Key Points
Follicular lymphoma-like cells found in healthy individuals accumulate within germinal centers in reactive lymphoid tissues.
Follicular lymphoma-like cells are nonproliferating cells in situ and in vitro.
Abstract
Follicular lymphoma (FL) is a B-cell neoplasm resulting from the transformation of germinal center (GC) B cells. Although t(14;18) and ectopic B-cell lymphoma 2 (BCL2) expression constitute the genetic hallmark of FL, t(14;18)pos B cells bearing genotypic and phenotypic features of FL cells can be found in the blood of most healthy individuals. Nevertheless, the localization of these FL-like cells (FLLCs) in nonmalignant GC-rich tissues and the functional consequences of BCL2 overexpression have not been evaluated thus far. Among 85 reactive lymph node (RLN) samples, 14% were found to contain high levels of t(14;18) by quantitative polymerase chain reaction. In t(14;18)hi RLNs, CD20posBCL2posCD10pos FLLCs consistently accumulated within the GC, essentially as nonproliferative CXCR4neg centrocytes. Moreover, they displayed a reduced response to proliferative stimuli in vitro. Altogether, our findings provide new insights into in situ FLLC functional properties and suggest that these cells have not acquired the ultimate genetic events leading to FL transformation.
Introduction
Follicular lymphoma (FL) is the most frequent indolent lymphoma and results from the malignant transformation of germinal center (GC) B cells.1 FL pathogenesis is a protracted, multistep oncogenic process, in which the first genetic hit is ascribed to the t(14;18) translocation that results from the illegitimate joining between the BCL2 proto-oncogene and the IGH locus. Although t(14;18) is the molecular hallmark of FL, t(14;18)pos B cells are found in the blood of most healthy individuals at a low frequency, indicating that ectopic expression of the antiapoptotic B-cell lymphoma 2 (BCL2) protein is not sufficient for tumor progression.2 Interestingly, transgenic overexpression of BCL2 results in a delay in cell cycle progression both in vitro and in mouse models.3-5 However, such an antiproliferative effect of BCL2 has never been evaluated in naturally occurring human t(14;18)pos B cells.
FL pathogenesis requires additional oncogenic events that are supposed to be acquired in the GC on exposure to the off-target effects of activation-induced cytidine deaminase, the key enzyme of antigen-driven antibody diversification. Such oncogenic events could already be detected in early FL lesions including FL in situ (FLIS).6,7 The frequency of t(14;18)pos circulating B cells increases with age and exposure to pesticides,8-11 reflecting the persistence and expansion of clonal populations of memory B cells bearing genotypic and phenotypic features of FL cells and called FL-like cells (FLLCs). Existence of t(14;18)pos precursor lymphoma-initiating cells has been recently supported by 2 reports of clonally related FL arising in both the donor and the recipient after allogeneic hematopoietic stem cell transplantation.12,13 Similarly, a clonal relationship between FL and FLLCs was reported by molecular backtracking in 3 FL cases for which prediagnostic blood samples were available.14 However, the connection between FLLCs and progression to overt FL remains elusive.
To further explore the in situ FLLC evolution, we identified a series of healthy individuals with GC-rich reactive lymph nodes (RLNs) displaying high levels of t(14;18), allowing us to visualize and characterize FLLCs in situ and to perform functional in vitro studies. We demonstrated that FLLCs are nonproliferating cells with specific enrichment within the GC.
Study design
For details, see supplemental Methods available on the Blood Web site.
Samples
Lymph node samples were collected from individuals recruited under institutional review board approval and the informed consent process according to the Declaration of Helsinki. Among them, hyperplastic RLNs from 85 individuals with nonmalignant diseases were both preserved as formalin-fixed paraffin-embedded tissues and rapidly dissociated and stored as frozen viable cells. When indicated, CD20pos B lymphocytes were purified using magnetic beads, whereas CD20posCD10posCXCR4pos Hoechstpos proliferating centroblasts and CD20posCD10posCXCR4negHoechstneg nonproliferating centrocytes were sorted on a FACSAria cell sorter (BD Biosciences).
Quantitative polymerase chain reaction and fluctuation polymerase chain reaction
Genomic DNA was extracted using the All Prep kit (Qiagen). The absolute quantification of t(14;18) was performed by real-time quantitative polymerase chain reaction (qPCR) for BCL2/JH and GAPDH.9 Standard curves were generated using serial dilutions of cloned BCL2/JH and GAPDH PCR products. As reported,13 the sensitivity of this technique is about 4 × 10−5. The χ2 test was used for analyzing differences between groups (GraphPad Prism software). Semiquantitative fluctuation PCR was performed on cell-sorted centroblasts and centrocytes, as previously described.10 The sensitivity of this technique is about 10−6.
