Key Points
GATA2 deficiency has a broad phenotype encompassing immunodeficiency, MDS/AML, pulmonary disease, and vascular/lymphatic dysfunction.
Early genetic diagnosis is critical to direct clinical management, prophylaxis, transplantation, and family screening.
Abstract
Haploinsufficiency of the hematopoietic transcription factor GATA2 underlies monocytopenia and mycobacterial infections; dendritic cell, monocyte, B, and natural killer (NK) lymphoid deficiency; familial myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML); and Emberger syndrome (primary lymphedema with MDS). A comprehensive examination of the clinical features of GATA2 deficiency is currently lacking. We reviewed the medical records of 57 patients with GATA2 deficiency evaluated at the National Institutes of Health from January 1, 1992, to March 1, 2013, and categorized mutations as missense, null, or regulatory to identify genotype-phenotype associations. We identified a broad spectrum of disease: hematologic (MDS 84%, AML 14%, chronic myelomonocytic leukemia 8%), infectious (severe viral 70%, disseminated mycobacterial 53%, and invasive fungal infections 16%), pulmonary (diffusion 79% and ventilatory defects 63%, pulmonary alveolar proteinosis 18%, pulmonary arterial hypertension 9%), dermatologic (warts 53%, panniculitis 30%), neoplastic (human papillomavirus+ tumors 35%, Epstein-Barr virus+ tumors 4%), vascular/lymphatic (venous thrombosis 25%, lymphedema 11%), sensorineural hearing loss 76%, miscarriage 33%, and hypothyroidism 14%. Viral infections and lymphedema were more common in individuals with null mutations (P = .038 and P = .006, respectively). Monocytopenia, B, NK, and CD4 lymphocytopenia correlated with the presence of disease (P < .001). GATA2 deficiency unites susceptibility to MDS/AML, immunodeficiency, pulmonary disease, and vascular/lymphatic dysfunction. Early genetic diagnosis is critical to direct clinical management, preventive care, and family screening.
Introduction
In 2011, heterozygous mutations in the hematopoietic transcription factor GATA2 were identified as the cause of 4 previously described clinical syndromes: monocytopenia and mycobacterial infections syndrome1,2 ; dendritic cell, monocyte, B, and natural killer (NK) lymphoid deficiency3,4 ; familial myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML)5 ; and Emberger syndrome (primary lymphedema with MDS).6,7 Interestingly, despite highly varied reasons for patient recruitment (disseminated mycobacterial infections, dendritic cell deficiency, familial MDS/AML, and primary lymphedema), there was considerable overlap among the clinical characteristics of all 4 cohorts. These syndromes are now recognized as different manifestations of a single genetic disorder with protean disease manifestations. More recently, GATA2 mutations have been identified in additional clinical cohorts including pediatric neutropenia,8 aplastic anemia,9 and the original case report of human NK cell deficiency.10,11
GATA2 mutations appear to cause loss of function of the mutated allele leading to haploinsufficiency.12 Germline mutations appear to arise spontaneously but are then transmitted with autosomal dominant inheritance.1,2 The clinical hallmarks of GATA2 deficiency include immunodeficiency with marked susceptibility to human papillomaviruses (HPVs) and nontuberculous mycobacteria (NTM), predisposition to MDS/AML, pulmonary alveolar proteinosis (PAP), and congenital lymphedema. The laboratory findings include severe circulating monocytopenia, dendritic cell cytopenia, and B and NK lymphocytopenia. These features overlap with other genetic and acquired syndromes. Mutations in TERT, TERC, CEBPA, and RUNX1 are also associated with familial MDS/AML.13 Severe HPV infection is also characteristic of epidermodysplasia verruciformis, DOCK8 deficiency, CXCR4 deficiency, and idiopathic CD4 lymphocytopenia.14 Inherited defects in the IL-12/interferon-gamma axis as well as interferon-gamma autoantibodies predispose to disseminated NTM infections.15,16 Granulocyte macrophage–colony-stimulating factor (GM-CSF) autoantibodies and mutations in the GM-CSF receptor may lead to PAP,17 whereas FLT4 and other mutations may underlie congenital lymphedema.18
Considerable clinical heterogeneity exists among patients with GATA2 deficiency. Age at disease onset ranges from early childhood to late adulthood, and clinical presentations range from asymptomatic to life-threatening infections, leukemia, and respiratory failure. The genetic lesions in GATA2 are also heterogeneous, clustering into 3 different groups: missense mutations and in-frame deletions involving the C-terminal zinc finger; nonsense mutations, frameshifts, and large gene deletions predicted to result in null alleles; and regulatory mutations within the enhancer region of intron 5.2,4,5,7,8,12,19,20 Genotype-phenotype correlations have been sought, but sample sizes and mutation distribution have been limiting. We report 57 patients with GATA2 deficiency in whom we performed comprehensive prospective characterization of the disease and looked for genotype-phenotype associations.
Methods
Patients
All patients or their guardians gave informed consent in accordance with the Declaration of Helsinki for Institutional Review Board–approved protocols at the National Institutes of Health (NIH) between January 1, 1992, and March 1, 2013. Probands were recruited through natural history protocols for the study of NTM infection, primary immune deficiency, or inherited bone marrow failure syndromes. In patients with a positive family history, a reasonable effort was made to identify and screen all available blood relatives. The medical records of 57 patients with mutation-proven GATA2 deficiency were reviewed and extracted onto a standardized form. All reported diagnostic testing was performed and reviewed internally according to standardized protocols. Data were included until chemotherapy for MDS/AML or hematopoietic stem cell transplantation (HSCT). Unless otherwise indicated, the only outcome evaluated posttransplant was survival.
Genotyping
Genomic DNA was isolated from peripheral blood, buccal swabs, or fibroblasts from all patients and available family members using the Puregene DNA isolation kit (Qiagen). Amplification and sequencing of all exons, flanking splice sites, and regulatory regions were performed as described previously.2,12 Missense mutations were analyzed using PolyPhen2 to predict deleterious effects of amino acid changes.21 Mutation groups were categorized as follows: “missense” includes all missense mutations and in-frame deletions in the C-terminal zinc finger predicted to allow production of a stable messenger RNA that is translated into a mutated protein; “null” includes nonsense and frameshift mutations and large gene deletions predicted to result in nonsense-mediated decay and no protein product; and “regulatory” includes mutations in the enhancer region of intron 5 with demonstrated reduced expression of wild-type protein.12,20 A fourth mutation group, designated “uniallelic,” includes phenotypically GATA2-deficient individuals with reduced or absent expression from a single GATA2 allele but without defined mutations.12
Diagnostic criteria
MDS, AML, and chronic myelomonocytic leukemia (CMML) were defined according to the 2008 World Health Organization criteria.22 The following were considered as severe viral infections: HPV or molluscum contagiosum infection with ≥10 warts/lesions or the presence of genital condyloma acuminata,8 herpes esophagitis or recalcitrant genital herpes infection, hemorrhagic varicella pneumonia, Epstein-Barr virus (EBV) viremia persisting for longer than 6 months or EBV+ tumors, and disseminated cytomegalovirus. Disseminated NTM and fungal infections were defined by biopsy-proven extrapulmonary involvement and identification in culture. PAP was defined by clinical presentation and demonstrated intra-alveolar accumulation of lipoproteinaceous material on lung biopsy. Lymphedema was considered in patients with chronic lower extremity and/or genital edema not attributable to other causes and severe enough to require treatment with compression stockings or lymphatic surgeries.
Statistical methods
The Fisher exact test was used to detect associations between basic demographics and genotype. For time-to-event analyses, Kaplan-Meier curves were used to estimate survival; log-rank tests and Cox regression, stratified on proband status and with robust variances, were used to detect associations among mutation groups. An exact log-rank was used for 1 rare end point (lymphedema). Wald tests using robust variance estimates to account for a within-kindred correlation were used to evaluate mean differences among groups for continuous markers. Skewed laboratory markers were log-transformed, and counts were offset by half the lower limit of detection to avoid logarithms of zero. Statistical analyses were performed using R (Version 2.14.0; Vienna: R Development Core Team). P values <.05 were not adjusted for multiple comparisons.
