In this issue of Blood, Matsumoto et al report that T cell–specific deletion of the cyclin-dependent kinase inhibitor p57Kip2 (p57) in mice leads to a block in T-cell development as a result of hyperactivation of the E2F-p53 pathway and demonstrate that the loss of p57 accelerates lymphomagenesis in the absence of p53.1
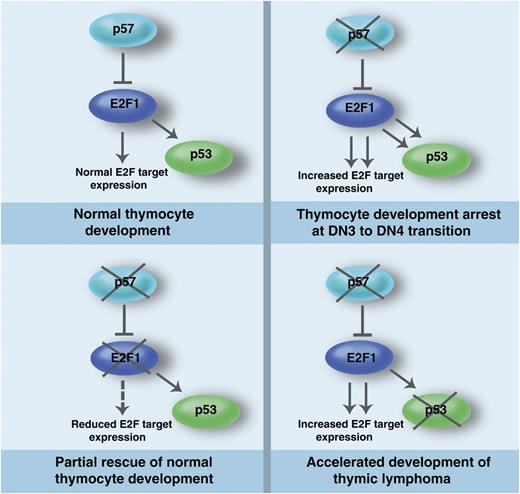
The p57-E2F1-p53 pathway in thymocyte development and lymphomagenesis. p57 regulates E2F1 to control E2F target gene expression and p53 activity during normal thymocyte development. In the absence of p57, increased E2F target expression and p53 hyperactivation contribute to the arrest of thymocyte development at the DN3 to DN4 transition. The developmental arrest of thymocytes lacking p57 is partially rescued by the loss of E2F1. Loss of p53 in thymocytes lacking p57 leads to acceleration of thymic lymphoma development, suggesting that the observed p53 hyperactivation in the absence of p57 executes an important tumor suppressor function in thymocytes. Professional illustration by Marie Dauenheimer.
The p57-E2F1-p53 pathway in thymocyte development and lymphomagenesis. p57 regulates E2F1 to control E2F target gene expression and p53 activity during normal thymocyte development. In the absence of p57, increased E2F target expression and p53 hyperactivation contribute to the arrest of thymocyte development at the DN3 to DN4 transition. The developmental arrest of thymocytes lacking p57 is partially rescued by the loss of E2F1. Loss of p53 in thymocytes lacking p57 leads to acceleration of thymic lymphoma development, suggesting that the observed p53 hyperactivation in the absence of p57 executes an important tumor suppressor function in thymocytes. Professional illustration by Marie Dauenheimer.
T cells are important components of the adaptive immune system central for cell-mediated immunity. All T cells originate from hematopoietic stem cells in the bone marrow and mature in the thymus. T-cell development involves progression of immature thymocytes that are double-negative (DN) for CD4 and CD8 (DN, CD4−CD8−) to the double-positive (DP, CD4+CD8+) thymocytes, which finally mature into single-positive (SP) CD4+ or CD8+ T cells. The DN thymocytes are further classified based by CD44 and CD25 expression: CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3), and CD44−CD25− (DN4). The differentiation of immature thymocytes into mature T cells involves several rounds of cell divisions and massive clonal expansion, indicating critical roles for cell-cycle regulators such as cyclin-dependent kinases (Cdks) and their inhibitors (CKIs) in T-cell development.2 For instance, Cdk6 and its cognate partner cyclin D3 are critical for normal thymocyte development at the DN stage and for lymphomagenesis.2
p57Kip2 is an imprinted gene that inhibits Cdk2, Cdk3, and Cdk1 and belongs to the Cdk interacting protein/kinase interacting protein (CIP/KIP) family of Cdk inhibitors (CKIs) that also includes p21Cip1/Waf1 and p27Kip1.3 Germ-line mouse knockouts for p57 die shortly after birth with phenotypes similar to Beckwith-Wiedemann syndrome (BWS) patients.4 When the p27 gene was knocked-in to the p57 locus in mice, many of the defects displayed by p57 knockouts were corrected, suggesting that the phenotypes of p57 knockout mice are largely attributable to its spatial and temporal expression patterns as well as to differences in the sensitivity of tissues to insufficient inhibition of Cdk activity and cell proliferation.5 Here Matsumoto et al show that conditional T cell–specific deletion of p57 leads to a differentiation block at the DN3 to DN4 transition, resulting in reduced thymic size and cellularity.1 The differentiation block and smaller thymic size in the absence of p57 is surprising and important given that p57 is a Cdk inhibitor whose deficiency is expected to promote increased cell proliferation. This supports the emerging notion that the roles of Cdks, cyclins, and Cdk inhibitors are not only limited to regulating cell proliferation but are critical for differentiation and survival in a cell type–specific manner.6 Matsumoto et al also demonstrate that the defects in thymocytes lacking p57 are caused by hyperactivation of E2F1 and p53 because deletion of these genes rescued the defects in thymocytes lacking p57 (see figure). This critical role of the p57-E2F1-p53 axis in thymic development is remarkably similar to its functions in brain development.7
p53 is mutated in most human cancers, and inactivation of the p53 tumor suppressor pathway is critical for oncogenic transformation. Mice lacking p53 succumb to thymic lymphomas and other cancers by 6 months of age.8 Matsumoto et al demonstrate that T cell–specific loss of p57 leads to a differentiation block at the DN3 stage and p53 hyperactivation, which accelerates lymphoma development in a p53-null background. This reveals a critical tumor suppressor function for p57 in T lymphocytes. Inactivation of the TCRβ gene enhancer in mice leads to a block in T-lymphocyte development at the CD44−CD25+ (DN3) stage, where recombination-activating genes (RAG) are expressed, and dramatically accelerates lymphomagenesis in a p53-null background.9 These lymphomas display evidence of RAG-dependent chromosomal translocations and amplifications associated with human hematologic malignancies, suggesting that mutations that lead to a block in lymphocyte development, at a stage associated with continued expression of RAG genes, can act as a potential precarcinogenic event.9 Based on the findings of this study, it is likely that persistent RAG expression in the thymocytes lacking p57 that are blocked at the DN3 stage may activate the DNA damage checkpoint, leading to the observed p53 hyperactivation, which acts as a tumor suppressor whose loss leads to rapid lymphoma development. Hence the differentiation block in T cells lacking p57 is possibly a critical mediator of the accelerated lymphoma development in a p53-null background. Loss of the CKI p57 leads to increased Cdk activity, which raises the question of the Cdk substrates in this context. Cdks are known to phosphorylate p53, E2F1, and definitely Rb (which represses E2F1 transcriptional activity), but it cannot be excluded that there are lymphomagenesis-specific Cdk substrate(s) to be uncovered. Because the knock-in of the p27 gene into the p57 locus corrected the defects in hematopoietic stem cells lacking p57,10 it would be interesting to check whether development of T cells lacking p57 can be similarly rescued. Future studies to determine the mechanism by which p57 loss leads to a differentiation block in thymocytes will be instructive for understanding its role in normal T-cell development as well as lymphomagenesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal