Key Points
A recombinant antibody recognizing the D1 domain of β2 glycoprotein I induces fetal loss and clot formation in animal models.
The CH2-deleted antibody fails to activate complement and prevents the procoagulant and proabortive effects of patient antibodies.
Abstract
A single-chain fragment variable (scFv) recognizing β2-glycoprotein 1 (β2GPI) from humans and other species was isolated from a human phage display library and engineered to contain an IgG1 hinge-CH2-CH3 domain. The scFv-Fc directed against β2GPI domain I-induced thrombosis and fetal loss, thus mimicking the effect of antibodies from patients with antiphospholipid syndrome (APS). Complement is involved in the biological effect of anti-β2GPI scFv-Fc, as demonstrated by its ability to promote in vitro and in vivo complement deposition and the failure to induce vascular thrombosis in C6-deficient rats and fetal loss in C5-depleted mice. A critical role for complement was also supported by the inability of the CH2-deleted scFv-Fc to cause vessel occlusion and pregnancy failure. This antibody prevented the pathological effects of anti-β2GPI antibodies from APS patients and displaced β2GPI-bound patient antibodies. The CH2-deleted antibody represents an innovative approach potentially useful to treat APS patients refractory to standard therapy.
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by recurrent vascular thrombosis and pregnancy morbidities because of the persistent presence of autoantibodies against phospholipid-binding proteins (aPL). The syndrome occurs either as a primary or secondary disorder in other autoimmune diseases, such as systemic lupus erythematosus. APS affects mainly young people and induces disability as a consequence of stroke or myocardial infarction.1 Some patients manifest a life-threatening form of APS characterized by the thrombotic occlusion of small vessels in various organs that develops over a short period and leads to multiorgan failure.2 Pregnancy morbidity includes recurrent miscarriages, intrauterine growth restriction, prematurity, and pre-eclampsia, resulting in significant social and economic costs.1,3
Data obtained from clinical studies and animal models support the involvement of antibodies in thrombus formation and miscarriage in APS. Although several PL-binding proteins have been identified as potential targets of aPL, β2-glycoprotein I (β2GPI) is considered the most relevant target antigen, and anti-β2GPI antibodies have been suggested to play a key pathogenic role in the disease.4-7 We recently reported a significant increase in fetal loss in pregnant mice immunized with human β2GPI following the injection of a fluorescein-labeled purified protein that binds selectively to decidual endothelial cells and trophoblasts.8 We also determined that the removal of anti-β2GPI antibodies from patient aPL-IgG using affinity chromatography significantly reduced the thrombotic effect in the rat mesenteric microcirculation.9
Although aPL antibodies exhibit a modest reactivity with the soluble target molecules, it is widely accepted that the antibodies interact preferentially with β2GPI bound to endothelial cells, platelets, monocytes, and trophoblasts, all of which contribute to the pathogenesis of APS.10,11 In particular, aPLs were recently suggested to recognize a conformational immunodominant epitope present on domain I (DI) of β2GPI.7 The mechanisms linking aPL to blood clotting and fetal loss include the inhibition of natural anticoagulants and fibrinolysis, the expression of adhesion molecules on activated endothelial cells, platelets, and monocytes, the inhibition of syncytium-trophoblast differentiation, and the promotion of decidual inflammation.10 However, the in vivo relevance of these observations mostly made in vitro remains to be fully established.
Compelling evidence for the critical role played by complement in APS has been obtained in animal models, beginning with the observation that mice deficient in C3 or treated with an inhibitor of the C3 convertase are protected from aPL-mediated fetal resorption and growth retardation. Subsequent studies led to the identification of C5a and the membrane attack complex as mediators of fetal loss and thrombus formation.9,12 Although most of these observations were made using aPL of undefined specificity, the role of complement in inducing aPL-mediated blood clots and fetal resorption has been confirmed using specific antibodies to β2GPI.8,9
In recent years, major efforts have been made to prevent aPL-mediated thrombus formation and miscarriages. Currently, anticoagulants, such as heparin or warfarin, are used to prevent vascular thrombi, and a combination of low-dose aspirin and low-molecular-weight heparin represents the first-line treatment of the obstetric complications of APS. However, despite the success of these therapeutic approaches, a significant proportion of patients (up to 30% of aPL-positive pregnant women in particular) do not benefit from these treatments.13-15
Given the pathogenic role attributed to anti-β2GPI antibodies, we adopted a novel therapeutic approach based on the use of a high affinity human monoclonal antibody to β2GPI that competes with the patients’ antibodies for binding to β2GPI and inhibits their ability to induce blood clots and fetal loss.
Methods
Purification and characterization of human and murine β2GPI
β2GPI was purified from human and mouse sera as previously published.16
Recombinant antibodies
The antibodies were selected from a phage display antibody library17 in 96-well plates coated with β2GPI after overnight incubation at 4°C. A positive clone (B2) was identified by enzyme-linked immunosorbent assay (ELISA), the VH and VL genes were sequenced, and the gene segments were assessed using the ImMunoGeneTics/V-QUEST tool in the ImMunoGeneTics information system.
Cloning and purification of recombinant antibodies
The single-chain fragment variables (scFvs) B2, anti-CD20, and anti-C518 (Adienne Pharma and Biotech) were subcloned into the pMB-SV519 vector containing the human IgG1 hinge-CH2-CH3 domains to obtain the human scFv-Fc format (MBB2, MBCD20, and MBC5). A CH2-deleted version of the scFv-Fc (ΔCH2) was generated as previously described20 using the following primers: Hu_CH3_sense, 5′ACGTGCTAGCCACACATGCCCACCGTGCGGTGGAGGCGGTTCAGGCGGAGGTGGCTCTGGGCAGCCCCGAGAACCACAGG3′;and Hu_CH3_anti, TGCTAAGCTTTTAAGTACTATCCAGGCCCAGCAGTGGGTTTGG.