Results
We identified 57 patients with GATA2 deficiency, 40 based on clinical presentation and 17 through family screening (Table 1). Fifty-four percent were female; 75% were white, 16% Hispanic, 7% African American, and 2% Asian (supplemental Table 1, available on the Blood Web site). Thirty-three mutations were characterized as missense, 12 null, 9 regulatory, and 3 uniallelic (Figure 1). No associations were observed between mutation group and gender (P = .58) or race (P = .39). Median age at first NIH visit was 30 years (age range, 4-76 years) with a median length of follow-up of 14 months (range, 0-180 months). Fifty-three patients (93%) had a past or current history of at least one of the following clinical manifestations at last follow-up: severe viral or NTM infection, MDS/AML, PAP, or lymphedema. Initial clinical presentations were variable: severe viral infections (32%), disseminated NTM infections (28%), MDS/AML (21%), lymphedema (9%), and invasive fungal infections (4%). Median age at initial presentation was 20 years but was highly variable (age range, 5 months to 78 years). Four patients (7%) had no apparent clinical manifestations as of the last follow-up (range, 5-55 years).
Genetic and clinical features of 57 patients with GATA2 deficiency
| Patient . | Gender . | Mutation* . | Mutation Group† . | Age at first illness‡ . | Bone marrow pathology . | Severe viral infections . | Mycobacterial, fungal, and other invasive infections . | Other clinical features . | Age at first and last NIH visit . | Reference . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Genomic . | Protein . | . | . | . | . | . | . | First . | Last . | . |
| 7.I.1 | F | Uniallelic expression | ——- | U | 51 | MDS, LGL | HPV | M kansasii | PAP, metastatic melanoma, DVT | 53 | 64§ | 1, 12 |
| 23.I.3 | M | Uniallelic expression | ——- | U | 21 | MDS | MAC | 22 | 26§ | 2, 12 | ||
| 29.I.1 | F | Uniallelic expression | ——- | U | 45 | MDS/AML, +8 | HPV | HT | 45 | 48 | 12 | |
| 13.I.1‖ | F | 1-200_871+527del2033ins7 | M1del290 | N | 40 | HPV | Lymphedema | 60 | 60 | 1, 2, 19 | ||
| 13.II.1 | M | 1-200_871+527del2033ins7 | M1del290 | N | 33 | MDS/AML, −7 | HPV, HSV | Histoplasmosis, MAC, Neosartorya udagawae | PAP, PAH | 33 | 34§ | 1, 2, 19 |
| 8.I.1 | M | 243_244delAinsGC | G81fs | N | 9 | MDS, smoldering myeloma | HPV | MAC, soft tissue aspergillosis | PAP, PAH | 27 | 36 | 1, 2, 12, 25 |
| 26.I.1 | F | 302delG | G101AfsX16 | N | 15 | MDS/AML, CMML | HSV, CMV | 21 | 22§ | 11 | ||
| 38.I.1 | F | 417dupT | V140CfsX44 | N | 7 | HPV | 24 | 24 | 11 | |||
| 27.I.1 | F | 586_593dup | G199LfsX21 | N | 31 | HPV | M kansasii | 46 | 46 | 12 | ||
| 20.I.3 | M | 769_778dup | Y260fsX24 | N | 13 | MDS, −7 | HPV, MCV | MAC, severe C difficile | Endocarditis,¶ CVA¶ | 13 | 17 | 2, 12, 25 |
| 22.I.1 | F | 941_951dup | A318fsX12 | N | 26 | MDS | HPV | M kansasii | PAP, HT | 26 | 27§ | 2, 12 |
| 39.I.1 | F | 988C>T | R330X | N | 6 | MDS, +8 | HPV, HSV, EBV | 25 | 25 | |||
| 39.I.2 | F | 988C>T | R330X | N | 6 | MDS, +8 | HPV, HSV, EBV | Salmonella enterocolitis, Neisseria meningitis | Lymphedema | 25 | 25 | |
| 32.I.1 | M | 989_992dup4 | L332TfsX53 | N | 5 mo. | MDS/AML, −7 +8 | Lymphedema, HT | 17 | 19 | 19 | ||
| 41.I.1 | F | 1009C>T | R337X | N | 9 | MDS | HPV, HSV | Lymphedema, HT | 44 | 44 | ||
| 6.I.1‖ | M | 1017+512del28 | ——- | R | 13 | Lymphedema | 66 | 66 | ||||
| 6.II.1 | F | 1017+512del28 | ——- | R | 24 | MDS | HPV | Group C streptococcal bacteremia/osteomyelitis, M tuberculosis | Early-onset breast cancer, miscarriage | 27 | 35 | 1, 11, 20 |
| 4.I.1‖ | M | 1017+572C>T | ——- | R | 78 | CMML, LGL | 72 | 79 | 1, 12 | |||
| 4.II.1 | F | 1017+572C>T | ——- | R | 26 | MDS, LGL | HPV, CMV | MAC, disseminated histoplasmosis, Serratia pneumonia | PAP, PAH, breast cancer, miscarriage | 38 | 53§ | 1, 11, 12 |
| 4.II.5‖ | F | 1017+572C>T | ——- | R | 19 | MDS, −7 | HPV | Miscarriage | 30 | 42 | 1, 12 | |
| 4.III.1‖ | M | 1017+572C>T | ——- | R | 30 | Lymphedema | 23 | 30 | ||||
| 4.III.2‖ | F | 1017+572C>T | ——- | R | – | 21 | 28 | |||||
| 11.II.1 | F | 1017+572C>T | ——- | R | 9 | HPV, VZV | Recurrent mucosal candidiasis | 30 | 31 | 1 | ||
| 25.II.1 | M | 1017+572C>T | ——- | R | 25 | MDS | HPV | M kansasii | 34 | 36 | 12 | |
| 24.I.1 | F | 1018-1G>A | Δ340-381 | M | 43 | MDS, LGL | HPV, VZV | MAC | 43 | 47 | 2 | |
| 5.II.1 | F | 1061C>T | T354M | M | 19 | MDS/AML, LGL | HPV | MAC, M abscessus | PAP, DVT/PE | 34 | 44§ | 1, 2 |
| 17.I.1 | M | 1061C>T | T354M | M | 28 | MDS, +8 | HPV | MAC | PAP | 32 | 34 | 2, 11 |
| 17.II.2‖ | M | 1061C>T | T354M | M | – | 4 | 5 | |||||
| 19.II.1 | M | 1061C>T | T354M | M | 20 | EBV | M chelonae | 20 | 22§ | 2 | ||
| 35.III.3 | M | 1061C>T | T354M | M | 20 | MDS, +8 | Necrotizing fasciitis, granulomatous lymphadenitis | DVT/PE | 32 | 33 | 5 | |
| 37.I.1 | F | 1081C>T | R361C | M | 26 | MDS | M kansasii, MAC | 32 | 33 | |||
| 12.I.1 | M | 1083_1094del12 | R361del4 | M | 13 | MDS, −7 | HPV, HSV, HCV | M kansasii, Candida esophagitis/synovitis | PVT | 25 | 28§ | 1, 2, 11 |
| 33.III.1 | F | 1099insG | D367GfsX15 | M | 17 | MDS | MAC, severe C difficile | 17 | 17 | |||
| 33.III.3‖ | F | 1099insG | D367GfsX15 | M | – | 10 | 10 | |||||
| 10.I.1 | F | 1113C>G | N371K | M | 12 | MDS/AML, −7 | VZV, HPV | MAC, severe C difficile, Candida esophagitis | Endocarditis, CVA, DVT, PVT miscarriage | 34 | 39 | 1, 2 |
