MECA32 (vasculature, green) with hindlimbs isolated from unirradiated mice (top panel) or day 2 post-4 Gy TBI (bottom panel). The marrow vasculature dilates after TBI. (D) Quantification of vascular area within MECA32+ marrow vessels by IHC. Vascular dilation occurs by day 1 after injury and remains constant from days 1 to 4 post-4 Gy TBI. (E) Quantification of Gp1Bβ+ MKs physically associated with MECA32+ vessels by femoral IHC prepared from unirradiated control mice (black) or days 1 to 4 post-4 Gy TBI (green). MK association with vasculature changes dynamically following radiation, increasing between days 0 and 1 and between days 2 and 3, but decreasing between days 3 and 4. (F) Quantification of Gp1Bβ+ MKs in the endosteal niche (within 100 μm of the endosteal surface within diaphysis) by IHC in unirradiated control mice (black) and days 1 to 4 post-4 Gy TBI (green). MKs increase in the endosteal niche between days 1 and 2, and decrease between days 2 and 3. Image processing and analysis is described in the “Immunohistochemistry” section. The vascular niche and endosteal niche measurements were not made in a mutually exclusive manner and there may be overlap in occupancy between these niches. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-12 total mice per group). Statistical analyses either comparing irradiated samples to unirradiated control (B and D) or indicated samples (E and F) were performed using a 2-tailed Student’s t test. Bar represents 100 μm (C). *P < .04; **P < .001.
Since MKs interact with sinusoidal endothelium, we also examined the effects of 4 Gy TBI on marrow vasculature by IHC. Most notable was a marked vascular dilation at day 1 post-TBI, with a 200% increase in the marrow area occupied by vasculature that persisted for at least 4 days following the injury (Figure 4C-D; supplemental Figure 2). Accompanying the early, rapid vascular dilation was a 30% increase in the MKs associated with sinusoids by IHC at day 1 (Figure 4E). The temporal overlap between the vascular changes and the shift in MK-vasculature association suggests that this initial change in MK niche occupancy is likely secondary to vascular dilation. However, within the subsequent timeframe in which vascular dilation remains constant, there were small, but significant changes in MKs in the vascular niche, suggesting additional factors may influence this altered localization (Figure 4E).
Given the development of an SDF-1 gradient toward the endosteum following TBI (Figure 3A,C), we also quantified MK distribution in the endosteal niche, defined as within 100 μm of the diaphyseal endosteum69 (Figure 4F). Strikingly, there was a 45% increase in radioresistant MKs in the endosteal niche between days 1 and 2 post-TBI (Figure 4F). MKs rapidly decreased in the endosteal niche by day 3 postinjury (Figure 4F), coincident with the diminished SDF-1 gradient (Figure 3A,C). Taken together, these results spatially and temporally implicate alterations in marrow SDF-1 after TBI in MK niche occupancy changes.
Stabilization of altered SDF-1 gradients post-TBI functionally regulate MK location and platelet production
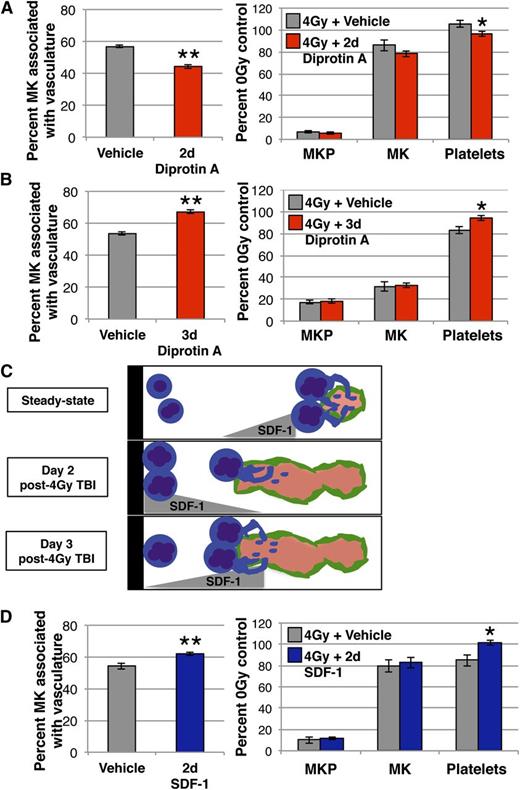