Immunohistochemical and immunohistofluorescence studies
Deparaffinized tissue sections were used for double immunohistochemical staining (PAX5/BCL2) and triple immunohistofluorescence staining (CD20/BCL2/CD10).
Proliferation assay
CD20pos purified B cells were stained with carboxyfluorescein succinimidyl ester (CFSE) and stimulated as previously described.15 After 4 days of culture, highly proliferative viable CFSEloDAPIneg and nonproliferative viable CFSEhiDAPIneg B cells were sorted before quantification of t(14;18) by qPCR.
Results and discussion
In humans, circulating FLLCs have been characterized at the phenotypic and molecular levels,9,10 but their in situ distribution within nonmalignant tissues with follicular hyperplasia and the functional consequences of endogenous BCL2 overexpression have not been evaluated.
To characterize FLLCs in situ, BCL2/JH translocation was quantified by qPCR on 85 consecutive RLNs. Twelve samples (14%) were found to be positive (supplemental Table 1), with a frequency ranging from 4 to 66 × 10−5, ie, 1 in 25 000 to 1 in 1 500 cells, within samples containing 38.7 ± 8% CD19pos B cells (data not shown). The prevalence of these t(14;18)hi samples was independent of gender but showed a significant correlation with age, reaching 28% in donors >50 years. A frequency of circulating FLLCs >1 in 25 000 mononuclear cells was previously reported in 4.6% of healthy adults, and these t(14;18)hi donors reached 9.2% >50 years, in agreement with our age-related prevalence increase.11,16 Overall, we identified 12 RLN with a frequency of FLLCs suitable for in situ visualization and/or functional studies and focused on 8 of them (RLN 1-8) for further analysis. We also selected 3 t(14;18)neg RLNs (RLN 9-11) as controls.
To visualize FLLCs in situ we first performed immunohistochemical staining for the pan-B-cell transcription factor PAX-5 and for BCL2, that is specifically down-regulated in normal GC B cells, except in the presence of the t(14;18) translocation.17 This approach revealed variable amounts of isolated PAX-5posBCL2pos B cells within the GC in t(14;18)hi RLNs, whereas such cells were essentially undetectable in the GC from t(14;18)neg samples (supplemental Figure 1). These GC PAX-5posBCL2pos cells visualized inside the GC could comprise IgDneg cells, corresponding to bona fide FLLCs, but also some IgDpos small lymphocytes invaginating from the mantle zone (data not shown). Moreover, because BCL2 is normally expressed in naive and memory B cells, such a strategy could not evaluate the presence of FLLCs outside the GC. We thus focused on CD10, a classical GC B-cell marker, and quantified cells coexpressing CD20, BCL2, and CD10 in 5 t(14;18)hi and 3 negative RLNs by immunohistofluorescence (Table 1; supplemental Figure 2). In fact, FLLCs have been suggested to resemble FL cells in retaining an atypical CD10posBCL2pos frozen GC-like phenotype outside the GC.2 We consistently detected more CD20posBCL2posCD10pos triple positive cells in t(14;18)hi than in t(14;18)neg RLNs. Interestingly, these cells were visualized both inside and outside the GC in t(14;18)hi RLNs. By contrast, in control t(14;18)neg samples, the few triple positive cells were exclusively located outside the GC and might correspond to rare CD27posBCL2posCD10pos memory B cells previously described in peripheral blood.9,18 However, the number of t(14;18)pos cells outside the GC was higher in t(14;18)hi than in negative RLNs and probably included post-GC FLLCs. In agreement, we detected by long-range PCR that the majority of t(14;18)hi RLNs displayed a class switch recombination to γ on the translocated allele (supplemental Figure 4). Most importantly, when normalizing the percentage of triple positive cells within GC to the surface occupied by the GC in corresponding samples, we pinpointed in 5 of 6 t(14;18)hi samples a clear enrichment (3.5- to 6.5-fold) of CD20posBCL2posCD10pos within the GC. This enrichment strongly suggests a preferential homing/retention into GC.