| 34.I.1‖ | F | 1116_1130del15 | C373del5 | M | 50 | MDS | Breast cancer | 51 | 51 | |||
| 34.II.1 | M | 1116_1130del15 | C373del5 | M | 14 | MDS, −7 | 15 | 15§ | ||||
| 34.II.2‖ | M | 1116_1130del15 | C373del5 | M | 13 | MDS, −7 | 15 | 15 | ||||
| 30.I.1‖ | M | 1163T>C | M388T | M | 65 | HPV | Oropharyngeal SCC, RCC | 76 | 77 | |||
| 30.II.1 | F | 1163T>C | M388T | M | 27 | HPV, HSV | M. kansasii, severe C difficile | PAP | 43 | 43 | ||
| 30.II.4‖ | F | 1163T>C | M388T | M | 30 | HPV | 52 | 52 | ||||
| 15.I.1 | F | 1186C>T | R396W | M | 7 | MDS, +8 | HSV | MAC, E histolytica | 8 | 13 | 1, 2, 11 | |
| 42.I.1 | M | 1186C>T | R396W | M | 18 | MDS | HPV, EBV | M szulgai, MAC | EBV+ spindle cell tumors | 24 | 24 | |
| 14.I.1 | F | 1187G>A | R396Q | M | 7 | EBV, HSV | 7 | 12 | 1 | |||
| 18.I.2 | F | 1187G>A | R396Q | M | 16 | MDS, +8 | M abscessus, nocardiosis | 16 | 25 | 2, 25 | ||
| 40.I.1‖ | M | 1187G>A | R396Q | M | – | 54 | 55 | |||||
| 40.II.1 | M | 1187G>A | R396Q | M | 18 | MDS, +8 | HPV, VZV | 23 | 23 | |||
| 40.II.2‖ | M | 1187G>A | R396Q | M | 16 | MDS, +8 | HPV | Desmoid tumor, DVT/PE¶ | 21 | 21 | ||
| 40.II.3‖ | M | 1187G>A | R396Q | M | 14 | MDS, +8 | HPV, VZV | HT | 17 | 17 | ||
| 31.II.1 | M | 1187G>A | R396Q | M | 27 | MDS | HPV | Granulomatous lymphadenitis, invasive aspergillosis | Sarcoidosis-like pulmonary process | 30 | 32 | |
| 31.II.2‖ | M | 1187G>A | R396Q | M | 29 | MDS | 29 | 29 | ||||
| 1.II.1 | F | 1192C>T | R398W | M | 20s | CMML | HPV, HSV, EBV | MAC | BOOP, EBV+ leiomyosarcomata, HT | 41 | 46§ | 1, 2 |
| 1.II.5‖ | F | 1192C>T | R398W | M | 18 | MDS, LGL | HPV, VZV | MAC, invasive aspergillosis | PAP, PAH, miscarriage | 36 | 49§ | 1, 2, 25 |
| 2.II.3 | M | 1192C>T | R398W | M | 34 | MDS/AML, +8, LGL | HPV | Histoplasmosis, MAC | CVA, HT | 37 | 39§ | 1, 2 |
| 3.I.1 | F | 1192C>T | R398W | M | 48 | CMML, LGL | HPV | M fortuitum, invasive aspergillosis, severe C difficile | PAP, PAH, HT, pancreatic cancer | 49 | 59§ | 1, 2 |
| 9.III.1 | M | 1192C>T | R398W | M | 8 | MDS | HPV | M fortuitum | 15 | 22 | 1, 2 | |
| 21.II.1 | M | 1192C>T | R398W | M | 32 | MDS | HPV, MCV | MAC | 32 | 34 | 2, 25 | |
| Patient . | Gender . | Mutation* . | Mutation Group† . | Age at first illness‡ . | Bone marrow pathology . | Severe viral infections . | Mycobacterial, fungal, and other invasive infections . | Other clinical features . | Age at first and last NIH visit . | Reference . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Genomic . | Protein . | . | . | . | . | . | . | First . | Last . | . |
| 7.I.1 | F | Uniallelic expression | ——- | U | 51 | MDS, LGL | HPV | M kansasii | PAP, metastatic melanoma, DVT | 53 | 64§ | 1, 12 |
| 23.I.3 | M | Uniallelic expression | ——- | U | 21 | MDS | MAC | 22 | 26§ | 2, 12 | ||
| 29.I.1 | F | Uniallelic expression | ——- | U | 45 | MDS/AML, +8 | HPV | HT | 45 | 48 | 12 | |
| 13.I.1‖ | F | 1-200_871+527del2033ins7 | M1del290 | N | 40 | HPV | Lymphedema | 60 | 60 | 1, 2, 19 | ||
| 13.II.1 | M | 1-200_871+527del2033ins7 | M1del290 | N | 33 | MDS/AML, −7 | HPV, HSV | Histoplasmosis, MAC, Neosartorya udagawae | PAP, PAH | 33 | 34§ | 1, 2, 19 |
| 8.I.1 | M | 243_244delAinsGC | G81fs | N | 9 | MDS, smoldering myeloma | HPV | MAC, soft tissue aspergillosis | PAP, PAH | 27 | 36 | 1, 2, 12, 25 |
| 26.I.1 | F | 302delG | G101AfsX16 | N | 15 | MDS/AML, CMML | HSV, CMV | 21 | 22§ | 11 | ||
| 38.I.1 | F | 417dupT | V140CfsX44 | N | 7 | HPV | 24 | 24 | 11 | |||
| 27.I.1 | F | 586_593dup | G199LfsX21 | N | 31 | HPV | M kansasii | 46 | 46 | 12 | ||
| 20.I.3 | M | 769_778dup | Y260fsX24 | N | 13 | MDS, −7 | HPV, MCV | MAC, severe C difficile | Endocarditis,¶ CVA¶ | 13 | 17 | 2, 12, 25 |
| 22.I.1 | F | 941_951dup | A318fsX12 | N | 26 | MDS | HPV | M kansasii | PAP, HT | 26 | 27§ | 2, 12 |
| 39.I.1 | F | 988C>T | R330X | N | 6 | MDS, +8 | HPV, HSV, EBV | 25 | 25 | |||
| 39.I.2 | F | 988C>T | R330X | N | 6 | MDS, +8 | HPV, HSV, EBV | Salmonella enterocolitis, Neisseria meningitis | Lymphedema | 25 | 25 | |
| 32.I.1 | M | 989_992dup4 | L332TfsX53 | N | 5 mo. | MDS/AML, −7 +8 | Lymphedema, HT | 17 | 19 | 19 | ||
| 41.I.1 | F | 1009C>T | R337X | N | 9 | MDS | HPV, HSV | Lymphedema, HT | 44 | 44 | ||
| 6.I.1‖ | M | 1017+512del28 | ——- | R | 13 | Lymphedema | 66 | 66 | ||||
| 6.II.1 | F | 1017+512del28 | ——- | R | 24 | MDS | HPV | Group C streptococcal bacteremia/osteomyelitis, M tuberculosis | Early-onset breast cancer, miscarriage | 27 | 35 | 1, 11, 20 |
| 4.I.1‖ | M | 1017+572C>T | ——- | R | 78 | CMML, LGL | 72 | 79 | 1, 12 | |||
| 4.II.1 | F | 1017+572C>T | ——- | R | 26 | MDS, LGL | HPV, CMV | MAC, disseminated histoplasmosis, Serratia pneumonia | PAP, PAH, breast cancer, miscarriage | 38 | 53§ | 1, 11, 12 |
| 4.II.5‖ | F | 1017+572C>T | ——- | R | 19 | MDS, −7 | HPV | Miscarriage | 30 | 42 | 1, 12 | |
| 4.III.1‖ | M | 1017+572C>T | ——- | R | 30 | Lymphedema | 23 | 30 | ||||
| 4.III.2‖ | F | 1017+572C>T | ——- | R | – | 21 | 28 | |||||
| 11.II.1 | F | 1017+572C>T | ——- | R | 9 | HPV, VZV | Recurrent mucosal candidiasis | 30 | 31 | 1 | ||
| 25.II.1 | M | 1017+572C>T | ——- | R | 25 | MDS | HPV | M kansasii | 34 | 36 | 12 | |
| 24.I.1 | F | 1018-1G>A | Δ340-381 | M | 43 | MDS, LGL | HPV, VZV | MAC | 43 | 47 | 2 | |
| 5.II.1 | F | 1061C>T | T354M | M | 19 | MDS/AML, LGL | HPV | MAC, M abscessus | PAP, DVT/PE | 34 | 44§ | 1, 2 |
| 17.I.1 | M | 1061C>T | T354M | M | 28 | MDS, +8 | HPV | MAC | PAP | 32 | 34 | 2, 11 |
| 17.II.2‖ | M | 1061C>T | T354M | M | – | 4 | 5 | |||||