We hypothesized that changes in the spatial localization of MKs between days 2 and 3 post-TBI were regulated by shifts in marrow SDF-1 production. In light of the importance of MK-vasculature association for efficient platelet production,21 we reasoned that alterations in MK niche occupancy would affect platelet production. To address this, we stabilized native SDF-1 with Diprotin A at day 2 post-TBI, when SDF-1 production is increased near the endosteum, and found a 20% decrease in MKs in the vascular niche (Figure 5A, left panel) and a small, but significant decrease in circulating platelets 24 hours later (Figure 5A, right panel). However, stabilization of SDF-1 at day 3 post-TBI, when there is no longer an endosteal SDF-1 gradient, has opposing effects, with significant increases both in MK-vasculature association (Figure 5B, left panel) and in platelet count (Figure 5B, right panel). These data support the concept that changes in the location of SDF-1 production at days 2 and 3 after TBI contribute to altered MK location in the marrow (Figure 5C). Additionally, these results demonstrate that the spatial distribution of MKs has functional consequences, as MKs leaving the vascular niche decreases circulating platelets while enhanced MK-vasculature association increases platelet numbers.
Changes in SDF-1 regulate MK spatial location and platelet production following 4 Gy TBI. (A) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after treatment with Diprotin A (red) or vehicle (gray) at day 2 post-4 Gy TBI. Diprotin A-mediated stabilization of SDF-1 at day 2 following TBI decreases platelets and MKs in the vascular niche. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments are expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 12 420 MKP/femur, 53 946 MK/femur, 470 × 103 platelets/μL. (B) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after treatment with Diprotin A (red) or vehicle (gray) at day 3 post-4 Gy TBI. Diprotin A-mediated stabilization of SDF-1 at day 3 increases platelets and MKs in the vascular niche. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 13 272 MKP/femur, 52 414 MK/femur, 491 × 103 platelets/μL. (C) Model depicting the regulation of MK niche occupancy in the marrow by SDF-1 at steady state (top panel) and days 2 (middle panel) and 3 (bottom panel) after 4 Gy TBI. At a steady state, Diprotin A-mediated stabilization of SDF-1 increases MKs in the vascular niche. At day 2 following TBI, when our studies reveal a gradient of SDF-1 toward the endosteum, stabilization of SDF-1 decreases both MK association with vasculature and the number of circulating platelets. In contrast, at day 3 post-TBI, when the endosteal SDF-1 gradient is lost, stabilization of SDF-1 resembles the steady-state condition with increased MKs in the vascular niche. (D) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after IV SDF-1 administration (blue) or vehicle (gray) at day 2 post-4 Gy TBI. At this time point, elevation of vascular SDF-1 with IV administration counteracts the endogenous endosteal SDF-1 gradient and leads to enhanced MK-vasculature association and increased circulating platelets. Mean absolute numbers for 0 Gy controls: 10 996 MKP/femur, 52 615 MK/femur, 459 × 103 platelets/μL. Error bars represent standard error of the mean of ≥3 independent experiments (n = 5-9 total mice per group). Statistical analyses comparing treated mice to vehicle controls were performed using a 2-tailed Student’s t test. *P ≤ .04; **P < .006.
Changes in SDF-1 regulate MK spatial location and platelet production following 4 Gy TBI. (A) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after treatment with Diprotin A (red) or vehicle (gray) at day 2 post-4 Gy TBI. Diprotin A-mediated stabilization of SDF-1 at day 2 following TBI decreases platelets and MKs in the vascular niche. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments are expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 12 420 MKP/femur, 53 946 MK/femur, 470 × 103 platelets/μL. (B) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after treatment with Diprotin A (red) or vehicle (gray) at day 3 post-4 Gy TBI. Diprotin A-mediated stabilization of SDF-1 at day 3 increases platelets and MKs in the vascular niche. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as percent of unirradiated control. Mean absolute numbers for 0 Gy controls: 13 272 MKP/femur, 52 414 MK/femur, 491 × 103 platelets/μL. (C) Model depicting the regulation of MK niche occupancy in the marrow by SDF-1 at steady state (top panel) and days 2 (middle panel) and 3 (bottom panel) after 4 Gy TBI. At a steady state, Diprotin A-mediated stabilization of SDF-1 increases MKs in the vascular niche. At day 2 following TBI, when our studies reveal a gradient of SDF-1 toward the endosteum, stabilization of SDF-1 decreases both MK association with vasculature and the number of circulating platelets. In contrast, at day 3 post-TBI, when the endosteal SDF-1 gradient is lost, stabilization of SDF-1 resembles the steady-state condition with increased MKs in the vascular niche. (D) Analysis of MK lineage kinetics (right panel) and Gp1Bβ+ MKs physically associated with MECA32+ vasculature (left panel) 24 hours after IV SDF-1 administration (blue) or vehicle (gray) at day 2 post-4 Gy TBI. At this time point, elevation of vascular SDF-1 with IV administration counteracts the endogenous endosteal SDF-1 gradient and leads to enhanced MK-vasculature association and increased circulating platelets. Mean absolute numbers for 0 Gy controls: 10 996 MKP/femur, 52 615 MK/femur, 459 × 103 platelets/μL. Error bars represent standard error of the mean of ≥3 independent experiments (n = 5-9 total mice per group). Statistical analyses comparing treated mice to vehicle controls were performed using a 2-tailed Student’s t test. *P ≤ .04; **P < .006.
Having shown that a transient increase in endosteal SDF-1 production directs MKs away from the vascular niche and negatively affects platelet production, we asked whether we could manipulate MK location and influence platelet output by altering SDF-1 gradients. To this end, we elevated vascular SDF-1 with acute IV administration at day 2 postinjury, when there is a native increase in endosteal SDF-1. Whereas Diprotin A-mediated stabilization of endogenous-produced SDF-1 at day 2 decreases both MK association with the vasculature and platelet production 24 hours later (Figure 5A), IV SDF-1 had opposing effects, increasing MKs in the vascular niche (Figure 5D, left panel) as well as circulating platelets (Figure 5D, right panel). Taken together, these data highlight the direct effects of rapidly changing SDF-1 gradients on MK spatial localization and thrombopoiesis.
Administration of SDF-1 improves radiation-induced thrombocytopenia in a manner additive with prior TPO administration
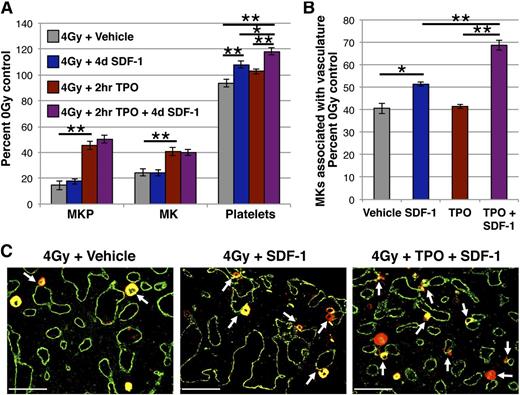
Our studies indicate that appropriately timed IV administration of SDF-1 can acutely increase platelet output. To test whether this strategy could be used to improve radiation-induced thrombocytopenia, we administered SDF-1 on day 4 after 4 Gy TBI, when thrombocytopenia begins to develop (Figure 4A), and our localization studies indicate a small decrease of MKs within the vascular niche (Figure 4E). With no change in MKP or MK number 24 hours following IV SDF-1 (day 5 post-TBI), there were increases in both platelet count and MKs associated with the vasculature (Figure 6A-C). However, these effects were less robust than those achieved with the same dose of SDF-1 in uninjured mice (Figure 1A). We hypothesized this was a consequence of fewer MKs present in the marrow to move to the vascular niche at this time point postinjury, and the SDF-1-mediated platelet increase may therefore depend on MK availability.