Distribution of CD20posBCL2posCD10pos triple positive cells in the GC of RLNs
| Sample* . | Triple positive cells/105 cells† . | Triple positive cells in GC . | GC surface (%)‡ . | Enrichment coefficient in GC§ . |
|---|---|---|---|---|
| RLN1 | 39 | 15 (38.5%) | 6.0 | 6.41 |
| RLN2 | 18 | 7 (39%) | 11.3 | 3.45 |
| RLN3 | 15 | 5 (33.3%) | 6.1 | 5.45 |
| RLN4 | 48 | 35 (72.9%) | 11 | 6.63 |
| RLN5 | 53 | 3 (5.7%) | 5.7 | 1 |
| RLN9 | 4 | 0 | 8.3 | 0 |
| RLN10 | 8 | 0 | 4.9 | 0 |
| RLN11 | 5 | 0 | 31.7 | 0 |
| Sample* . | Triple positive cells/105 cells† . | Triple positive cells in GC . | GC surface (%)‡ . | Enrichment coefficient in GC§ . |
|---|---|---|---|---|
| RLN1 | 39 | 15 (38.5%) | 6.0 | 6.41 |
| RLN2 | 18 | 7 (39%) | 11.3 | 3.45 |
| RLN3 | 15 | 5 (33.3%) | 6.1 | 5.45 |
| RLN4 | 48 | 35 (72.9%) | 11 | 6.63 |
| RLN5 | 53 | 3 (5.7%) | 5.7 | 1 |
| RLN9 | 4 | 0 | 8.3 | 0 |
| RLN10 | 8 | 0 | 4.9 | 0 |
| RLN11 | 5 | 0 | 31.7 | 0 |
RLN 1-5 and RLN 9-11 samples correspond to t(14;18)hi and t(14;18)neg LNs, respectively. The frequency of t(14;18) was determined by qPCR on dissociated RLN cells.
Number of CD20posBCL2posCD10pos triple positive cells visualized by immunohistofluorescence. For each sample, between 1 and 3 large (2-5 mm2) representative areas including GC were chosen (corresponding to 7 × 104 to 2 × 105 cells per sample) for counting.
Percentage of the analyzed area occupied by GC zones (defined as areas of CD20posCD10posBcl2neg cells).
Enrichment coefficient = (% triple positive cells in GC)/(% GC surface).
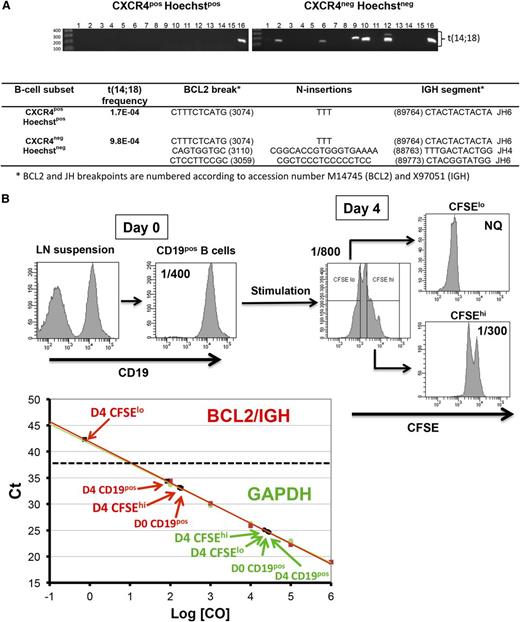
Although FLLCs accumulate within the GC of t(14;18)hi RLNs, they presented as scattered cells without proliferation foci. In addition, high t(14;18) frequency was not correlated to high GC expansion, making it unlikely that resident FLLCs contributed significantly to follicular hyperplasia. These observations raised the question of the proliferation potential of FLLCs within the hyperproliferative GC environment. To address this issue in the absence of any specific marker allowing the sorting of FLLCs, we first purified from t(14;18)hi RLN proliferative centroblasts vs nonproliferative centrocytes based on their differential membrane expression of CXCR419 and staining with Hoechst dye (supplemental Figure 3). Interestingly, CD20posCD10posCXCR4posHoechstpos centroblasts contained a lower frequency of t(14;18)pos B cells than their nonproliferative centrocyte counterparts as determined by sensitive fluctuation PCR (Figure 1A). In addition, different B-cell clones could be detected in centrocytes, including the unique one detected in centroblasts. To better understand the lack of proliferation of FLLCs in situ, purified B cells from 3 t(14;18)hi RLNs were stained with CFSE and stimulated in vitro during 4 days using an optimal cocktail to activate both naive and memory B cells.15 Highly proliferative (CFSElo) and nonproliferative (CFSEhi) B cells were then sorted, and the frequency of the BCL2/JH translocation was determined (Figure 1B). As shown in supplemental Table 2, t(14;18)pos cells were systematically found in CFSEhi nonproliferative cells, whereas they could not be detected in CFSElo proliferative cells. Altogether, these data demonstrate that human nonmalignant B cells with naturally occurring t(14;18) accumulate within a poorly proliferative B-cell compartment and support the hypothesis of an antiproliferative effect of BCL2, as previously shown in mouse and human BCL2-transgenic B cells.3-5
