In this issue of Blood, Spinner et al and Dickinson et al describe a surprising breadth of clinical and laboratory findings associated with germ-line mutation of the gene encoding the GATA2 transcription factor.1 ,2
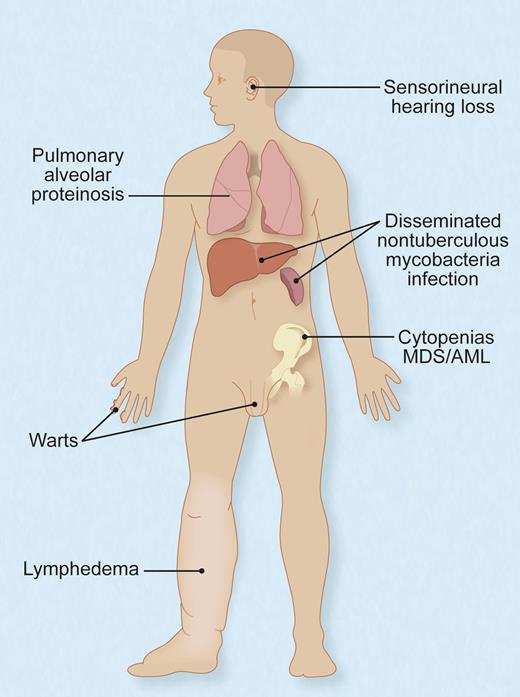
Protean manifestations of germ-line, heterozygous mutations of GATA2. Not all individuals have all, or even any, of these features, and their timing and sequence of occurrence and complementation by hematopoietic stem cell transplant are not fully understood. Adapted with permission from Figure 6 in the article by Spinner et al.1 Professional illustration by Debra T. Dartez.
Protean manifestations of germ-line, heterozygous mutations of GATA2. Not all individuals have all, or even any, of these features, and their timing and sequence of occurrence and complementation by hematopoietic stem cell transplant are not fully understood. Adapted with permission from Figure 6 in the article by Spinner et al.1 Professional illustration by Debra T. Dartez.
Recognition of a new disease does not happen very often nowadays, but, thanks to the power of molecular genetics, a constellation of findings bordering on both the rare and the common are united under the etiological umbrella of GATA2.
In 2011, multiple groups identified heterozygous germ-line mutations in GATA2 as the basis for autosomal dominant forms of acute myeloid leukemia (AML) and myelodysplasia (MDS)3 ; Emberger syndrome comprising MDS, lymphedema, and warts4 ; the MonoMAC syndrome of monocytopenia and nontuberculous mycobacterial infection5 ; and dendritic cell, monocyte, and B and natural killer lymphoid deficiency (DCML) with vulnerability to viral infections.6 Shortly thereafter, GATA2 mutations were recognized as frequent in congenital neutropenia7 and pediatric MDS,8 as well as other bone marrow failure syndromes where additional causes were previously known.
Although the overlapping manifestations and some of the additional clinical features were noted anecdotally, it has not been until the 2 reports of a large series of patients described here that the diversity of the clinical presentation of GATA2 deficiency becomes apparent.
Spinner et al report on 57 patients from 40 different families with GATA2 mutations who were all evaluated at the US National Institutes of Health Clinical Center. They document additional associated findings including deafness, pulmonary alveolar proteinosis, erythema nodosum, miscarriage, hypothyroidism, thrombosis, and more (see figure). Yet, they also show that some patients may remain asymptomatic well into their adult years.
Dickenson et al studied 30 European patients from 16 different families and contributed a careful definition of GATA2 deficiency’s associated cellular characteristics. They similarly found that some patients with GATA2 mutation lack a phenotype, corroborating that cellular deficiency may evolve over time. However, even before problems emerge, there is, in some cases, an elevation of Flt3 ligand, which can be a marker of stress hematopoiesis. They point out that the DCML immunophenotype resembles a pattern of terminal differentiation seen with aging and chronic viral infection. Indeed, they demonstrate a tendency toward clonal myelopoiesis, consistent with GATA2s role in maintaining stem cell longevity.
Both papers provide important insights into the pathophysiology and clinical features of this disorder and are packed with practical recommendations aimed at a multidisciplinary approach to patient care, involving screening and diagnosis, as well as treatment, including prophylaxis for infectious complications and hematopoietic stem cell transplantation. That so many patients with a seemingly rare disorder could be identified and evaluated in such a relatively short period of time would indicate that heritable GATA2 mutations might not be uncommon.
As with all good studies, there are more questions than answers. Without robust genotype-phenotype correlation, how much of the varied clinical manifestations, including differences among individuals within the same family sharing a common germ-line mutation, are due to environment—particularly infectious exposures—vs modifier genes and acquired mutations either as a second hit to GATA2 or other genes? What is cause and what is effect? In other words, an orthodox interpretation might suggest that cytopenias are contributing to infections, yet the possibility that an overtaxed response may be helping to drive malignant transformation remains intriguing, though untested.
GATA2 joins the ranks of other transcription factors,9 namely RUNX1, CEBPA, and PAX5,10 central to development and hematopoiesis, where germ-line mutations confer Mendelian predisposition to leukemia. In addition to MDS and AML, RUNX1 mutation is also associated with thrombocytopenia and platelet functional defects and CEBPA mutation with eosinophilia. Thus far, there are no extramedullary complications known to occur with PAX5 mutations associating with familial acute lymphoblastic leukemia, although, perhaps, greater scrutiny is warranted.
The categorical unity of leukemia predisposition genes may support the concept that, fundamentally, leukemia derives from loss of transcriptional control of the hierarchic differentiation program during blood cell development and may augur well for therapies directed toward promoting cellular differentiation. Further definition of the consequences of heritable deficiency of GATA2 and its cohort of other transcription factors should continue to enlighten understanding of benign and malignant hematopoiesis, as well as flesh out associated clinical features extending beyond blood.
Conflict-of-interest disclosure: The author declares no competing financial interests.