Acute administration of IV SDF-1 improves radiation-induced thrombocytopenia and is an additive with earlier TPO administration. (A) MK lineage kinetics at day 5 post-4 Gy TBI in mice treated with IV SDF-1 at day 4 (blue), 0.3 μg IP TPO (red) at 2 hours, both 2-hour TPO and day 4 SDF-1 (purple), or vehicle control (gray). IV SDF-1 increases platelet count following radiation injury. TPO administration at 2 hours post-TBI increases MK and MKP numbers, and IV SDF-1 at day 4 following initial TPO treatment results in an additive improvement in circulating platelets. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as a percentage of unirradiated control. Mean absolute numbers for 0 Gy controls: 10 939 MKP/femur, 50 595 MK/femur, 473 × 103 platelets/μL. (B) Quantification of Gp1Bβ+ MKs in the vascular niche (physically associated with MECA32+ vessels) by femoral IHC prepared on day 5 post-4 Gy from irradiated vehicle control mice (gray), or mice treated with IV SDF-1 at day 4 (blue), 0.3 μg IP TPO (red) at 2 hours, or both 2-hour TPO and day 4 SDF-1 (purple). Percentage of MKs associated with vasculature (IHC) are normalized to per femur values (imaging flow cytometry). Mean 20 104 MK/femur associated with vasculature for 0 Gy controls. Sequential TPO and SDF-1 administration increases MKs in the vascular niche above either treatment alone. (C) Representative images of femoral marrow IHC for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) at day 5 post-4 Gy with hindlimbs isolated from vehicle controls (left panel), mice receiving IV SDF-1 at day 4 (middle panel), and mice receiving 2-hour TPO followed by day 4 SDF-1 (right panel) as described above. White arrows indicate examples of MKs physically associated with MECA32+ vessels. Images were acquired and processed as described in the “Immunohistochemistry” section. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-11 total mice per group). Statistical analyses comparing indicated platelet samples in panel A were performed by one-way analysis of variance with Tukey’s multiple comparisons test, and all other indicated comparisons were performed by a 2-tailed Student’s t test. Bar represents 100 μm (C). *P < .05; **P < .006.
Acute administration of IV SDF-1 improves radiation-induced thrombocytopenia and is an additive with earlier TPO administration. (A) MK lineage kinetics at day 5 post-4 Gy TBI in mice treated with IV SDF-1 at day 4 (blue), 0.3 μg IP TPO (red) at 2 hours, both 2-hour TPO and day 4 SDF-1 (purple), or vehicle control (gray). IV SDF-1 increases platelet count following radiation injury. TPO administration at 2 hours post-TBI increases MK and MKP numbers, and IV SDF-1 at day 4 following initial TPO treatment results in an additive improvement in circulating platelets. MKP (colony assay) and MK (imaging flow cytometry) numbers are normalized to per femur values and all compartments expressed as a percentage of unirradiated control. Mean absolute numbers for 0 Gy controls: 10 939 MKP/femur, 50 595 MK/femur, 473 × 103 platelets/μL. (B) Quantification of Gp1Bβ+ MKs in the vascular niche (physically associated with MECA32+ vessels) by femoral IHC prepared on day 5 post-4 Gy from irradiated vehicle control mice (gray), or mice treated with IV SDF-1 at day 4 (blue), 0.3 μg IP TPO (red) at 2 hours, or both 2-hour TPO and day 4 SDF-1 (purple). Percentage of MKs associated with vasculature (IHC) are normalized to per femur values (imaging flow cytometry). Mean 20 104 MK/femur associated with vasculature for 0 Gy controls. Sequential TPO and SDF-1 administration increases MKs in the vascular niche above either treatment alone. (C) Representative images of femoral marrow IHC for Gp1Bβ (MKs, red) and MECA32 (vascular endothelium, green) at day 5 post-4 Gy with hindlimbs isolated from vehicle controls (left panel), mice receiving IV SDF-1 at day 4 (middle panel), and mice receiving 2-hour TPO followed by day 4 SDF-1 (right panel) as described above. White arrows indicate examples of MKs physically associated with MECA32+ vessels. Images were acquired and processed as described in the “Immunohistochemistry” section. Error bars represent standard error of the mean of ≥3 independent experiments (n = 3-11 total mice per group). Statistical analyses comparing indicated platelet samples in panel A were performed by one-way analysis of variance with Tukey’s multiple comparisons test, and all other indicated comparisons were performed by a 2-tailed Student’s t test. Bar represents 100 μm (C). *P < .05; **P < .006.