| 19.II.1 | M | 1061C>T | T354M | M | 20 | EBV | M chelonae | 20 | 22§ | 2 | ||
| 35.III.3 | M | 1061C>T | T354M | M | 20 | MDS, +8 | Necrotizing fasciitis, granulomatous lymphadenitis | DVT/PE | 32 | 33 | 5 | |
| 37.I.1 | F | 1081C>T | R361C | M | 26 | MDS | M kansasii, MAC | 32 | 33 | |||
| 12.I.1 | M | 1083_1094del12 | R361del4 | M | 13 | MDS, −7 | HPV, HSV, HCV | M kansasii, Candida esophagitis/synovitis | PVT | 25 | 28§ | 1, 2, 11 |
| 33.III.1 | F | 1099insG | D367GfsX15 | M | 17 | MDS | MAC, severe C difficile | 17 | 17 | |||
| 33.III.3‖ | F | 1099insG | D367GfsX15 | M | – | 10 | 10 | |||||
| 10.I.1 | F | 1113C>G | N371K | M | 12 | MDS/AML, −7 | VZV, HPV | MAC, severe C difficile, Candida esophagitis | Endocarditis, CVA, DVT, PVT miscarriage | 34 | 39 | 1, 2 |
| 34.I.1‖ | F | 1116_1130del15 | C373del5 | M | 50 | MDS | Breast cancer | 51 | 51 | |||
| 34.II.1 | M | 1116_1130del15 | C373del5 | M | 14 | MDS, −7 | 15 | 15§ | ||||
| 34.II.2‖ | M | 1116_1130del15 | C373del5 | M | 13 | MDS, −7 | 15 | 15 | ||||
| 30.I.1‖ | M | 1163T>C | M388T | M | 65 | HPV | Oropharyngeal SCC, RCC | 76 | 77 | |||
| 30.II.1 | F | 1163T>C | M388T | M | 27 | HPV, HSV | M. kansasii, severe C difficile | PAP | 43 | 43 | ||
| 30.II.4‖ | F | 1163T>C | M388T | M | 30 | HPV | 52 | 52 | ||||
| 15.I.1 | F | 1186C>T | R396W | M | 7 | MDS, +8 | HSV | MAC, E histolytica | 8 | 13 | 1, 2, 11 | |
| 42.I.1 | M | 1186C>T | R396W | M | 18 | MDS | HPV, EBV | M szulgai, MAC | EBV+ spindle cell tumors | 24 | 24 | |
| 14.I.1 | F | 1187G>A | R396Q | M | 7 | EBV, HSV | 7 | 12 | 1 | |||
| 18.I.2 | F | 1187G>A | R396Q | M | 16 | MDS, +8 | M abscessus, nocardiosis | 16 | 25 | 2, 25 | ||
| 40.I.1‖ | M | 1187G>A | R396Q | M | – | 54 | 55 | |||||
| 40.II.1 | M | 1187G>A | R396Q | M | 18 | MDS, +8 | HPV, VZV | 23 | 23 | |||
| 40.II.2‖ | M | 1187G>A | R396Q | M | 16 | MDS, +8 | HPV | Desmoid tumor, DVT/PE¶ | 21 | 21 | ||
| 40.II.3‖ | M | 1187G>A | R396Q | M | 14 | MDS, +8 | HPV, VZV | HT | 17 | 17 | ||
| 31.II.1 | M | 1187G>A | R396Q | M | 27 | MDS | HPV | Granulomatous lymphadenitis, invasive aspergillosis | Sarcoidosis-like pulmonary process | 30 | 32 | |
| 31.II.2‖ | M | 1187G>A | R396Q | M | 29 | MDS | 29 | 29 | ||||
| 1.II.1 | F | 1192C>T | R398W | M | 20s | CMML | HPV, HSV, EBV | MAC | BOOP, EBV+ leiomyosarcomata, HT | 41 | 46§ | 1, 2 |
| 1.II.5‖ | F | 1192C>T | R398W | M | 18 | MDS, LGL | HPV, VZV | MAC, invasive aspergillosis | PAP, PAH, miscarriage | 36 | 49§ | 1, 2, 25 |
| 2.II.3 | M | 1192C>T | R398W | M | 34 | MDS/AML, +8, LGL | HPV | Histoplasmosis, MAC | CVA, HT | 37 | 39§ | 1, 2 |
| 3.I.1 | F | 1192C>T | R398W | M | 48 | CMML, LGL | HPV | M fortuitum, invasive aspergillosis, severe C difficile | PAP, PAH, HT, pancreatic cancer | 49 | 59§ | 1, 2 |
| 9.III.1 | M | 1192C>T | R398W | M | 8 | MDS | HPV | M fortuitum | 15 | 22 | 1, 2 | |
| 21.II.1 | M | 1192C>T | R398W | M | 32 | MDS | HPV, MCV | MAC | 32 | 34 | 2, 25 | |
BOOP, bronchiolitis obliterans organizing pneumonia; CMV, cytomegalovirus; CVA, cerebrovascular accident; DVT, deep vein thrombosis; HCV, hepatitis C virus; HSV, herpes simplex virus; HT, hypothyroidism; LGL, large granular lymphocytosis; MAC, M avium complex; MCV, molluscum contagiosum virus; PE, pulmonary embolism; PVT, portal vein thrombosis; RCC, renal cell carcinoma; VZV, varicella zoster virus; −7, monosomy 7; +8, trisomy 8.
All mutations refer to isoform NM_001145661, protein changes reference NP_116027.
Mutation groups are defined as follows: M, missense, includes all missense mutations, in-frame deletions, and insertions/deletions predicted to escape nonsense-mediated decay; N, null, includes insertions/deletions and nonsense mutations predicted to result in nonsense mediated decay and no protein product; R, regulatory, includes mutations in the intron 5 enhancer with demonstrated reduced expression of wild-type protein; U, uniallelic, includes undefined mutations with reduced or absent transcription from 1 allele by cDNA analysis.
First illness is defined as severe infection, MDS/AML, PAP, or lymphedema.
Deceased.
Relative of proband.
Complication developed posttransplant.
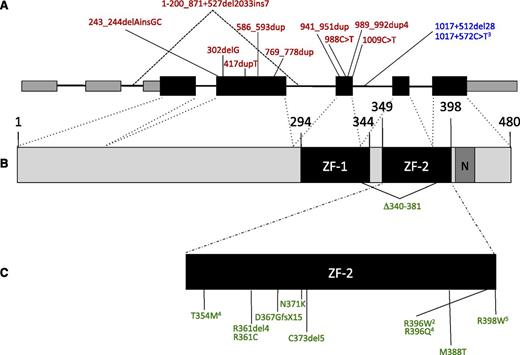
GATA2 mutations. (A) Genomic organization of GATA2 showing the 2 5′-untranslated and 5 coding exons. Larger boxes represent coding regions. Insertion/deletion and nonsense mutations predicted to result in null alleles are shown above in red. Regulatory mutations in intron 5 are shown in blue. (B) Protein domains of GATA2, showing N- and C-terminal zinc fingers (ZF-1, ZF-2) and nuclear localization signal (N). (C) Missense mutations, in-frame deletions, and frameshift mutations predicted to escape nonsense-mediated decay are shown in green. Superscript numerals indicate the number of independent mutations.
GATA2 mutations. (A) Genomic organization of GATA2 showing the 2 5′-untranslated and 5 coding exons. Larger boxes represent coding regions. Insertion/deletion and nonsense mutations predicted to result in null alleles are shown above in red. Regulatory mutations in intron 5 are shown in blue. (B) Protein domains of GATA2, showing N- and C-terminal zinc fingers (ZF-1, ZF-2) and nuclear localization signal (N). (C) Missense mutations, in-frame deletions, and frameshift mutations predicted to escape nonsense-mediated decay are shown in green. Superscript numerals indicate the number of independent mutations.
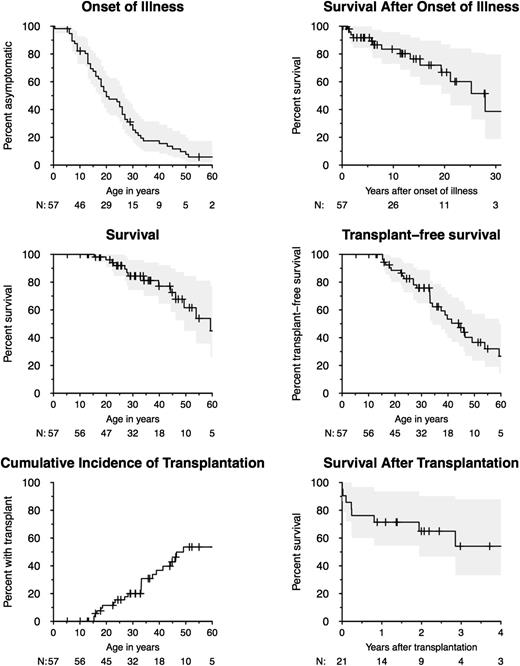
The proportion of patients without symptoms was 50% by age 20 years, 25% by age 30 years, and 16% by age 40 years (Figure 2). Survival time declined soon after onset of symptoms, with 91% estimated survival time by 5 years after onset, 84% by 10 years, and 67% by 20 years. Overall survival rate was 96% by age 20 years, 77% by age 40 years, and 45% by age 60 years. Twenty-one patients underwent allogeneic HSCT for MDS/AML, PAP, and/or recurrent infection (age range at transplantation, 15-49 years). The proportion of patients surviving without HSCT was 89% by age 20 years, 76% by age 30 years, and 53% by age 40 years. Posttransplant survival rate was 72% by 1 year, 65% by 2 years, and 54% by 4 years after HSCT.
Onset of illness, survival, and transplantation. Kaplan-Meier curves are shown for onset of illness (first severe infection, myelodysplasia/leukemia, PAP, or lymphedema), survival after onset of illness, overall survival, and transplant-free survival for 57 individuals. Cumulative incidence of transplantation is shown accounting for death as a competing event. Posttransplant survival is shown for 21 individuals who underwent transplantation. The number of individuals under follow-up is indicated under the X axis. Confidence intervals of 95% are shaded in gray.
Onset of illness, survival, and transplantation. Kaplan-Meier curves are shown for onset of illness (first severe infection, myelodysplasia/leukemia, PAP, or lymphedema), survival after onset of illness, overall survival, and transplant-free survival for 57 individuals. Cumulative incidence of transplantation is shown accounting for death as a competing event. Posttransplant survival is shown for 21 individuals who underwent transplantation. The number of individuals under follow-up is indicated under the X axis. Confidence intervals of 95% are shaded in gray.
Clinical features
Hematologic.
Hemograms and lymphocyte phenotyping from the initial NIH evaluation were available for 55 and 51 patients, respectively. B lymphocytopenia (86%), NK lymphocytopenia (82%), and monocytopenia (78%) were common and profound (supplemental Figure 1). CD4 lymphocytopenia (51%) and neutropenia (47%) were also common but were generally less marked. Pancytopenia was observed in the setting of MDS and monocytosis in patients with CMML. Of 50 patients who underwent bone marrow biopsies, 42 (84%) met diagnostic criteria for MDS with most marrows demonstrating multilineage dysplasia and <5% blasts (supplemental Table 2). In contrast to de novo MDS, which is typically characterized by bone marrow hypercellularity, most bone marrows were hypocellular for age with increased reticulin fibrosis (Figure 3A).23 Atypical megakaryocytes were identified in 92% of patients, even in those without overt MDS (Figure 3B-D).23,24 Forty-eight percent had abnormal bone marrow cytogenetics, including trisomy 8 in 24% and monosomy 7 in 16%. There were 14% of patients whose condition evolved into AML, and 8% met criteria for CMML. Increased populations of CD3+CD8+CD56+ large granular lymphocytes were detected in 16% of patients.
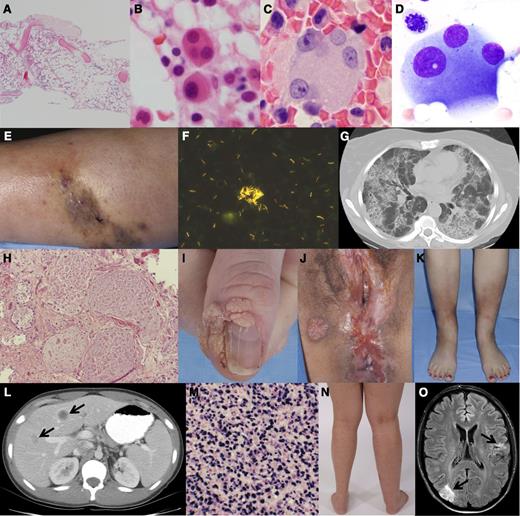
Clinical, pathologic, and radiographic features of GATA2 deficiency. (A) Hypocellular bone marrow with trilineage hypoplasia (36-year-old woman), bone marrow core biopsy hematoxylin and eosin stain. (B-D) Atypical megakaryocytes, small mononuclear (B), and dysplastic forms with separated nuclear lobes (B-D); taken from bone marrow core biopsies (B,C) and aspirate smear (D). (E) Cutaneous M abscessus infection and (F) skin biopsy AFB smear showing abundant mycobacteria. (G) Computed tomography scan of the chest showing the typical crazy paving pattern of PAP and (H) lung biopsy hematoxylin and eosin stain showing alveolar filling with lipoproteinaceous material. (I) Recalcitrant periungual warts, (J) perineal condyloma, and vulvar/anal intraepithelial neoplasia. (K) Panniculitis/erythema nodosum involving the anterior shins. (L) Computed tomography scan of the abdomen with multiple hypodense liver lesions (arrows) and (M) liver biopsy showing EBV-related spindle cell tumor with in situ hybridization of EBV-encoded messenger RNA using EBV probe antifluorescein antibody and bond polymer refine detection. (N) Unilateral lymphedema of the right lower extremity. (O) Magnetic resonance imaging of the brain with embolic infarcts in the right occipital and left parietal lobes (arrows) in the setting of culture-negative endocarditis.
Clinical, pathologic, and radiographic features of GATA2 deficiency. (A) Hypocellular bone marrow with trilineage hypoplasia (36-year-old woman), bone marrow core biopsy hematoxylin and eosin stain. (B-D) Atypical megakaryocytes, small mononuclear (B), and dysplastic forms with separated nuclear lobes (B-D); taken from bone marrow core biopsies (B,C) and aspirate smear (D). (E) Cutaneous M abscessus infection and (F) skin biopsy AFB smear showing abundant mycobacteria. (G) Computed tomography scan of the chest showing the typical crazy paving pattern of PAP and (H) lung biopsy hematoxylin and eosin stain showing alveolar filling with lipoproteinaceous material. (I) Recalcitrant periungual warts, (J) perineal condyloma, and vulvar/anal intraepithelial neoplasia. (K) Panniculitis/erythema nodosum involving the anterior shins. (L) Computed tomography scan of the abdomen with multiple hypodense liver lesions (arrows) and (M) liver biopsy showing EBV-related spindle cell tumor with in situ hybridization of EBV-encoded messenger RNA using EBV probe antifluorescein antibody and bond polymer refine detection. (N) Unilateral lymphedema of the right lower extremity. (O) Magnetic resonance imaging of the brain with embolic infarcts in the right occipital and left parietal lobes (arrows) in the setting of culture-negative endocarditis.
Infectious.