The polymerase chain reaction fragments cut with NheI and HindIII were cloned into the PMB-SV5 vector to create the pMB-ΔCH2 vector. All recombinant antibodies were subcloned into the pUCOE vector21 for expression in stable transfected CHO-s cells and purified using a HiTrap protein G column (GE Healthcare) as described.21
Sera
Sera were obtained from patients with an APS history and a medium-high anti-β2GPI antibody titer (supplemental Table 1, available on the Blood Web site) and from various animal species. The IgG were purified from patient sera as indicated for the scFv-Fc. Pooled normal human sera from AB+ blood donors was used as source of complement. Informed consent was obtained from patients and blood donors.
Antibody binding assays
The interaction of antibodies with β2GPI was measured by ELISA using γ-irradiated polystyrene plates (Maxi-Sorp Nunc-Immunoplates) coated with human or mouse β2GPI (10 μg/mL) and alkaline phosphatase (AP)-conjugated anti-human IgG (Sigma-Aldrich) as a secondary antibody.8 The domain specificity of the anti-β2GPI scFv-Fc was investigated by ELISA using recombinant domains kindly provided by Dr P. G. De Groot (Amsterdam, The Netherlands).22
To displace β2GPI-bound patient antibodies, immobilized human β2GPI (10 μg/mL) was first incubated with patient serum (1:200) for 30 minutes at room temperature and then with MBB2ΔCH2 (2 µg/100 µL) for an additional 30 minutes at room temperature. The residual β2GPI-bound patient antibody was measured by ELISA using a murine monoclonal IgG (Serotec) that recognizes the CH2 domain absent in MBB2ΔCH2 and AP-conjugated goat anti-mouse IgG (Sigma-Aldrich).
Anticardiolipin antibodies were detected as described by Tincani and Meroni,23 and the antiprothrombin antibodies were identified using a commercial kit (INOVA Diagnostics).
Binding of MBB2 to cell surface β2GPI
Human umbilical vein endothelial cells (HUVECs)24 and the trophoblast cell line BeWo (CLS GmbH) were grown to confluence in 96-well tissue culture plates (Costar) in RPMI 1640 medium (Gibco, Invitrogen) supplemented with 10% fetal calf serum (FCS; Gibco, Invitrogen). After overnight culture in FCS-free medium, the cells were exposed to human β2GPI (5 μg/mL) or 20% FCS for 90 minutes at 37°C followed by scFv-Fc for 1 hour at room temperature. The bound antibodies were detected with AP- or horseradish peroxidase-conjugated goat anti-human IgG (Sigma-Aldrich) for the HUVECs and BeWo cells, respectively.
Biosensor analysis
The affinity measurements for the anti-β2GPI were performed using a Biacore X100 biosensor (GE Healthcare) on a carboxymethyldextran-coated sensor chip (CM5). Purified IgGs and scFv molecules were diluted in PBS and injected over the sensor chip at a flow rate of 30 µL/min, with 180 seconds of analyte contact over the surface and 600 seconds of dissociation. The chip surface was regenerated between samples by the injection of 10 mM glycine (pH 2.0) for 60 seconds at a flow rate of 30 µL/minute. Each sample was analyzed in triplicate.
Evaluation of complement activation
Complement activation by antibodies bound to solid phase-adsorbed β2GPI was evaluated by incubating the antibody-β2GPI complex with AB+ pooled sera for 30 minutes at 37°C. C1q and C4 deposition was detected by specific goat antibodies (Quidel), and C9 binding was revealed using the murine monoclonal antibody aE11 against C9 neoantigen kindly provided by Prof T. E. Mollnes (Oslo, Norway).25 The AP-conjugated anti-goat IgG or anti-mouse IgG (Sigma-Aldrich) was used as secondary antibodies.
Animal models
Antibody-induced thrombus formation was evaluated in male Wistar rats and in both wild-type and C6−/− PVG male rats (270-300 g) as previously described.9 Briefly, the animals were anesthetized 4 hours after intraperitoneal injection of lipopolysaccharide (LPS) from Escherichia coli O55:B5 (2.5 mg/kg body weight; Sigma-Aldrich). Rhodamine 6G (Sigma-Aldrich) (0.025 mg/kg/min) was infused into the femoral vein at a rate of 0.25 mL/h 30 minutes prior to the 30-minute infusion of scFv-Fc (1 mg/mL), patient IgG (10 mg/mL), or MBB2ΔCH2 followed by patient IgG (10 mg/mL) into the carotid artery. At least 5 microvascular areas were analyzed for the formation of fluorescent platelet-leukocyte aggregates that partially or completely occluded the vessels.
The effect of antibodies on pregnancy outcome was evaluated in female BALB/c mice (6-8 weeks) receiving vein infusions of either scFv-Fc (10 μg/100 µL saline) or patient IgG (50 μg/100 µL saline) on the day of vaginal plug formation (day 0). The animals were euthanized on day 15, and the resorbed fetuses were identified by their small size and necrotic or hemorrhagic appearance compared with normal embryos. The results are presented as percentage of fetal loss and individual fetus weight in each mouse. Complement was depleted by intraperitoneal injection of neutralizing MBC5 (100 μg/400 µL saline) 3 times a week. The protective effect of MBB2ΔCH2 was evaluated in mice receiving intravenous patient IgG (50 μg/100 µL saline) on day 0 of pregnancy and intraperitoneal injections of MBB2ΔCH2 (50 μg/200 µL saline) on days 0, 5, and 10. The results are presented as fetal weight and as percentage of fetal loss.
