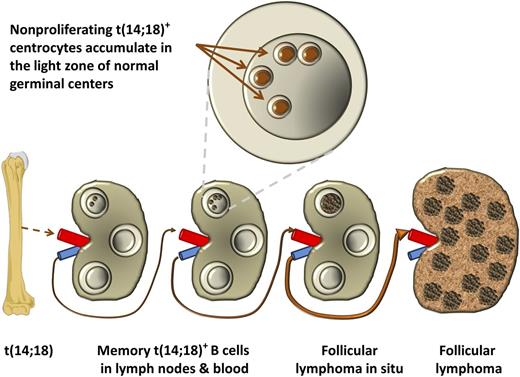
In this issue of Blood, Tellier et al report on extremely rare t(14;18)-positive cells within reactive lymph nodes of normal individuals. They convincingly show that these cells preferentially reside as nonproliferating cells in germinal centers.1
Tellier et al1 propose that the rare naïve t(14;18)-positive B cells as produced from erroneous VDJ recombinations in bone marrow precursor B cells may preferentially home germinal centers of lymph nodes where they reside as nonproliferating cells. Several rounds of reentry in this compartment in which the cells accumulate DNA damage may lead to functionally important gene mutations and consequently to the development of a so-called follicular lymphoma in situ and ultimately to overt follicular lymphoma.
Tellier et al1 propose that the rare naïve t(14;18)-positive B cells as produced from erroneous VDJ recombinations in bone marrow precursor B cells may preferentially home germinal centers of lymph nodes where they reside as nonproliferating cells. Several rounds of reentry in this compartment in which the cells accumulate DNA damage may lead to functionally important gene mutations and consequently to the development of a so-called follicular lymphoma in situ and ultimately to overt follicular lymphoma.
Using primitive nested polymerase chain reaction methodologies, Limpens et al was the first to find t(14;18)-carrying cells in reactive lymphoid tissues of otherwise healthy persons.2 Later work by the same and other groups showed that these rare translocations can be regularly found in blood B cells of normal adults with some associations to age and exposure to pesticides.3 The risk in developing follicular lymphoma is estimated to be very low; however, recent data showed that individuals with a frequency of >1 translocation-positive cell in 104 leukocytes (approximately 1 in 500 B cells) have a 23-fold increased risk in developing follicular lymphoma over a period of 15 years.4
The t(14;18) is derived from erroneous recombination of variable, diverse and joining (VDJ) gene segments in precursor B cells, and previously it was thought that circulating t(14;18)+ cells should be naive B cells. In 2007, Roulland et al provided a surprising publication showing that these cells, just like follicular lymphoma cells, represent memory B cells that have undergone somatic hypermutations and abortive class switching, proving that they are postgerminal center memory B cells.5
In 2002 Cong et al identified a novel entity called “follicular lymphoma in situ” in which individual germinal centers of otherwise normal lymph nodes are heavily colonized by translocation-positive B cells.6 This in situ lymphoma is found in ∼2% of all individuals in which lymph nodes are removed for other reasons than lymphoma diagnostics.7 These rare lesions have already accumulated several genetic alterations underway to follicular lymphoma and obviously may have little to do with the circulating t(14;18)+ cells that are present in so many normal adults.8,9
Tellier et al provide an important missing link between these circulating t(14;18)-positive memory B cells in healthy individuals and their origin from bone marrow precursor B cells by the in situ analysis of t(14;18)+ cells in lymph nodes of healthy individuals. As in peripheral blood, these cells are identifiable in a sizeable proportion of individuals. Using triple staining for CD20, CD10, and B-cell lymphoma 2 (BCL2) and cell sorting for CD20, CD10, and C-X-C chemokine receptor 4 (CXCR4; a marker of centroblasts), translocations are mainly seen in the CXCR4 dim staining centrocytes. Moreover, by labeling for carboxyfluorescein succinimidyl ester (CFSE) before in vitro B-cell stimulation and flow sorting, they are exclusively found in the strongly CFSE-positive nonproliferating cells. The authors suggest that this nonproliferating state is caused by aberrant BCL2 overexpression.
The current paper suggests a preferential homing to germinal centers where these cells undergo somatic mutations and class switching and also further steps in the development of (pre)malignant clones, incidentally giving rise to a follicular lymphoma in situ (see figure). The next, even more challenging step will be to get these rare cells in hand to further investigate them by mutation analysis and in functional assays.
Conflict-of-interest disclosure: The author declares no competing financial interests.