FLLCs are nonproliferative cells. (A) Distribution of the t(14;18)pos cells within proliferative CD20posCD10pos CXCR4posHoechstpos centroblasts vs CD20posCD10posCXCR4negHoechstneg nonproliferative centrocytes cell sorted from a t(14;18)hi RLN7 sample. Genomic DNA from each GC subsets was tested using a 2-step fluctuation nested PCR assay consisting of 16 replicates. BCL2/IGH translocations were sequenced. Multiple size bands revealed oligoclonality. (B) CFSE-labeled CD20pos purified B cells from RLN4 were stimulated for 4 days. Highly proliferative viable CFSEloDAPIneg and nonproliferative viable CFSEhiDAPIneg B cells were then sorted before quantification of t(14;18) by qPCR. Standard curves were generated from cloned BCL2/IGH (red) and GAPDH (green) PCR products, and it was demonstrated that detection of the BCL2/IGH transcript from the CFSEhi population was above the CFSElo cell population, where it remained below the quantification threshold of 1/25 000 cells. NQ, not quantifiable.
FLLCs are nonproliferative cells. (A) Distribution of the t(14;18)pos cells within proliferative CD20posCD10pos CXCR4posHoechstpos centroblasts vs CD20posCD10posCXCR4negHoechstneg nonproliferative centrocytes cell sorted from a t(14;18)hi RLN7 sample. Genomic DNA from each GC subsets was tested using a 2-step fluctuation nested PCR assay consisting of 16 replicates. BCL2/IGH translocations were sequenced. Multiple size bands revealed oligoclonality. (B) CFSE-labeled CD20pos purified B cells from RLN4 were stimulated for 4 days. Highly proliferative viable CFSEloDAPIneg and nonproliferative viable CFSEhiDAPIneg B cells were then sorted before quantification of t(14;18) by qPCR. Standard curves were generated from cloned BCL2/IGH (red) and GAPDH (green) PCR products, and it was demonstrated that detection of the BCL2/IGH transcript from the CFSEhi population was above the CFSElo cell population, where it remained below the quantification threshold of 1/25 000 cells. NQ, not quantifiable.
In conclusion, our in situ visualization of FLLCs in nonmalignant RLNs with follicular hyperplasia demonstrates for the first time that FLLCs are not randomly distributed but display preferential homing within the GC, a property shared with FL and FLIS cells that accumulate as poorly proliferative centrocytes in the early stage of the disease. FLIS have been previously proposed as the in situ counterpart of circulating FLLCs,20 but we demonstrate here that such t(14;18)pos B cells could accumulate within the GC even in the absence of classical FLIS lesions characterized as homogeneous follicles exhibiting strong CD20, CD10, BCL6, and BCL2 positivity.21 Scattered GC FLLCs thus potentially represent an earlier precursor stage in FL pathogenesis. Altogether, our findings provide new insights into the dynamics of FLLC progression and the connection between FLLC and FL.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Patrick Tas, Céline Pangault, and the Centre de Ressources Biologiques–Santé of Rennes' hospital for providing high-quality samples and Gersende Caron for cell sorting.
This work was supported by research grants from the Institut National du Cancer (INCa libre PL06-10 and Programmes d'Actions Intégrées de Recherche Lymphome 2008-019), the Ligue Contre le Cancer (Equipe Labellisée 2013), and institutional grants from Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique.
Authorship
Contribution: J.T., C. Menard, and S.R. designed and performed research and analyzed data; N.M., C. Monvoisin, and L.C. performed research; B.N. contributed to the study design; P.G. contributed to the study design, analyzed data, and wrote the paper; and C.S. and K.T. designed and supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudine Schiff, Centre d'Immunologie de Marseille-Luminy, Case 906, F-13288 Marseille Cedex 09, France; e-mail: schiff@ciml.univ-mrs.fr; and Karin Tarte, INSERM U917, Faculté de Médecine, 2 Avenue du Pr Léon Bernard, F-35043 Rennes, France; e-mail: karin.tarte@univ-rennes1.fr.
References
Author notes
J.T. and C.M. contributed equally to this work.
C.S. and K.T. contributed equally to this work.