Treatment with the cytokine TPO increases the number of MKs in the marrow and has been shown to improve MK lineage recovery when administered after sublethal TBI in mice.12,13,70 In our model, administration of TPO 2 hours following 4 Gy TBI resulted in a >200% expansion of MKPs, a 65% increase in MKs, and a small, but not statistically significant increase in platelet count (Figure 6A). Mice receiving TPO at 2 hours followed by SDF-1 at day 4 had an additive increase in circulating platelets compared with either treatment alone (Figure 6A), and a corresponding 70% increase in MKs associated with the vasculature (Figure 6B-C). These data suggest that the platelet increase accompanying SDF-1-mediated localization of MKs to the vascular niche is dependent, at least in part, on the number of MKs available to relocate. Additionally, our results indicate that a sequential combination of TPO, which increases MK number, followed by SDF-1, which enhances MK localization in the thrombopoietic niche and platelet output, may be a potent strategy to acutely improve thrombocytopenia in the setting of radiation injury.
Discussion
The working paradigm of megakaryopoiesis and thrombopoiesis has been primarily cytokine-driven, based on the potent ability of TPO to increase MK numbers and accelerate MK maturation, thereby indirectly enhancing platelet production.13,14,71 Elegant studies by Avecilla et al20 identified the chemokine SDF-1 as a TPO-independent promoter of thrombopoiesis. However, the extended timeframe of vascular SDF-1 elevation in these studies complicates the evaluation of in vivo effects on MK chemotaxis vs a direct role for SDF-1 and/or endothelium in MK lineage maturation.20 Furthermore, there are conflicting in vitro reports concerning a role for SDF-1 in MKP proliferation and MK maturation.22,72,73 Here, we directly addressed the acute effects of exogenous SDF-1 using a single IV administration, which only circulates for minutes to hours.64 With a quantitative IHC approach, we found MKs redistribute to the vasculature within 1 hour of SDF-1 administration (Figure 1E). Importantly, this rapid SDF-1-mediated association of MKs with the vasculature is also accompanied by an increase in circulating platelets apparent at 24 hours (Figure 1). In vitro treatment of MKs with SDF-1 does not yield an increase in platelets (data not shown), suggesting that SDF-1 directly contributes to MK movement to the vasculature, whereas the increase in thrombopoiesis is likely due to instructive cues from the vascular niche.4,18,19,74
To our knowledge, these studies are the first to demonstrate that natively produced SDF-1 directs MK migration to the vascular niche (Figure 2). For the stabilization of endogenous SDF-1, we used tripeptide Diprotin A to inhibit DPP4, a peptidase that inactivates SDF-1.66,75 This strategy has been used to stabilize SDF-1 in several contexts, including the homing and engraftment of hematopoietic stem cells and the recruitment of marrow-derived progenitors to sites of cardiac ischemia for myocardial regeneration.68,76-78 DPP4 was recently shown to truncate several cytokines, including IL-6, and is predicted to truncate TPO.79 While we cannot exclude effects of stabilization of these cytokines by Diprotin A, the short timeframe of our experiments coupled with the lack of change in the number or ploidy distribution of MKs (Figure 1A; supplemental Figure 3) supports SDF-1 as the mediator of spatial changes following Diprotin A treatment. Longer term inhibition of DPP4 may uncover cytokine-driven effects on the MK lineage as Dpp4−/− mice have increased numbers of MKPs and MKs.80
CXCR4 can be internalized after SDF-1 stimulation, and either recycled to the membrane or degraded.81 Unexpectedly, we found that acute stimulation with SDF-1 resulted in a small, but significant increase in CXCR4 on the surface of MKs both in vitro and in vivo, as well as following stabilization of SDF-1 in vivo (Figure 1F-G and 2B). It has been reported that SDF-1 induces CXCR4 promoter activity59 and activated nuclear factor κB, which is downstream of SDF-1/CXCR4 signaling in MKs,26 can also induce CXCR4 transcription.58 In addition, SDF-1/CXCR4 signaling in MKs induces vascular endothelial growth factor production,26 which can increase MK surface CXCR4.34 Moreover, the observed increase in surface CXCR4 could result from the translocation of intracellular stores of CXCR4 to the cell surface, which has been reported in hematopoietic progenitor cells.82 Following radiation injury, the upregulation of MK surface CXCR4 could also be due to microenvironmental hypoxia through HIF-1α83 (Figure 4B).