Major infections affected 82% of patients (supplemental Table 3). Severe viral infections were the most common (70%), particularly HPV (63%) presenting with recalcitrant warts, condylomata, and/or dysplasia. Severe herpesvirus infections were present in 35% of patients: recurrent herpes stomatitis, esophagitis, or genital infection in 16%; severe varicella in 11%; persistent EBV viremia in 11%; and cytomegalovirus pneumonia or disseminated disease in 4%. Severe cutaneous molluscum contagiosum infections developed in 2 patients. Disseminated NTM infections were present in 53% of patients, but this may reflect a referral bias of our institution (Figure 3E-F). NTM infections included both slow-growing (Mycobacterium avium complex, M kansasii, and M szulgai) and rapid-growing mycobacteria (M fortuitum, M abscessus, and M chelonae). Other severe bacterial infections were observed in 49%: bacteremia in 21%, skin and soft tissue infection in 19%, pneumonia in 14%, and colitis in 9%. Clostridium difficile infections were particularly severe, including 1 fatality, 1 colectomy in childhood, and 2 cases of toxic megacolon. Severe fungal infections were observed in 16%: invasive aspergillosis in 9%, disseminated histoplasmosis in 5%, and recalcitrant mucosal candidiasis in 5%. After HSCT, no patients had reactivation of preexisting NTM or fungal infections, and most patients demonstrated marked clearing of HPV lesions.25
Pulmonary.
Biopsy-proven PAP was identified in 10 adults (18%), all of whom lacked anti-GM-CSF autoantibodies and whose disease was refractory to subcutaneous and inhaled GM-CSF therapy (Figure 3G-H). Five patients with PAP (9%) went on to have subsequent pulmonary arterial hypertension (PAH). After HSCT, all patients with PAP/PAH demonstrated significant improvement in pulmonary function, including weaning from supplemental oxygen and medications for PAH.25 Even among patients without PAP, pulmonary function and radiographic appearance were typically abnormal (supplemental Table 4). Of 38 patients who received pulmonary function testing, 30 (79%) had diffusion defects and 24 (63%) had ventilatory defects, including obstruction (39%), restriction (11%), and mixed patterns (13%). Structural abnormalities identified on chest computed tomography included nodules, reticular opacities, ground-glass opacities, subpleural blebbing, “crazy paving,” and paraseptal emphysema. Surprisingly, despite profound circulating monocytopenia, alveolar macrophages were abundant in bronchoalveolar lavage fluids.
Dermatologic.
Dermatologic disease was often an indicator of underlying infection or malignancy (supplemental Table 5). Recurrent warts were present in the majority of patients (53%) and were often the initial manifestation of disease. Common and flat warts were present on the extremities (39%) and/or genitalia (32%) (Figure 3I-J) and were typically refractory to cauterization, cryotherapy, topical imiquimod, 5-fluorouracil, corticosteroids, and laser therapies. Biopsy-proven panniculitis/erythema nodosum was seen in 30% of patients and was commonly associated with underlying NTM or fungal infection (Figure 3K). Skin cancers, including basal cell carcinoma, squamous cell carcinoma (SCC), and malignant melanoma, developed in 6 patients (11%), half of whom had multiple cancers. Sweet syndrome was seen in 3 patients in the setting of underlying MDS/AML.
Neoplastic.
The majority of solid tumors were related to underlying viral infections. HPV-related dysplasia was observed in 35% of patients and ranged from squamous intraepithelial lesions/Bowenoid papulosis to invasive SCC (supplemental Table 6). Most patients with genital dysplasia required recurrent laser ablative surgeries, loop electrosurgical excision procedures, or radical surgery. HPV-related head and neck cancers were also observed, including SCC of the oral commissure and oropharynx. Unusual EBV+ mesenchymal tumors were observed in 2 patients: one with widely disseminated EBV+ leiomyosarcomata,1 and another with EBV+ spindle cell tumors of the liver (Figure 3L-M). Notably, of 18 women ≥35 years of age at last follow-up, 4 (22%) had breast cancers, one of whom had a BRCA2 mutation. Other solid tumors included adenocarcinoma of the pancreas, renal cell carcinoma, and a locally invasive desmoid tumor of the chest wall.
Cardiovascular and lymphatic.
Chronic lymphedema was present in 6 patients (11%) and could involve the unilateral or bilateral lower extremities and genitalia (Figure 3N). Severe cases developed in infancy or childhood, were complicated by recurrent cellulitis, and often required lymphatic surgeries. Other cases only first manifested in adolescence or adulthood and were managed conservatively with compression stockings. Venous thromboses, including deep vein thrombosis, pulmonary embolism, portal vein thrombosis, and catheter-related thrombosis, were observed in 14 patients (25%), half of whom had multiple events (supplemental Table 7). Lupus anticoagulant was transiently positive (ie, negative on repeat testing) in 7 of 14 patients with thrombotic events. One patient had mutations in Factor V Leiden and methylenetetrahydrofolate reductase. Two patients experienced culture-negative endocarditis (1 posttransplant) with demonstrated mitral valve vegetations and subsequent embolic strokes (Figure 3O). Thrombotic events also developed posttransplant in 2 patients.
Other clinical findings.
Other associated clinical findings included sensorineural hearing loss, miscarriage, and idiopathic hypothyroidism. Of 25 patients with age-adjusted audiograms, 19 (76%) had mild to severe sensorineural hearing loss typically involving the high frequency range (supplemental Table 8). However, prior aminoglycoside exposure for disseminated NTM infections may confound this relationship. Follow-up audiograms on 9 patients found 5 with progressive deterioration. Of 43 known pregnancies in our cohort, 14 (33%) resulted in miscarriage (supplemental Table 9). Notably, all women who miscarried demonstrated marked NK lymphocytopenia. Idiopathic hypothyroidism requiring thyroid hormone replacement was observed in 14% of patients.
Phenotype clustering
Certain clinical phenotypes were frequently encountered in combination with others (supplemental Figure 2). For example, all patients with PAP had comorbid immunodeficiency with recurrent viral and NTM infections. Nine of 10 patients with PAP also had comorbid MDS. The majority of patients with MDS also had a history of recurrent infections with viruses, NTM, or both. However, MDS without associated immunodeficiency developed in 6 patients. Of 6 patients with lymphedema, 4 had recurrent warts and/or MDS characteristic of Emberger syndrome. However, 2 patients with lymphedema had no other identifiable clinical manifestations. Four patients had a history of severe viral infections with no other identifiable manifestations.
Phenotypic variation within kindreds
Certain phenotypes tended to cluster within families (supplemental Figure 3). For example, in kindred 40, the proband and his siblings each experienced warts and MDS with trisomy 8. Their family history was also significant for multiple family members with MDS/AML and HPV-related cancer. Interestingly, however, their father carries the exact same mutation but is clinically asymptomatic. Other families demonstrated marked clinical heterogeneity. For example, the proband in kindred 4 first presented at age 26 years with disseminated M avium complex and went on to have disseminated histoplasmosis, MDS, PAP/PAH, and multiple malignancies of the skin and breast. Her sister first presented at age 19 years with genital HPV infection and later had MDS with monosomy 7 requiring HSCT. However, in contrast to their severe presentations in adolescence and early adulthood, their father was clinically silent until age 78 years, at which time he had monocytosis and bone marrow changes consistent with CMML. The proband’s 2 children who carry the same genetic mutation are currently asymptomatic except for lower-extremity lymphedema in her son.
Genotype-phenotype associations
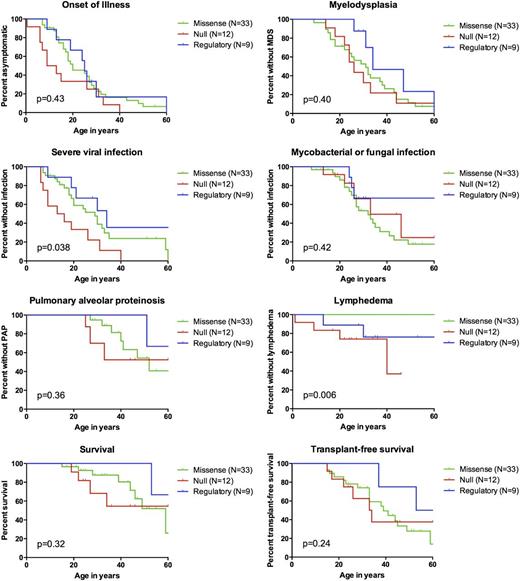
We compared Kaplan-Meier disease-free survival curves among patients with missense, null, and regulatory mutations for the following end points: onset of illness, MDS/AML, severe viral infection, NTM/fungal infection, PAP, lymphedema, survival, and transplant-free survival (Figure 4). Severe viral infections were more common and had earlier-onset in those with null mutations (P = .038). Lymphedema was only observed in those with null or regulatory mutations, but not in those with missense mutations (exact log rank P = .006). No other end points differed significantly among mutation groups.