Immunofluorescence analysis
The mesenteric tissue samples harvested from rats and the snap-frozen samples of mouse placentae prepared as previouslydescribed8,9 were analyzed for C3 deposition using goat anti-rat C3 (MP-Cappel) and fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse C3 (MP-Cappel,) and the presence of C9 using rabbit anti-rat C9 (a gift of Prof P. Morgan, Cardiff, United Kingdom)9,26,27 and rabbit anti-mouse C9 kindly provided by Prof M. D. Daha (Leiden, The Netherlands),8,28 followed by FITC-conjugated secondary antibodies (Dako). MBB2 binding to trophoblasts and endothelial cells in human placenta was analyzed by immunofluorescence incubating the cells with MBB2 (50 µg/mL), followed by FITC-conjugated F(ab′)2 goat anti-human IgG (Sigma-Aldrich), and with mAb anti-human CK7 or mAb anti-human vWF (Dako), followed by Cy3-conjugated F(ab′)2 goat anti-mouse IgG (Jackson ImmunoResearch). The slides were examined using a DM2000 fluorescence microscope (Leica).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 for Windows. The fetal resorption and vessel occlusion data and the ELISA results were analyzed using the Mann-Whitney test. The fetal weights were analyzed using the Student t test. The data were expressed as mean ± standard deviation (SD) and as median and interquartile range for parametric and nonparametric variables, respectively. P < .05 was considered statistically significant.
The in vivo procedures were performed in compliance with the guidelines of European (86/609/EEC) and Italian (D.L.116/92) laws and were approved by the Italian Ministry of University and Research and the Administration of the University Animal House. This study was conducted in accordance with the Declaration of Helsinki.
Results
Isolation and characterization of anti-human β2GPI recombinant antibody
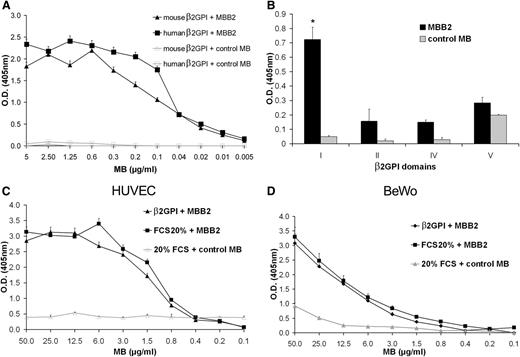
An scFv specific for human β2GPI was isolated from a human phage display library. The sequence analysis of the scFv variable regions revealed that the VH genes belonged to the IGVH3 gene family, specifically the IGHV3-33 gene containing a 16-amino acid CDR3 sequence. The VL region was encoded by the IGLV3-19 gene containing an 11-amino acid CDR3. Once isolated, the scFv was further engineered into the scFv-Fc format (MBB2) containing the hinge-H2-CH3 domains of human IgG1. Further analysis of MBB2 binding to immobilized β2GPI using ELISA demonstrated a strong reactivity of the antibody with the target antigen to a concentration of 20 ng/mL (Figure 1A) and a modest interaction with soluble β2GPI (supplemental Figure 1). The IgG purified from an APS patients was found to be 1000-fold less potent than MBB2. Furthermore, MBB2 did not react with solid phase-bound prothrombin and failed to exhibit lupus anticoagulant activity. The antibody reacted equally well with the murine β2GPI molecule (Figure 1A) and also with cardiolipin-bound β2GPI from several species including rat, mouse, pig, goat, and fetal calf (supplemental Figure 2). To identify the target epitope of MBB2, recombinant single domains of the β2GPI protein were used, and, testing 4 of the 5 domains, the antibody was shown to bind to the DI domain (Figure 1B). The binding of MBB2 to DI was confirmed by Dr Michael Mahler (INOVA Diagnostics, San Diego, CA) using a novel solid phase chemiluminescent assay (BioFlash; INOVA Diagnostics)29 and by Dr Anisur Rahman using solid phase-bound recombinant DI (described in Ioannou et al30 ).
Analysis of MBB2 binding to purified β2GPI. (A) Human or mouse β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates was incubated with different concentrations of MBB2 or control minibody (MB) for 90 minutes at room temperature, and the bound scFv-Fc was measured by ELISA. (B) Binding of MBB2 to human β2GPI domains (10 μg/mL). (C-D) Binding of MBB2 or control MB to HUVECs or BeWo cells grown to confluence in 96-well tissue culture plates. The cells were incubated with β2GPI (5 μg/mL) or 20% FCS prior to exposure to the scFv-Fc, and the bound antibodies were measured using ELISA. The data were analyzed with the Mann-Whitney test. The results are expressed as mean ± SD of 3 different experiments performed in triplicate. *P < .05 vs control MB.
Analysis of MBB2 binding to purified β2GPI. (A) Human or mouse β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates was incubated with different concentrations of MBB2 or control minibody (MB) for 90 minutes at room temperature, and the bound scFv-Fc was measured by ELISA. (B) Binding of MBB2 to human β2GPI domains (10 μg/mL). (C-D) Binding of MBB2 or control MB to HUVECs or BeWo cells grown to confluence in 96-well tissue culture plates. The cells were incubated with β2GPI (5 μg/mL) or 20% FCS prior to exposure to the scFv-Fc, and the bound antibodies were measured using ELISA. The data were analyzed with the Mann-Whitney test. The results are expressed as mean ± SD of 3 different experiments performed in triplicate. *P < .05 vs control MB.