Our studies reveal spatial fluctuations in SDF-1 transcript production in the marrow following sublethal TBI that dynamically regulate MK location. Despite loss of the vast majority of nucleated marrow cells following TBI, we confirmed a global increase in marrow SDF-1 transcripts (Figure 3B). These data are in-line with the induction of SDF-1 expression after radiation injury in rodents and in humans, through HIF1α-dependent and HIF1α-independent pathways.39,46,84-86 More relevant for chemotactic function, we identified a transient elevation of SDF-1 transcripts near the endosteum at day 2 following injury that directs MKs away from the vascular niche (Figures 3C and 5A). Evidence pointing to a cell population that may contribute to altered SDF-1 distribution comes from the work of Dominici et al,39 Olson et al,47 and Caselli et al87 who described an expansion of osteoblasts arising from a subset of radioresistant mesenchymal cells at the endosteal surface concomitant with MK relocation following lethal TBI. Mesenchymal stromal and progenitor cells are some of the highest producers of SDF-1 in the marrow at steady state29,31,32 and are relatively radioresistant,88 suggesting these populations may participate in marrow microenvironmental changes following TBI. Further investigation to specifically identify the SDF-1–producing cells pertinent for MKs at steady state and postinjury may lead to new strategies to influence platelet production by controlling MK niche occupancy. Additionally, while this work focused on the thrombopoietic vascular niche because of its functionality for MKs, it remains an open question whether alterations in microenvironmental SDF-1 following sublethal radiation injury affect the location or function of other hematopoietic cells. This may be particularly relevant for hematopoietic stem and progenitor cells, which are regulated by SDF-129,31,32,89 and have been found in close proximity to diverse marrow microvessels,90 including preferentially arteriolar vasculature in the endosteal region.91
Significantly, our studies establish that acute manipulation of SDF-1 has functional consequences for MK location and platelet production. We tested the validity of this concept in a model of radiation-induced thrombocytopenia. While SDF-1 increases MK association with the vasculature and improves thrombocytopenia, there is an additive increase in circulating platelets if the number of marrow MKs is increased with TPO administration prior to SDF-1 treatment. These data suggest a potential sequential therapeutic approach combining an agent that first increases the number of MKs in the marrow followed by an agent that shifts their location to the vascular niche to promote platelet production. Additionally, the ability to acutely elevate circulating platelets within 24 hours may have therapeutic relevance in certain scenarios as the current clinically available Mpl agonists require 5 days to begin elevating platelet counts.92,93
Taken together, our data indicate that SDF-1 acutely regulates MK location in the bone marrow. At steady state, SDF-1 directs MKs to the vascular niche, resulting in thrombopoiesis. Following radiation injury, dynamic changes in microenvironmental SDF-1 alter the location of radioresistant MKs in the marrow. Ultimately, we demonstrate that rational manipulation of SDF-1 can acutely improve thrombocytopenia by enhancing MK migration to the thrombopoiesis-promoting vascular niche.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jennifer McLaughlin for assistance with tissue sectioning, and gratefully acknowledge the technical support of Dr Tim Bushnell and Flow Cytometry Core Facility at the University of Rochester Medical Center.
This work was supported by funding from The National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (R01AI080401 and U19AI091036) (J.P.), NIH, National Institute of Diabetes and Digestive and Kidney Diseases (F30DK100164) (L.M.N.), and the Michael Napoleone Foundation. L.M.N. is a trainee in the Medical Scientist Training Program funded by the NIH, National Institute of General Medical Sciences (T32GM07356).
Authorship
Contribution: L.M.N. designed and performed experiments, analyzed data, and wrote the manuscript; K.H.F. and P.D.K. designed and performed experiments; K.E.M. designed experiments and analyzed data; and J.P. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Palis, Department of Pediatrics, University of Rochester Medical Center, Center for Pediatric Biomedical Research, 601 Elmwood Ave, Box 703, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.