Genotype-phenotype associations. Kaplan-Meier curves are shown for patients with missense (green, N = 33), null (red, N = 12), and regulatory mutations (blue, N = 9). Patients with uniallelic expression (N = 3) are not shown. Mutation group was associated with the risk for viral infection (P = .038) and lymphedema (exact log-rank test P = .006). No other end points differed by mutation group. Unless otherwise noted, P values were calculated using Cox regression stratified on proband status, with robust standard errors to adjust for kindred clusters.
Genotype-phenotype associations. Kaplan-Meier curves are shown for patients with missense (green, N = 33), null (red, N = 12), and regulatory mutations (blue, N = 9). Patients with uniallelic expression (N = 3) are not shown. Mutation group was associated with the risk for viral infection (P = .038) and lymphedema (exact log-rank test P = .006). No other end points differed by mutation group. Unless otherwise noted, P values were calculated using Cox regression stratified on proband status, with robust standard errors to adjust for kindred clusters.
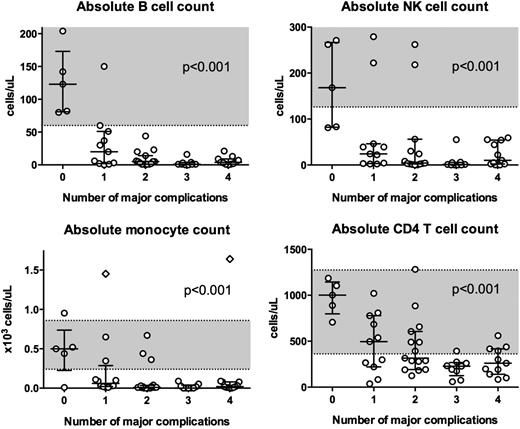
Blood counts and disease severity
We developed a scoring system to estimate disease severity or progression on the basis of the number of major complications. The following complications were considered as major, given their associated morbidity and mortality: MDS/AML, severe viral infection, NTM/fungal infection, and PAP. Initial presenting B-cell, NK-cell, monocyte, and CD4 T-cell counts were compared among individuals with 0, 1, 2, 3, or 4 of these complications at the time of clinical presentation (Figure 5). Among individuals with no major complications, median blood counts were within the normal range. However, as complications mounted, median blood counts declined significantly for all cell lines (P < .001).
Blood counts and disease severity. Absolute B cell, NK cell, monocyte, and CD4+ T cell count at first NIH visit for patients with 0, 1, 2, 3, or 4 of the following major complications: MDS/leukemia, severe viral infection, disseminated mycobacterial or fungal infection, and PAP. Monocyte counts and lymphocyte phenotyping were available for 55 and 51 patients, respectively. Shaded regions indicate normal blood count ranges from the NIH Department of Laboratory Medicine: absolute CD20+ B cell count 60 to 539 cells/μL; absolute NK cell count 126 to 729 cells/μL; absolute monocyte count 0.24 to 0.86 x103 cells/μL; and absolute CD4+ T-cell count 362 to 1275 cells/μL. Diamonds indicate individuals with monocytosis in the setting of CMML. Horizontal bars indicate medians. Vertical bars indicate interquartile ranges. Blood counts were significantly associated with the number of major complications (P < .001). P values were obtained using linear regression with robust standard errors to adjust for kindred clusters. Analysis was done for the log-transformed cell counts, with offsets of half the lower limit of detection for B cells, NK cells, and monocytes to account for those cases in which the numbers were zero.
Blood counts and disease severity. Absolute B cell, NK cell, monocyte, and CD4+ T cell count at first NIH visit for patients with 0, 1, 2, 3, or 4 of the following major complications: MDS/leukemia, severe viral infection, disseminated mycobacterial or fungal infection, and PAP. Monocyte counts and lymphocyte phenotyping were available for 55 and 51 patients, respectively. Shaded regions indicate normal blood count ranges from the NIH Department of Laboratory Medicine: absolute CD20+ B cell count 60 to 539 cells/μL; absolute NK cell count 126 to 729 cells/μL; absolute monocyte count 0.24 to 0.86 x103 cells/μL; and absolute CD4+ T-cell count 362 to 1275 cells/μL. Diamonds indicate individuals with monocytosis in the setting of CMML. Horizontal bars indicate medians. Vertical bars indicate interquartile ranges. Blood counts were significantly associated with the number of major complications (P < .001). P values were obtained using linear regression with robust standard errors to adjust for kindred clusters. Analysis was done for the log-transformed cell counts, with offsets of half the lower limit of detection for B cells, NK cells, and monocytes to account for those cases in which the numbers were zero.
Discussion
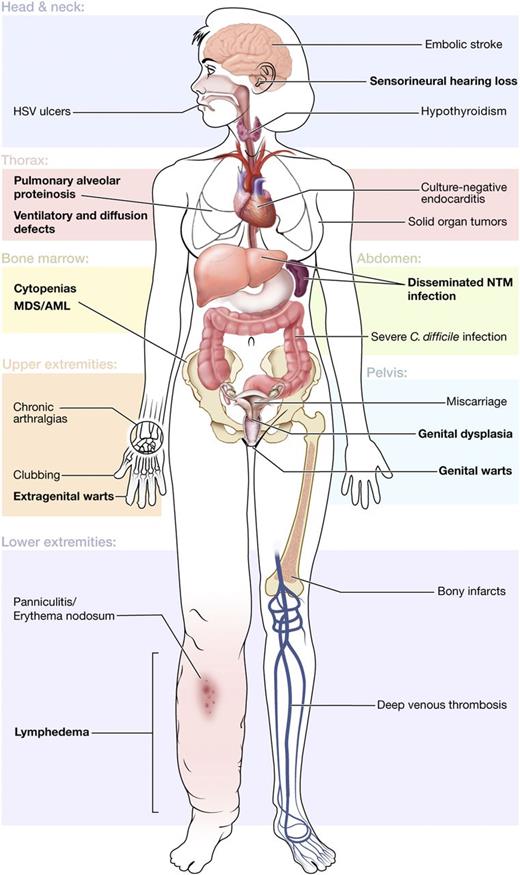
We systematically reviewed 57 patients with GATA2 deficiency and identified a broad phenotype encompassing MDS/AML, immunodeficiency, pulmonary disease, vascular/lymphatic dysfunction, and additional clinical associations (Figure 6). Recognition of these clinical features is critical to facilitate early genetic diagnosis and to direct appropriate clinical management, preventive care, and family screening.
Clinical features of GATA2 deficiency by organ system. Common clinical findings are shown by the affected organ system. Primary disease features are indicated in bold.
Clinical features of GATA2 deficiency by organ system. Common clinical findings are shown by the affected organ system. Primary disease features are indicated in bold.
GATA2 belongs to a family of zinc finger transcription factors that are critical regulators of gene expression in hematopoietic cells.26 Although GATA1 functions primarily in erythropoiesis27 and megakaryopoiesis28 and GATA3 in T-cell lymphopoiesis,29 GATA2 is particularly critical for the genesis and function of hematopoietic stem and progenitor cells and thus all subsequent blood cell lineages.30-32 GATA2 haploinsufficiency perturbs normal hematopoietic stem cell homeostasis in murine models.33 The high prevalence of cytopenias and bone marrow failure in our cohort supports a similar defect in human GATA2 haploinsufficiency. It remains unclear why monocytes, dendritic cells, B cells, and NK cells are preferentially depleted, but profound cytopenias of all of these lineages appears to be highly specific for GATA2 deficiency.