To determine whether MBB2 interacts with cell-bound β2GPI, HUVECs and BeWo cells were exposed to purified β2GPI to allow surface binding of the protein and subsequently incubated with the scFv-Fc. MBB2, unlike the control antibody, bound to the 2 cell types loaded with β2GPI (Figure 1C-D). The modest signal observed on the BeWo cells exposed to the highest concentration of the control antibody was compatible with the expression of IgG receptor on these cells.31 These data were confirmed by immunofluorescence analysis of the distribution pattern of MBB2 in sections of first and third trimester placentae (supplemental Figure 3).
The affinity measurement of MBB2 determined by surface plasmon resonance revealed a significant binding of MBB2 to immobilized β2GPI much higher than that of the patient IgG, whereas both the IgG from a blood donor and the control MBC5 failed to bind to β2GPI (supplemental Figure 4). Kinetic analysis of the interaction between MBB2 and β2GPI allowed calculation of a KD of 1.1 × 10−8 M with an association rate constant Ka (1/Ms) of 3.3 × 106 and a dissociation rate constant Kd (1/s) of 3.64 × 10−2.
In vivo effect of MBB2 on thrombus formation and fetal loss
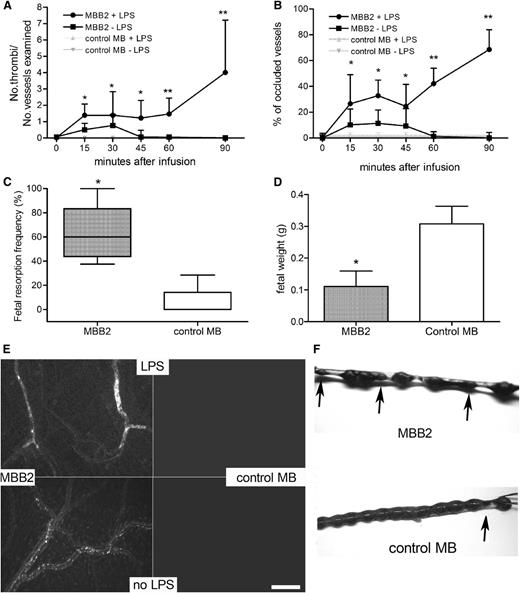
The ability of MBB2 to cause blood clots and fetal loss was investigated in animal models to establish the potential clinical relevance of antibodies exhibiting this specificity in APS patients. The antibody was injected into rats primed with LPS, and the procoagulant effect was monitored in the mesenteric microvessels using intravital microscopy. As shown in Figure 2A, MBB2 induced clot formation that followed a biphasic pattern, exhibiting an early phase characterized by platelet-leukocyte microaggregates visible 10 minutes after antibody infusion. The transient vessel occlusion caused by these aggregates was followed by a second phase characterized by a progressive increase in thrombus size resulting in permanent occlusion of the blood vessels (Figure 2A-B,E). The MBB2 injected into unprimed rats caused small leukocyte-platelet conjugates that disappeared after 60 minutes, whereas the control antibody was completely ineffective (Figure 2A-B,E).
Analysis of the procoagulant and proabortive effects of MBB2. (A) Thrombus formation and (B) vessel occlusion were monitored by intravital microscopy at different time intervals in rats treated or untreated with LPS and infused with MBB2 or control MB. The results are expressed as a ratio between the number of thrombi and the number of microvessels examined and as a percentage of occluded microvessels. (C) Percentage of fetal loss and (D) fetal weight in mice treated with MBB2 or control MB (10 μg/100 µL saline). (E) Sections of rat mesenteric tissue showing vessel occlusion with thrombi in LPS-treated rats receiving MBB2 and small cell aggregates in MBB2-treated animals in the absence of LPS. Both thrombi and cell aggregates were undetectable in the animals treated with control MB. Scale bar equals 50 μm. (F) Representative examples of uteri from a MBB2-treated pregnant mouse showing resorbed fetuses (indicated by arrows) and from a control MB-treated mouse containing live pups. The procoagulant effect of the antibodies was evaluated in 3 rats for each treatment protocol, and the proabortive activity of MBB2 and control MB was tested on ≥5 pregnant mice for each antibody. The data of fetal resorption and vessel occlusion were analyzed with the Mann-Whitney test, whereas the Student t test was used to analyze the data of the fetal weights. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01 vs control MB.
Analysis of the procoagulant and proabortive effects of MBB2. (A) Thrombus formation and (B) vessel occlusion were monitored by intravital microscopy at different time intervals in rats treated or untreated with LPS and infused with MBB2 or control MB. The results are expressed as a ratio between the number of thrombi and the number of microvessels examined and as a percentage of occluded microvessels. (C) Percentage of fetal loss and (D) fetal weight in mice treated with MBB2 or control MB (10 μg/100 µL saline). (E) Sections of rat mesenteric tissue showing vessel occlusion with thrombi in LPS-treated rats receiving MBB2 and small cell aggregates in MBB2-treated animals in the absence of LPS. Both thrombi and cell aggregates were undetectable in the animals treated with control MB. Scale bar equals 50 μm. (F) Representative examples of uteri from a MBB2-treated pregnant mouse showing resorbed fetuses (indicated by arrows) and from a control MB-treated mouse containing live pups. The procoagulant effect of the antibodies was evaluated in 3 rats for each treatment protocol, and the proabortive activity of MBB2 and control MB was tested on ≥5 pregnant mice for each antibody. The data of fetal resorption and vessel occlusion were analyzed with the Mann-Whitney test, whereas the Student t test was used to analyze the data of the fetal weights. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01 vs control MB.