The presence of neutropenia and CD4 lymphocytopenia in roughly half of our patients suggests that idiopathic neutropenia and idiopathic CD4 lymphocytopenia may be important populations to screen. Indeed, targeted screening of 14 probands from the French Severe Chronic Neutropenia Registry identified 6 with GATA2 mutations.8 Pediatric MDS represents another critical population to screen, with GATA2 mutations identified in 8 (16%) of 51 patients with MDS and monosomy 7 in the European Working Group of MDS and JMML in Childhood.34 In the absence of cytogenetic abnormalities or overt dysplasia, the hypoplastic bone marrow of GATA2 deficiency may also be initially diagnosed as aplastic anemia.9,35 Vigilant bone marrow monitoring is prudent in patients with GATA2 deficiency, as the development of excess blasts or abnormal cytogenetics may influence the timing of HSCT. Somatic mutations in ASXL1 have also been implicated in leukemic transformation and might accelerate the timing for HSCT.36,37 Importantly, family members should be screened for GATA2 mutations before serving as bone marrow donors for an affected proband.
Susceptibility to severe viral infections in GATA2 deficiency likely reflects the deficit and/or dysfunction of NK cells, which are critical for antiviral immunity.11 Systemic interferon alpha improves NK cell cytotoxicity in vitro and may be beneficial for patients with refractory HPV or herpesvirus infections.11 Likewise, dysfunction of monocytes/macrophages may predispose to intramacrophagic pathogens, such as NTM and Histoplasma capsulatum. Given their marked susceptibility to HPV and NTM, we empirically recommend early HPV vaccination and prophylactic azithromycin for all patients with GATA2 deficiency. However, HSCT offers the only definitive cure and should be considered in patients with severe, recurrent, or treatment-refractory infections.25
An association between PAP and hematologic malignancies has long been appreciated17 ; GATA2 deficiency unifies both entities and may help explain this association. Given the abundance of alveolar macrophages in lavage fluids from our patients, PAP in GATA2 deficiency must reflect alveolar macrophage dysfunction rather than a quantitative deficit. Indeed, GATA2 has been shown to regulate alveolar macrophage phagocytosis.38 We identified PAH only in the setting of PAP, likely reflecting that progressive lung disease can drive arterial dysfunction. Patients with PAP who lack anti-GM-CSF autoantibodies should be screened for GATA2 mutations, particularly patients with comorbid immunodeficiency and/or MDS/AML. HSCT has been reported to markedly improve pulmonary function in patients with PAP, presumably via reconstitution of the alveolar macrophage compartment.25 The high prevalence of diffusion and ventilatory defects in our cohort may reflect alveolar filling, emphysematous changes, fibrosis, or bronchiectasis secondary to recurrent pulmonary infection.
Careful dermatologic and gynecologic monitoring are prudent in view of the high prevalence of dysplastic HPV lesions and skin cancers. Panniculitis and Sweet syndrome should raise clinical suspicion for underlying infection or malignancy, respectively. EBV+ mesenchymal tumors, as seen in advanced HIV/AIDS, indicate a profound host defect that may also be NK cell mediated. The relationship of GATA2 deficiency to other cancers is less clear, but the development of multiple breast cancers and other solid tumors is concerning, particularly in light of the putative role of NK cells in tumor surveillance and the high frequency of somatic GATA3 mutations in human breast cancers.39,40
GATA2 was first identified as an endothelial transcription factor, and its haploinsufficiency perturbs normal vascular development.20,41-43 Thrombotic events in one quarter of patients suggest an association between GATA2 deficiency and coagulopathy, more likely the result of endothelial rather than coagulation abnormalities. In the lymphatic endothelium, GATA2 is involved in the development of lymphatic valves, deficiency of which likely underlies lymphedema.19 Endocarditis may reflect GATA2 haploinsufficiency in the endocardium and/or impaired overlap with GATA4 function, which is involved in cardiogenesis.44-46 Importantly, the development of thrombotic events and endocarditis in the early posttransplant period suggests that HSCT may not immediately ameliorate cardiovascular risks.
GATA2 is required for development of the semicircular canals and perilymphatic space of the vestibular system,47 and its haploinsufficiency may lead to hearing loss. GATA2 also interacts with GATA3, haploinsufficiency of which is associated with congenital deafness.48 Although hearing loss is confounded by aminoglycoside exposure in our cohort, it was also present in several patients without prior exposure. GATA2-deficient patients are likely more susceptible to ototoxic insults, which should inform the use of aminoglycosides in these patients.
The 33% prevalence of miscarriage in our cohort exceeds the 15% to 20% rate suspected in the general population.49 Dysfunction of uterine NK cells, which regulate trophoblast invasion and uterine spiral artery angiogenesis, may be one potential mechanism.50,51 GATA2 expression may also be impaired in trophoblast giant cells, which are critical for embryonic implantation and placental function.52-54 Vascular dysfunction may also contribute to miscarriage.
Hypothyroidism has affected 14% of patients, which may reflect GATA2-mediated transcriptional activation of the thyrotropin β gene.55 However, thyroid dysfunction is relatively common, and further studies are necessary to determine the mechanism of this association.
The marked clinical heterogeneity of GATA2 deficiency is multifactorial. Severe viral infections and lymphedema were more common in individuals with null mutations, suggesting an effect of genotype on clinical phenotype. This may explain why the great majority of patients previously reported with Emberger syndrome have nonsense and frameshift mutations or large gene deletions predicted to result in null alleles.7,19 However, in our series, even among individuals within the same family, there was considerable variation in clinical phenotype, largely negating a generalized genotype-phenotype association. Contiguous gene deletion syndromes may be a somewhat separate entity, as described previously.19 The distinction between the roles of background genetics and environmental exposure will await work in animal models. Clearly environmental factors, such as exposure to endemic mycoses and mycobacteria, influence certain infections. ASXL1 is emerging as an important “second hit” associated with transformation from MDS to overt leukemia.36,37
The clinical spectrum of our cohort was necessarily limited by the referral bias of our center. Many probands were recruited through natural history protocols for the study of NTM infections or primary immune deficiency. However, the addition of 17 relatives identified through family screening was an important opportunity to obtain a more complete picture of the clinical phenotype. Our cohort was also likely underrepresented for those with acute and fatal presentations, representing possible length bias and causing an optimistic bias in survival estimates.56 These potential biases can only be addressed through broader testing for GATA2 mutations and a prospective study in additional cohorts.
Our pathophysiological understanding of GATA2 deficiency is primitive but rapidly growing. GATA2 interacts closely with GATA1 in the development of the hematopoietic compartment and GATA3 in trophoblastic development.26 It underlies the development of circulating myeloid dendritic cells but seems to allow infections or cancer only after a prolonged latency.57 Finally, although haploinsufficiency is clearly pathological and leads to myeloid leukemia in a substantial number of cases, gain-of-function mutations are also associated with myeloid leukemias, indicating that the proper regulation of GATA2 is both critical and its range quite narrow.58 It is now clear that GATA2 deficiency is a protean disorder that unites susceptibility to immunodeficiency, bone marrow failure, pulmonary disease, and vascular/lymphatic dysfunction. It causes cases of familial and sporadic MDS/AML, aplastic anemia, neutropenia, primary immune deficiency, and lymphedema, the full extent of which is yet to be determined. Early genetic diagnosis is essential for appropriate disease management, prophylaxis, transplantation, and family screening.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases and National Cancer Institute. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: M.A.S. and L.A.S. collected and analyzed clinical data and made the figures; A.P.H. performed genetic sequencing and created Figure 1; P.A.S. and W.G. performed statistical analysis; K.R.C. and D.C.A. reviewed bone marrow biopsies and cytogenetics; C.S.Z., C.C.B., C.M.G., E.W.C., A.F.F., K.N.O., G.U., J.R.D., C.D.S., A.M.Z., R.J.C., N.K.G., B.P.A., J.C.-R., D.D.H., and S.M.H. provided clinical care and patient samples; M.A.S. and L.A.S. wrote the manuscript; and A.P.H., P.A.S., K.R.C., C.C.B., E.W.C., N.K.G., B.P.A., E.M.M., J.S.O., and S.M.H. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven M. Holland, CRC B3-4141, MSC 1684, Bethesda, MD 20892-1684; e-mail: smh@nih.gov.
References
Author notes
M.A.S. and L.A.S. contributed equally to this study.