In a second set of experiments, we investigated the effect of MBB2 on the pregnancy outcome in mice. A single injection of antibody (10 μg) induced a significant increase in the fetal resorption rate with a mean value of 60%, which was significantly higher than the 10% mean value observed in the animals treated with a similar amount of control scFv-Fc. Similarly, the anti-β2GPI antibody affected fetal growth, causing a substantial decrease in fetal weight (Figure 2C,D,F). No difference was found in the intra-animal fetal weight variation between MBB2- and control-treated mice that did not exceed 10% in all groups.
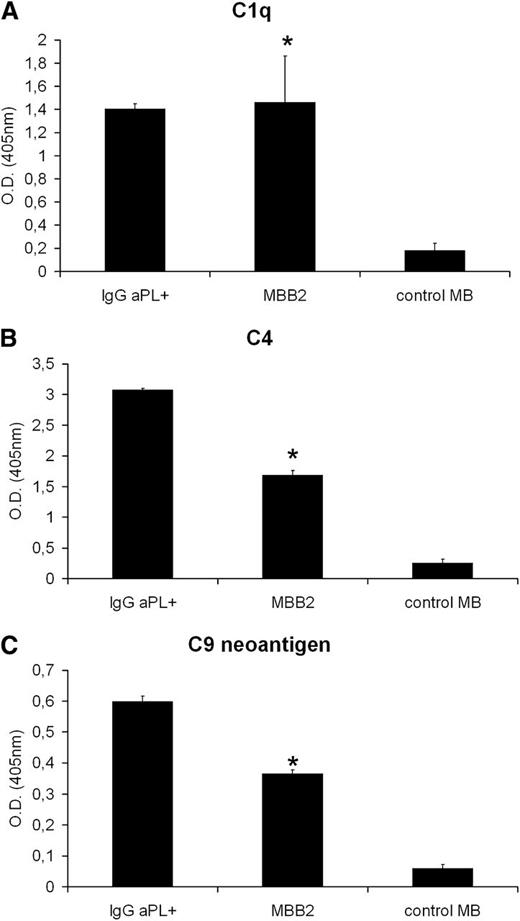
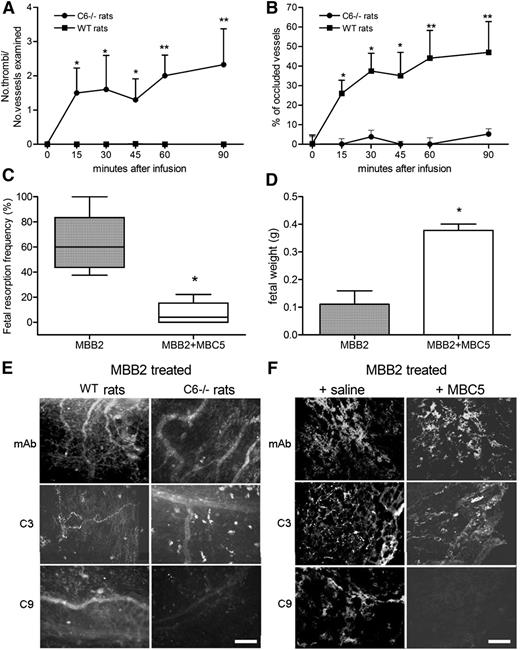
Complement is required for the in vivo effect of MBB2
To investigate the contribution of complement to the pathogenic effect of MBB2, we first analyzed the ability of scFv-Fc bound to immobilized β2GPI to activate complement in the presence of normal human sera MBB2 triggered the classical pathway, as suggested by the binding of C1q and C4, and the assembly of the terminal complement complex, demonstrated by the deposition of C9 (Figure 3). The role of the effector phase of complement activation in thrombus formation induced by MBB2 was evaluated in LPS-primed C6-deficient rats. The antibody had a negligible procoagulant effect in these rats, and the analysis of the mesenteric vessels revealed deposits of IgG and C3 in the absence of C9 (Figure 4A-B,E). The contribution of complement to MBB2-induced fetal loss was analyzed in pregnant mice that received C5 neutralizing antibody prior to the administration of the anti-β2GPI scFv-Fc. This treatment resulted in the inhibition of C9 deposition at the implantation sites and was associated with a significant decrease in fetal loss and an increase in fetal weight (Figure 4C-D,F).
Complement activation by MBB2. Human β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates was first incubated with either MBB2 (1 µg/mL) or control MB (1 µg/mL) or IgG aPL+ isolated from a patient with APS for 90 minutes at room temperature and then with 1:100 fresh human serum for 30 minutes at 37°C. The bound C1q (A), C4 (B), and C9 (C) were identified by ELISA using goat antibodies to C1q and C4 and the monoclonal antibody aE11 to C9 neoantigen. The data were analyzed with the Mann-Whitney test. The experiments were performed in triplicate, and the data are expressed as mean ± SD, *P < .05 vs control MB.
Complement activation by MBB2. Human β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates was first incubated with either MBB2 (1 µg/mL) or control MB (1 µg/mL) or IgG aPL+ isolated from a patient with APS for 90 minutes at room temperature and then with 1:100 fresh human serum for 30 minutes at 37°C. The bound C1q (A), C4 (B), and C9 (C) were identified by ELISA using goat antibodies to C1q and C4 and the monoclonal antibody aE11 to C9 neoantigen. The data were analyzed with the Mann-Whitney test. The experiments were performed in triplicate, and the data are expressed as mean ± SD, *P < .05 vs control MB.
Analysis of the procoagulant and proabortive effect induced by MBB2 in complement-deficient or complement-depleted animals. (A) Thrombus formation and (B) vessel occlusion were monitored by intravital microscopy at different time intervals in LPS-primed C6−/− and wild-type (WT) PVG rats receiving MBB2. Percentage of (C) fetal loss and (D) fetal weight in normal and C5-depleted mice treated with MBB2. Immunofluorescence analysis of (E) rat mesenteric tissue and (F) fetal implantation sites in normal and complement-deficient animals treated with MBB2 for the deposition of scFv-Fc, C3, and C9. Scale bars equal 50 μm. The procoagulant and the proabortive effects of MBB2 were evaluated and analyzed as described in Figure 2. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01.
Analysis of the procoagulant and proabortive effect induced by MBB2 in complement-deficient or complement-depleted animals. (A) Thrombus formation and (B) vessel occlusion were monitored by intravital microscopy at different time intervals in LPS-primed C6−/− and wild-type (WT) PVG rats receiving MBB2. Percentage of (C) fetal loss and (D) fetal weight in normal and C5-depleted mice treated with MBB2. Immunofluorescence analysis of (E) rat mesenteric tissue and (F) fetal implantation sites in normal and complement-deficient animals treated with MBB2 for the deposition of scFv-Fc, C3, and C9. Scale bars equal 50 μm. The procoagulant and the proabortive effects of MBB2 were evaluated and analyzed as described in Figure 2. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01.
Failure of non–complement-fixing antibody to β2GPI to induce in vivo effect
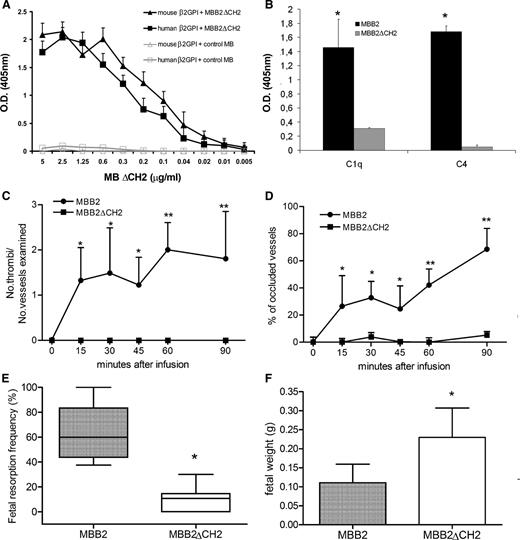
Because the CH2 domain of IgG1 contains a binding site for C1q, we generated a deleted version of scFv-Fc MBB2 lacking this domain (MBB2ΔCH2) to further confirm the role of complement in mediating the pathogenic effect of the parent antibody. The ELISA analysis showed that MBB2ΔCH2 and MBB2 interacted equally well with β2GPI (Figure 5A); however, once bound, MBB2ΔCH2 was unable to activate complement (Figure 5B). The modified molecule injected into normal rats pretreated with LPS exhibited negligible procoagulant activity and showed a pattern of prothrombotic effects that resembled observations in C6-deficient rats, emphasizing the important contribution of complement activation to the thrombotic events (Figure 5C-D). Similarly, MBB2ΔCH2 did not affect pregnancy outcome in terms of fetal weight and fetal loss, which were compromised severely by MBB2 (Figure 5E-F).
Binding and in vivo effect of MBB2ΔCH2. (A) Human or mouse β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates were incubated with different concentrations of either MBB2ΔCH2 or control MB, and the bound scFv-Fc was measured using ELISA. (B) Deposition of C1q and C4 to bound MBB2ΔCH2 and MBB2 was evaluated as indicated in the legend to Figure 3. (C) Thrombus formation and (D) vessel occlusion monitored by intravital microscopy at different time intervals in LPS-primed normal rats receiving either MBB2ΔCH2 or MBB2. Percentages of (E) fetal loss and (F) fetal weight in pregnant mice treated with either MBB2ΔCH2 or MBB2. The procoagulant and proabortive effects of MBB2 and MBB2ΔCH2 were evaluated and analyzed as reported in Figure 2. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01.
Binding and in vivo effect of MBB2ΔCH2. (A) Human or mouse β2GPI (10 μg/mL) immobilized on γ-irradiated polystyrene plates were incubated with different concentrations of either MBB2ΔCH2 or control MB, and the bound scFv-Fc was measured using ELISA. (B) Deposition of C1q and C4 to bound MBB2ΔCH2 and MBB2 was evaluated as indicated in the legend to Figure 3. (C) Thrombus formation and (D) vessel occlusion monitored by intravital microscopy at different time intervals in LPS-primed normal rats receiving either MBB2ΔCH2 or MBB2. Percentages of (E) fetal loss and (F) fetal weight in pregnant mice treated with either MBB2ΔCH2 or MBB2. The procoagulant and proabortive effects of MBB2 and MBB2ΔCH2 were evaluated and analyzed as reported in Figure 2. The data of vessels occlusion and fetal weight are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05, **P < .01.
MBB2ΔCH2 prevents the in vivo effect induced by patients’ IgG
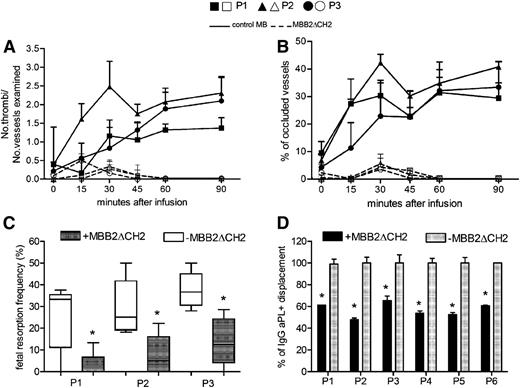
The finding that the CH2-deleted antibody failed to activate complement despite the strong interaction with immobilized β2GPI led us to consider the possibility that MBB2ΔCH2 competes with the anti-β2GPI antibodies from APS patients, preventing their pathogenic effect. To investigate this possibility, the procoagulant activity of patient IgG in LPS-primed rats was compared with that of a mixture of MBB2ΔCH2 and IgG anti-β2GPI. As shown in Figure 6A-B, the CH2-deleted antibody reduced thrombus formation and vessel occlusion to levels previously obtained using control IgG.9 The difference in thrombus formation and vessel occlusion between the rats receiving IgG aPL+ alone and the animals treated with both IgG aPL+ and MBB2ΔCH2 reached statistical significance 15 minutes after the infusion of the IgG from patient 2 and 30 minutes after the injection of the IgG from the other 2 patients. The same molecule administered to pregnant mice significantly reduced fetal death induced by anti-β2GPI IgG from APS patients (Figure 6C). To reproduce more closely the clinical situation of pregnant women with circulating IgG aPL+, MBB2ΔCH2 was injected into pregnant mice 3 days after the administration of the IgG from 1 APS patient. This treatment was still effective in preventing pregnancy loss with a significant reduction (P = .03) of the fetal resorption rate from 31% (±13%) to 14% (±10%).
Control of the pathogenic effect of IgG aPL+ by MBB2ΔCH2. (A) Thrombus formation and (B) vessel occlusion observed in rats that received IgG (10 mg/mL) purified from 3 APS patients (P1, ▪ and □; P2, ▲ and △; P3, ● and ○) and either MBB2ΔCH2 (dashed lines, empty symbols) or control MB (2 mg/mL, continuous lines, filled symbols). The difference in thrombus formation and vessel occlusion between the rats treated with both IgG aPL+ and MBB2ΔCH2 reached statistical significance 15 minutes after infusion of the IgG from patient 2 and 30 minutes after the injection of the IgG from the other 2 patients. (C) Percentage of fetal loss in pregnant mice receiving patient IgG and MBB2ΔCH2. (D) Displacement of patient IgG bound to β2GPI by MBB2ΔCH2 evaluated as described in Methods. The procoagulant and proabortive effects of MBB2 and MBB2ΔCH2 were evaluated and analyzed as reported in Figure 2. The results of vascular occlusion are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05.
Control of the pathogenic effect of IgG aPL+ by MBB2ΔCH2. (A) Thrombus formation and (B) vessel occlusion observed in rats that received IgG (10 mg/mL) purified from 3 APS patients (P1, ▪ and □; P2, ▲ and △; P3, ● and ○) and either MBB2ΔCH2 (dashed lines, empty symbols) or control MB (2 mg/mL, continuous lines, filled symbols). The difference in thrombus formation and vessel occlusion between the rats treated with both IgG aPL+ and MBB2ΔCH2 reached statistical significance 15 minutes after infusion of the IgG from patient 2 and 30 minutes after the injection of the IgG from the other 2 patients. (C) Percentage of fetal loss in pregnant mice receiving patient IgG and MBB2ΔCH2. (D) Displacement of patient IgG bound to β2GPI by MBB2ΔCH2 evaluated as described in Methods. The procoagulant and proabortive effects of MBB2 and MBB2ΔCH2 were evaluated and analyzed as reported in Figure 2. The results of vascular occlusion are expressed as mean ± SD; percentages of fetal loss are expressed as median ± interquartile range presented as box plots. *P < .05.
Because aPL circulates in the blood at the time of embryo implantation when β2GPI is detected on the surface of trophoblasts and decidual endothelial cells,8 we believe that MBB2ΔCH2 should displace antibodies already bound to β2GPI to produce a beneficial effect in patients with APS. To address this issue, immobilized β2GPI was incubated with patient IgG for 30 minutes and subsequently exposed to MBB2ΔCH2. The residual aPL bound to β2GPI was measured using a secondary antibody directed against the CH2 domain of IgG that recognized the patient antibodies but not the CH2-deleted antibody. The results presented in Figure 6D demonstrate that the mutant antibody removed approximately 40% to 50% of the patient IgG. The failure to displace all the bound antibodies may be explained by the fact that the polyclonal antibodies circulating in APS patients are likely directed against other β2GPI epitopes in addition to DI as recently reported by our group.29
Discussion
Unraveling the mechanisms involved in the development of vascular thrombi and pregnancy loss in APS patients is critically important for the development of therapeutic strategies to control these pathologic conditions. The results of this study indicate that this goal is achievable using a modified version of a non–complement-fixing human antibody that recognizes the DI domain of β2GPI and prevents the procoagulation and proabortion activities of patient antibodies. In contrast, the parent antibody mimicked the pathogenic effects of aPL antibodies.
MBB2, similar to the patient antibodies, reacted with immobilized β2GPI and, to a much lesser extent, with the soluble molecule, suggesting that the epitope recognized by the scFv-Fc in the DI domain was exposed mainly because of its binding to the plastic wells or to the cell membranes. These results are consistent with the observation that β2GPI circulates in plasma in a circular form in which domain V physically interacts with domain I and shields the epitope recognized by MMB2.32-34
Our in vivo findings demonstrate that MBB2 mimicked the activity of aPL antibodies, leading to the induction of blood clots and pregnancy failure, and support the concept that the DI domain represents the major target of pathogenic antibodies. These data corroborate previous observations in clinical studies and indicate that the antibodies to DI detected frequently in APS patients are more often correlated with a history of thrombosis than the antibodies to the other domains in the molecule.22,29,35 Similarly, Ioannou and colleagues reported an inhibitory effect of soluble DI on the antibody-mediated increase in thrombus size in mice.7 Despite the distinct clinical and histopathological features of thrombosis and fetal demise, the pathological events leading to the onset of these 2 clinical manifestations are initiated by the antibody specific for the DI domain of β2GPI, although the contribution of antibodies with different specificities cannot be ruled out.10,11 The only difference between the onsets of these 2 diseases lies in the requirement for proinflammatory stimuli to induce the formation of thrombi, whereas prior stimulation is not required for antibody-mediated fetal loss.
Although the mechanisms implicated in the development of these 2 clinical conditions have not been fully clarified, the diseases share the complement system as a key triggering mediator. The contribution of complement activation products to the MBB2-mediated pathological events was suggested by the in vitro and in vivo observations of complement deposition on the antibody-β2GPI complexes bound to the ELISA plates or to damaged tissues. The involvement of the terminal pathway of complement activation is further supported by the failure of MBB2 to reproduce the thrombosis and the fetal loss induced in normal animals using the C6-deficient rats or the mice depleted of C5. C5a has been identified as an important mediator of fetal loss because its interaction with the C5a receptor on neutrophils leads to the release of tissue factor12,36 ; however, the contribution of the terminal complement complex cannot be totally excluded based on the detection of C9 at sites of fetal injury. In contrast, the finding that thrombotic vessel occlusion induced by aPL and, in this study, by anti-β2GPI was markedly reduced in C6-deficient rats9 and mice37 suggests a dominant role of this complex in blood clot formation.
An interesting finding of this investigation was the loss of the procoagulant and proabortive effects of MBB2 observed using the variant antibody lacking the CH2 domain, which is required for C1q binding and complement activation. This observation provides additional evidence supporting a critical role of the complement system in animal models of APS. Because MBB2ΔCH2 retained the ability of the parent antibody to bind to β2GPI, it is not surprising that the antibody effectively prevented the pathogenic effect induced by aPL. The finding that MBB2ΔCH2 displaced patient antibodies bound to β2GPI due to its higher affinity for β2GPI suggests that this variant molecule may be an effective tool to prevent vascular thrombosis and fetal loss in pregnant patients with circulating antibodies to β2GPI. A synthetic peptide derived from cytomegalovirus, which mimics the V domain of β2GPI and competes with this molecule for binding to target cells, was shown to reduce the size of thrombi38,39 and the incidence of fetal loss in mice treated with IgG-aPL.40 Although the ability of the peptide to displace bound β2GPI has not been investigated, the removal of cell-bound molecules should be considered with caution because this may affect fetal development and pregnancy outcomes, as suggested by the defect in embryo implantation and placental morphogenesis observed in β2GPI−/− mice.41 This would not be the case for MBB2ΔCH2, which simply removes the patient antibodies, leaving the bound β2GPI untouched. A peptide that shares sequence similarity with a region in domain I/II of β2GPI, as well as a recombinant DI and its mutant, have been reported to reduce the thrombus size and fetal loss induced by IgG-aPL; however, the ability of the peptides to displace β2GPI-bound antibodies has yet to be established.42-44 A tetravalent conjugate of domain 1 of β2GPI was reported to induce tolerance reducing the level of anti-β2GPI antibodies in immunized mice.45 The ability of this approach to prevent blood clot and fetal loss was not investigated, although the finding that 35% of antibody was still detected in mice receiving the maximum amount of the conjugate raises some doubt that it may be effective in protecting APS patients from these clinical manifestations.
In conclusion, we presented evidence that a human monoclonal antibody directed against β2GPI DI exhibits complement-dependent procoagulant and proabortive effects, and a variant of this antibody, lacking the CH2 domain, is effective in preventing blood clot formation and fetal loss induced by aPL. This antibody could represent a useful tool to prevent recurrent pregnancy loss in APS patients unresponsive to standard therapy and to treat life-threatening conditions such as the catastrophic APS variant.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The contribution of Sabrina Boscolo and Mara Saccomano is gratefully acknowledged.
This work was supported by grants from the Ministry of Health (Ricerca Finalizzata RC41/08 e RC 01/09), Ministry of University (PRIN MFXE7L_004), and Fondazione Casali (F.T.) and from Ricerca Corrente “Target molecolari della APS” 22-07-2010, Istituto di Ricovero e Cura a Carattere Scientifico Istituto Auxologico Italiano, Milan, Italy (P.L.M.).
Authorship
Contribution: C.A., D.S., P.L.M., and F.T. designed the experiments and wrote the manuscript; M.O.B. and C.G. isolated β2GPI and performed the in vitro binding experiments; P.D. performed the in vivo experiments and the immunofluorescence analysis; F.G. performed the biosensor analysis; R.B. analyzed the implantation sites and contributed to the preparation of the figures; P.M. prepared the neutralizing antibody to C5 and monitored the anti-C5 treated mice for complement depletion; and F.P. performed the statistical analysis of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Tedesco, Department of Life Sciences, University of Trieste, via Valerio, 28–34127 Trieste, Italy; e-mail: tedesco@units.it.
References
Author notes
C.A. and P.D. contributed equally to this work.
P.L.M. and F.T. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal